Abstract
Objectives
We assessed the methodological quality and prognostic accuracy of clinical decision rules (CDR) in emergency department (ED) syncope patients.
Methods
We searched 5 electronic databases, reviewed reference lists of included studies and contacted content experts to identify articles for review. Studies that derived or validated CDRs in ED syncope patients were included. Two reviewers independently screened records for relevance, selected studies for inclusion, assessed study quality and abstracted data. Random effects meta-analysis was used to pool diagnostic performance estimates across studies that derived or validated the same CDR. Between study heterogeneity was assessed with I-squared statistic (I2), and subgroup hypotheses were tested using a test of interaction.
Results
We identified 18 eligible studies. Deficiencies in outcome (blinding) and inter-rater reliability assessment were the most common methodological weaknesses. Meta-analysis of the San Francisco Syncope Rule (SFSR) [sensitivity 86% (95%CI 83-89); specificity 49% (95%CI 48-51)] and the Osservatorio Epidemiologico sulla Sincope nel Lazio (OESIL) risk score [sensitivity 95% (95%CI 88-98); specificity 31% (95%CI 29-34)]. Subgroup analysis identified study design [prospective, diagnostic odds ratio (DOR) 8.82 (95%CI 3.5-22) vs. retrospective, DOR 2.45 (95%CI 0.96-6.21)] and ECG determination [by evaluating physician, DOR 25.5 (95%CI 4.41-148) vs. researcher or cardiologist, DOR 4 (95%CI 2.15-7.55)] as potential explanations for the variability in SFSR performance.
Conclusion
The methodological quality and prognostic accuracy of CDRs for syncope is limited. Differences in study design and ECG interpretation may account for the variable prognostic performance of the SFSR when validated in different practice settings.
Keywords: syncope, clinical decision rules
Introduction
Rationale
Syncope is a symptom of cerebral hypoperfusion1 and is defined as a short, sudden, self-terminating episode of transient loss of consciousness with failure to maintain postural tone.1, 2 In the United States it accounts for 0.6% of hospital admissions3 and up to 1.4% of all Emergency Department (ED) visits.4-8
Patients with syncope who present to the ED can be classified as “stable” or “unstable,” depending on their initial presentation (e.g. ongoing chest pain, upper GI bleeding), vital signs (e.g. hypotension, hypoxia) or ECG abnormalities (e.g. ischemic changes).9 “Unstable” syncope patients represent up to 70% of serious clinical events (myocardial infarction, arrhythmias, hemorrhage) in syncope patients who present to the ED.5, 10, 11
On the other hand, the evaluation and diagnosis of “stable” ED patients with syncope is more challenging. Most patients are well-appearing and asymptomatic upon arrival,12 and there are often no witnesses to the event. Emergency physicians are often unable to obtain a detailed and accurate history of the event. Finally, there are a myriad of possible etiologies – from benign to life-threatening – that may present as syncope. The concern for occult myocardial infarction and transient non-identified arrhythmias in “stable” patients with syncope may lead to hospitalization, with no clear effect on clinical outcome.2 Evaluation and management of patients with syncope represent a significant economic burden. Between 2000 and 2005 the cost of syncope admissions exceeded 10 billion dollars, with a median cost of hospitalization of $8579.3
Time constraints and lack of available diagnostic ancillary studies for evaluation of syncope in the ED make determining the cause and prognosticating the short-term outcome a difficult task. The reported diagnostic yield of the ED evaluation is extremely variable among studies (20%-70%).13-16 Thus, analogous to the evaluation of patients with chest pain,2 the focus of the ED assessment has shifted from diagnosis to risk stratification based on clinical factors.17
Clinical decision rules (CDR) are tools designed to assist clinicians in making decisions at the bedside. They are derived from original research and incorporate important predictors of outcome from the history, physical examination, and basic diagnostic tests. CDRs can be used to risk-stratify patients, using the probability of an adverse outcome to inform the course of action, including the need for further testing or observation.18, 19 Investigators have developed several clinical decision tools and risk scores to predict short and long term adverse outcomes in ED syncope patients. The primary purpose of these prediction rules is to aid clinical decision making and to safely determine patient disposition.
Objectives
In this review we sought to assess the methodological quality and prognostic accuracy of studies that derived or validated CDRs or risk scores that predict adverse outcomes in adult patients presenting to the ED with syncope.
Methods
The report of this systematic review and meta-analysis is consistent with recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)20 as applicable to diagnostic accuracy reviews. A protocol was developed with input from methodologists with expertise in systematic reviews (M.H.M., V.M.M.) and a content expert (W.W.D.) and is available upon request.
Search Strategy
An expert reference librarian (P.J.E.) designed and conducted a comprehensive literature search with input from the lead author (L.A.S.). The search strategy incorporating medical subject headings and keywords related to clinical prediction rules (clinical prediction guides, decision support techniques, algorithms, multivariate analyses, logistic models, risk assessment) and syncope (fainting, loss of consciousness, drop attack, near syncope). We searched the following databases: Medline (1966 to November 2009), EMBASE (1988 to November 2009), CINAHL, Web of Science (1993-2009), Cochrane Database of Systematic Reviews, Cochrane Controlled Trials register and the Database of Reviews of Effectiveness. No language restrictions were applied to the search strategy. The Medline (Ovid interface) search strategy is displayed in Appendix 1.
Abstracts from the American College of Emergency Physicians, American Academy of Emergency Medicine and the Society for Academic Emergency Medicine were hand-searched from 2007-2009. Experts in syncope and clinical decision rule development were consulted for additional published or unpublished reports. We also reviewed the bibliographies of all retrieved articles to identify potentially relevant articles not identified in the electronic search strategy.
Selection of Studies for Inclusion
The following inclusion criteria were applied according to published methodological standards for clinical prediction rules21: (1) prospectively or retrospectively derived or validated clinical decision rules or risk scores that predict subsequent adverse events in patients with syncope; (2) Patients presenting with syncope or near syncope to the ED. Syncope was defined as a sudden transient loss of consciousness with loss of postural tone that is brief, self-limiting and resolves without medical intervention;1, 2 (3) Based on original research, and (4) inclusion of 3 or more variables from the history, physical examination, and basic diagnostic tests. In order to conduct a more informative review, we did not exclude studies based on the timing of outcome assessment. Clinical practice guidelines and editorials were excluded. They were, however, used as potential bibliographic sources of eligible primary studies. In view of the inconsistent definition of syncope throughout the medical literature,22 we excluded studies including patients with other causes of transient loss of consciousness such as seizures, vertigo, hypoglycemia, dizziness, head trauma, coma, shock and other states of altered mental status. Studies that enrolled patients outside of the ED (i.e., hospital ward or outpatient facilities) were excluded.
Study Selection
Two reviewers (L.A.S and M.F.B) individually screened all titles and abstracts identified from the search strategy (phase I). Selection was based on potential relevance to the review and according to the predetermined inclusion and exclusion criteria. Reviewers were not blinded to the names of the authors, institutions, journal of publication, and/or results. Full manuscripts were obtained for all titles and abstracts considered to be potentially relevant by at least one reviewer.
Two reviewers (L.A.S and M.F.B) working independently assessed the full text articles for eligibility (phase II). Disagreements were resolved by consensus or by consulting a third co-investigator (E.P.H). We calculated chance-adjusted agreement for full text inclusion using kappa statistics with 95% confidence intervals.
Quality Assessment
Quality assessment of the clinical decision rules and risk scores was performed at the level of the rule itself and at the level of each study. The rules were classified according to a hierarchy of evidence for clinical decision rules (Table 3).23 Each rule was assigned a level (1-4) according to the strength of evidence. The individual studies were appraised using methodological standards for the development of clinical decision rules in emergency medicine.21 Answers were dichotomized as “yes” and “no/unclear.” Two reviewers (L.A.S and M.F.B) independently evaluated the quality of each included study, and chance-adjusted agreement was determined (kappa, 95% CI).
Table 3. Hierarchy of evidence for clinical decision rules.
| Level 1 | Rules that can be used in a wide variety of settings with confidence that they can change clinical behavior and improve patient outcomes | At least 1 prospective validation in a different population and 1 impact analysis, demonstrating change in clinician behavior with beneficial consequences. |
| Level 2 | Rules that can be used in various settings with confidence in their accuracy | Demonstrated accuracy in either 1 large prospective study including a broad spectrum of patients and clinicians or validated in several smaller settings that differed from one another. |
| Level 3 | Rules that clinicians may consider using with caution and only if patients in the study are similar to those in the clinician's clinical setting. | Validated in 1 narrow prospective sample |
| Level 4 | Rules that need further evaluation before they can be applied in the clinical setting. | Derived, but not validated or validated in split samples, large retrospective databases or by statistical techniques. |
Data Abstraction
Two authors independently abstracted data using a standardized data abstraction form. We abstracted the following data from each article: year of publication, setting, objective, predictor variables included, population characteristics (age, sex, past medical history, and admission rate), outcome measures, prevalence of adverse outcomes and duration of follow up. We also abstracted data needed for 2×2 contingency table analysis.
Author Contact and Missing Data
We contacted the corresponding author and last author for unclear or missing data and confirmed the correctness of the email address by a MEDLINE search of recent articles. If data were presented as a linear risk score, the author was contacted to provide enough information to convert it to a binary risk system. If a study had insufficient data for meta-analysis and no response was obtained after sending 2 emails, a phone call, and written communication to the corresponding author and last author, the study was excluded from the quantitative synthesis. Data were entered into Microsoft Excel (Microsoft Corporation).
Data Analysis
Due to the anticipated clinical heterogeneity between available clinical decision rules (different predictor variables, length of follow-up and outcome measures), meta-analysis was restricted a priori to studies that derived or validated the same CDR. Diagnostic test characteristics were calculated using publicly available Meta-DiSc statistical software (Unit of Clinical Biostatistics of the Ramon y Cajal Hospital).24 Using random effects meta-analyses, we pooled the sensitivities, specificities, likelihood ratios, and diagnostic odds ratios and estimated 95% confidence intervals for the outcomes of CDRs with two or more studies. The diagnostic odds ratio (DOR) of a test describes the ratio of the odds of a positive result in patients with disease compared to patients without disease.25 Inconsistency among studies was assessed using the I2 statistic, which indicates the proportion of variability in study estimates due to between-study heterogeneity. I2 values of 25%, 50% and 75% indicate low, moderate and high heterogeneity, respectively.26
Subgroup and sensitivity analyses
We performed a priori subgroup analyses to explain potential heterogeneity among included studies. We hypothesized that heterogeneity in prognostic performance could potentially arise from differences in study design, outcome period, ECG definition, ECG determination, and inclusion of “unstable” syncope patients. We tested these hypotheses using a test for interaction.27 Sensitivity analysis were conducted using a bivariate random-effects model in which the sensitivities and specificities were simultaneously analyzed to derive pooled likelihood ratios, rather than pooling the likelihood ratios directly across studies.28
Results
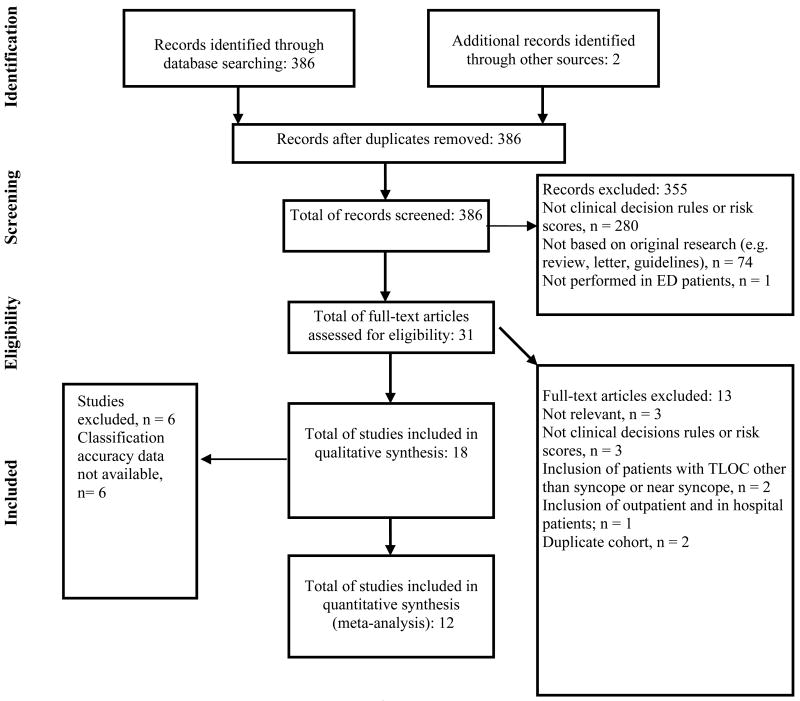
The electronic search strategy identified 388 records (Figure 1). Two articles (accepted for publication) were obtained from the primary authors.29, 30 Two duplicate records were removed. Three hundred and fifty five records did not meet inclusion criteria and were excluded. The full text of 31 potentially eligible articles was obtained for review. Full-text review identified 18 studies meeting inclusion criteria (κ= 0.84, 95% CI 62-100), representing 9 different CDRs and risk scores. Sufficient data to construct a 2×2 contingency table were directly available from the article in 10 studies, 5-7, 10, 29-35 and additional data were provided by the authors for 2 studies.32, 36 The twelve studies included in the quantitative analysis represent 5 different clinical prediction tools and risk scores.
Figure 1. Flow diagram of study selection process.
Study Characteristics
Characteristics of the included studies are shown in Tables 1 and 2. The 18 studies comprised at total of 10,994 patients. Nine studies were conducted in the United States,5-7, 10, 34, 36-38 1 in Canada,35 3 in Italy,29, 32, 39 2 in Australia,33, 40 2 in the United Kingdom30, 33, 40, 41 and 1 in Switzerland.16 There were 5 derivation studies,6, 34, 36, 37, 39 9 validation studies5, 7, 10, 31, 33, 35, 40, 41 and 4 derivation/validation studies.16, 30, 32, 38 Three studies validated more than one rule.29, 30, 41 Overall, there were 5,708 patients from derivation studies and 5,286 patients from validation studies. Thirteen studies assessed outcomes within 30 days of the index visit5-7, 10, 29-31, 33-37, 41 1 study predicted outcomes within 6 months,40 2 studies within one year,32, 38 and 1 study did not specify the duration of follow-up.16
Table 1. General Study Information (n = 18).
| Study | Population | Predictor Variables Included | Outcome Measures | Outcome Period |
|---|---|---|---|---|
| Cardiac ischemia in syncope | ||||
| Georgeson et al 1992 37 | 251 ED patients (men >30 yrs., women > 40 yrs.) with syncope and no chest pain, but complaints consistent with ACS, from six hospitals in the US. | ECG, arm, neck, shoulder or throat pain, Hx of exercise induced-angina and rales. | Acute cardiac ischemia | 48 hours |
| Risk stratification of syncope | ||||
| Martin et al 1997 38 | 252 ED patients with syncope from a single academic institution in the US for derivation; 374 ED patients with syncope for internal validation (same institution). | ECG, Hx of ventricular arrhythmia, Hx of CHF and age >45 years | Arrhythmia or 1-year mortality | 1 year |
| Risk score to predict arrhythmias in unexplained syncope | ||||
| Sarasin et al 2003 16 | 175 adult ED patients with unexplained syncope from a single academic hospital in Switzerland for derivation; 267 adult ED patients with unexplained syncope from tertiary care hospital in the US for validation. | ECG, Hx of CHF and age > 65 years | Cardiac arrhythmias | Not specified |
| OESIL Risk Score | ||||
| Colivicci et al 2003 32 | 270 ED patients (>12 years) with syncope from 6 community hospitals in Italy; 328 ED syncope patients from 2 hospitals in Italy for validation. | ECG, Age >65 years, Hx of cardiac dz., and no prodrome | Death from any cause | 12 months |
| OESIL validation: Hing et al 2005 40 | 100 adult ED patients with syncope from a single tertiary referral hospital in Australia. | ECG, Age >65 years, Hx of cardiac dz., and no prodrome | Adverse cardiac outcome: ischemic heart disease, arrhythmias and cardiac death. | 3-6 months |
| ROSE pilot study (OESIL, SFSR): Reed et al 2007 41 | 99 ED patients (≥16 years) with syncope from a single hospital in the United Kingdom. | SFSR: ECG, dyspnea, systolic BP <90, Hct <30%, and Hx of CHF : OESIL: ECG, Age >65 years, Hx of cardiac dz., and no prodrome | Death, AMI, arrhythmias, PE, hemorrhage, stroke, subarachnoid hemorrhage, acute procedure | 1 week, 1 month and 3 months |
| SFSR | ||||
| SFSR derivation: Quinn et al 2004 6 | 684 ED patients with syncope or near syncope from a single large academic hospital in the US. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | Death, MI, arrhythmia, PE, stroke, subarachnoid hemorrhage, hemorrhage, ED return visit and hospitalization | 7 days |
| SFSR validation: Quinn et al 2006 5 | 791 ED patients with syncope or near syncope from a single large academic hospital in the US. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | SFSR outcome measures | 30 days |
| SFSR external validation: Sun et al 2007 10 | 477 adult ED patients with syncope or near syncope from a single urban academic hospital in the US. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | SFSR outcome measures | 7 days |
| SFSR validation: Cosgriff et al 2007 33 | 89 adult ED patients with syncope or near syncope from a teaching hospital in Australia. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | SFSR outcome measures | 7 days |
| SFSR external validation: Birnbaum et al 2008 31 | 713 ED adult patients with syncope or near syncope from a single urban academic hospital. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | SFSR outcome measures | 7 days |
| SFSR application: Schladenhaufen et al 2008 7 | 517 elderly ED patients (≥65 years) with syncope or near syncope from a community teaching hospital. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | SFSR outcome measures | 7 days |
| SFSR external validation: Thiruganasambandamoorthy et al 2009 35 | 505 ED patients (≥16 years) with syncope from a single center urban hospital in Canada. | ECG, dyspnea, Hct <30%, systolic BP <90 and Hx of CHF | SFSR outcome measures | 30 days |
| SFSR and OESIL validation: Dipaola et al (in press) 29 | 488 adult ED patients with syncope within previous 48 hours from 2 general hospitals in Italy. | SFSR: ECG, dyspnea, systolic BP <90, Hct <30%, and Hx of CHF : OESIL: ECG, Age >65 years, Hx of cardiac dz, and no prodrome | Death, need for major therapeutic procedures and early readmission to the hospital | 10 days |
| Boston Syncope Rule | ||||
| Boston Syncope Rule validation: Grossman et al 2007 34 | 293 adult ED patients with syncope from a large urban teaching hospital in the US. | ACS sign/symptoms, conduction disease, worrisome cardiac hx, valvular heart disease, hx of familial sudden death, abnormal vital signs, volume depletion, primary CNS event | Critical intervention or an adverse outcome | 30 days |
| STePS | ||||
| STePS derivation: Costantino et al 2008 39 | 670 adult ED with syncope within previous 48 hours from 4 general hospitals in Italy. | Short term: ECG, concomitant trauma, no prodrome and male gender Long term: age >65, Hx of neoplastic procedures, stroke, SHD and VA | Death, need for major therapeutic procedures and early readmission to the hospital | 10 days |
| Syncope Risk Score | ||||
| Syncope Risk Score derivation: Sun et al 2009 36 | 2,584 older adult ED patients with syncope or near syncope from a regional managed care system of 3 hospitals in the US. | ECG, age>90 years, male, hx of arrhythmia, triage systolic BP>160, and elevated troponin I | MI, arrhythmia, pacemaker or cardiac defibrillator placement, PE, stroke, hemorrhage or acute procedure | 30 days |
| ROSE Study | ||||
| ROSE derivation and validation: Reed et al 2010 30 | 529 ED patients (≥16 years) with syncope from a single large tertiary hospital in the United Kingdom for the derivation; 538 ED patients (≥16 years) for internal validation. | ECG, BNP ≥300, bradycardia ≤50, Hgb ≤9, chest pain, O sat ≤94%, and + stool for occult blood test | Death, AMI, arrhythmias, PE, hemorrhage, stroke, subarachnoid hemorrhage, acute procedure | 1 month |
Derivation and validation cohorts; adult, >18 years; SFSR, San Francisco Syncope Rule; OESIL, Osservatorio Epidemiologico sulla Sincope nel Lazio; STePS, Short-Term prognosis of Syncope Study; ROSE, Risk Stratification of Syncope in the Emergency Department; SOB, shortness of breath; ECG, electrocardiogram; BP, Blood pressure; Hx, past medical history; CHF, congestive heart failure; MI,: myocardial infarction; PE, pulmonary embolism; ED, Emergency Department; ACS, acute coronary syndrome; Dz, disease; SHD, structural heart disease; VA, ventricular arrhythmias.
Table 2. Study Characteristics (n = 18).
| Study (n) | Definition of syncope | Adverse outcomes | Adverse outcomes identified outside ED | Admitted | |
|---|---|---|---|---|---|
| Georgeson et al 1992 37 (251) | Sudden TLOC with loss of postural tone. Included patients with near syncope and found on floor | 7% | NS | 70% | |
| Martin et al 1997 38∓ (252; 374) | Sudden TLOC with loss of postural tone and spontaneous recovery. | 26%;13% | NS | NS | |
| Sarasin et al 2003 16∓ (175; 267) | Sudden TLOC with loss of postural tone and spontaneous recovery. | 17%;18% | NS | NS | |
| Colivicci et al 2003 32∓ (270; 328) | Sudden TLOC with loss of postural tone and spontaneous recovery. | 11%; 9% | NS | NS | |
| Hing et al 2005 40 (100) | Brief TLOC | 23% | NS | 45% | |
| Reed et al 2007 41 (99) | NS | 11% | NS | 44% | |
| Quinn et al 2004 6 (684) | TLOC with return to baseline neurologic function | 12% | NS | 55% | |
| Quinn et al 2006 5 (791) | TLOC with return to baseline neurologic function | 14% | 7% | 59% | |
| Sun et al 2007 10 (477) | Sudden TLOC | 12% | 3% | 51% | |
| Cosgriff et al 2007 33 (89) | TLOC with return to baseline neurologic function | 11% | NS | 39% | |
| Birnbaum et al 2008 31 (713) | Sudden TLOC | 9% | NS | 83% | |
| Schladenhaufen et al 2008 7 (517) | NS | 19% | NS | 60% | |
| Thiruganasambandamoorthy et al 2009 35 (676) | Sudden TLOC with prompt and complete recovery | 10% | 5% | 12% | |
| Dipaola et al (in press) 29 (488) | Sudden TLOC with loss of postural tone and spontaneous recovery. | 5% | 5% | 34% | |
| Grossman et al 2007 34 (384) | Sudden and brief TLOC with loss of postural tone and spontaneous recovery. | 23% | 4% | 69% | |
| Costantino et al 2008 39 (670) | Sudden TLOC with loss of postural tone and spontaneous recovery. | NS | 6% | 33% | |
| Sun et al 2009 36 (2,584) | Sudden TLOC | 18% | 7% | 43% | |
| Reed et al 2010 30 (550; 550) ∓ | Sudden and brief TLOC with loss of postural tone and spontaneous recovery. | 8%; 7% | 4%; 2% | 48%; 53% | |
Derivation and validation studies; TLOC, transient loss of consciousness; NS, not specified
There were 4,510 patients (41%) admitted to the hospital. There were 1,437 (13%) patients who had an adverse outcome, including 832 (15%) in the derivation cohorts and 605 (11%) in the validation cohorts. Three hundred and eighty four patients (3%) suffered an adverse outcome that was identified after the initial ED evaluation. In the SFSR studies (n= 5468) there were 522 (10%) adverse outcomes, and in the OESIL risk score studies there were 148 (9%) outcomes. The mean age of patients in the included studies ranged from 50 to 79 years. Seven studies included patients less than 18 years of age.5, 6, 30, 32, 35, 38, 41 The weighted mean age of the 11,032 patients included in the qualitative analysis was 62 years, and 5,908 (55%) were female. Of the studies that reported elements from the past medical history, a total of 42% had hypertension, 23% coronary artery disease, 8% congestive heart failure, and 12% diabetes mellitus.
Methodological Quality of Clinical Decision Rules
The hierarchy of evidence for clinical decision rules23 is described in Table 3. Of the 9 clinical decision rules and risk scores included, none were classified as level 1evidence. Two clinical decision rules6, 32 met criteria for level 2 evidence and the remaining clinical decision rules or risk scores were derived but not validated36, 37, 39 or applied to populations without clinicians using the rule16, 30, 34, 38 and constitute level 4 evidence.
Methodological Quality of Individual Studies
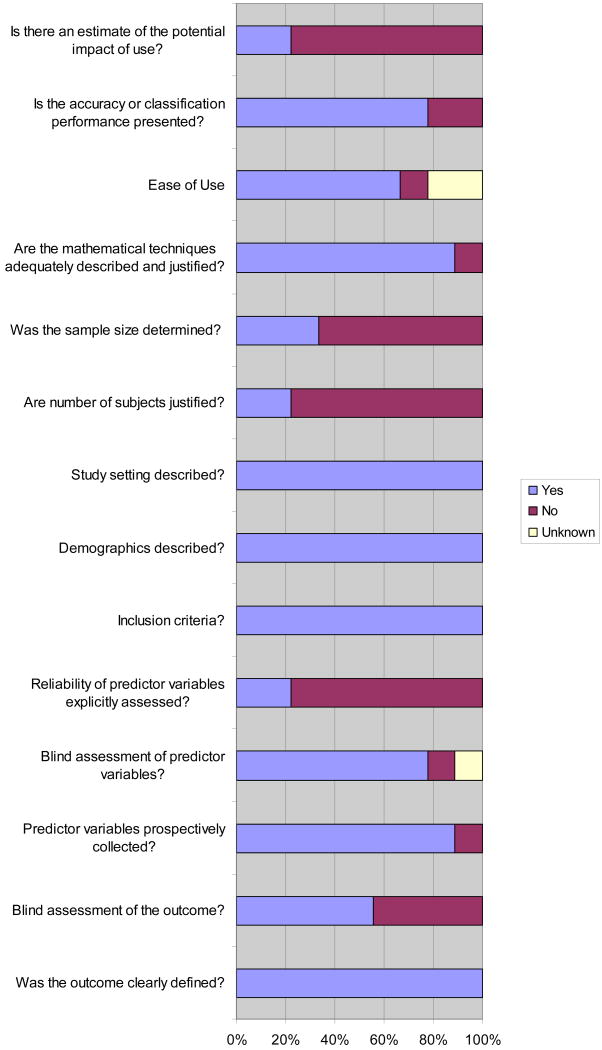
The methodological quality of the derivation studies is shown in Figure 2. Interobserver reliability for the methodological quality of derivation studies was 92.5% (95%CI 84-100). Blinded assessment of outcome was reported in 5 studies.6, 30, 34, 36, 37 Explicit assessment of the reliability of the predictor variables (i.e. kappa statistics) was reported in two studies.6, 36 Three studies justified the sample size based on mathematical techniques and reported calculation of the sample size.6, 30, 36
Figure 2. Methodological quality assesment of derivation studies (n= 9).
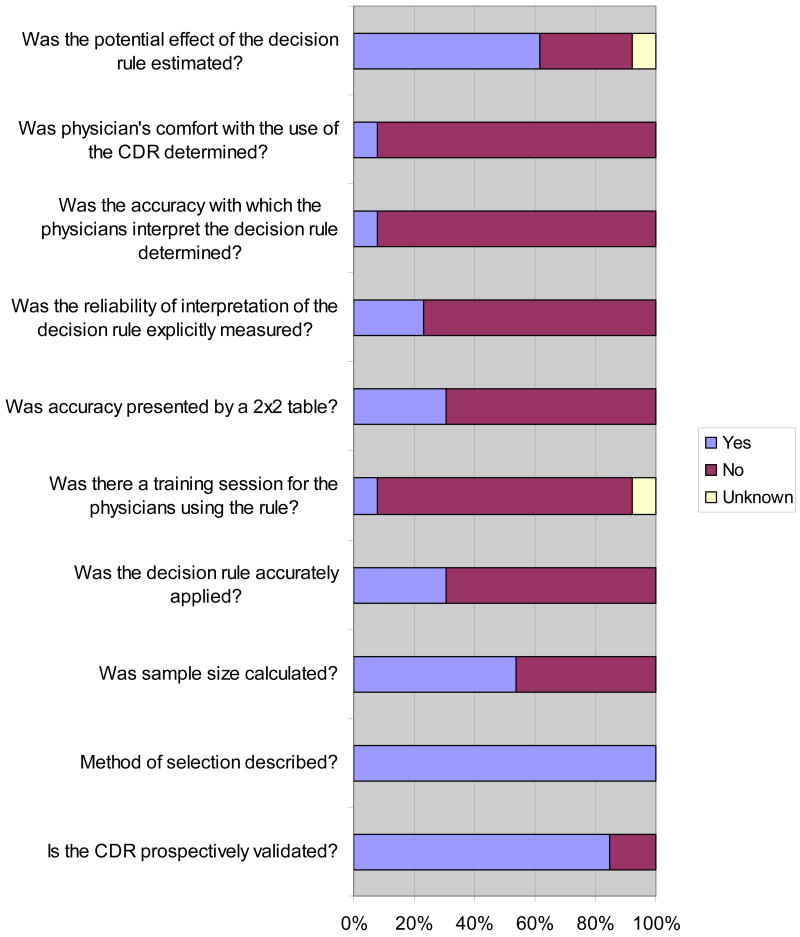
The methodological quality of the validation studies is shown in Figure 3. Interobserver reliability for the validation studies was 84.9% (95%CI 76-94). All validations except two7, 35 prospectively validated one or more clinical decision rules. One study reported having a training session for the physicians using the rule.10 None of the studies accurately applied the CDR in their validation.
Figure 3. Methodological quality assessment of validation studies (n= 13).
Diagnostic Accuracy of Individual Studies
The diagnostic accuracy of each individual study is shown in Table 5.
Table 5. Subgroup analysis of SFSR and OESIL risk score studies.
| Sensitivity % (95%CI); I2% |
Specificity % (95%CI); I2% |
LR Positive (95%CI); I2 % |
LR Negative (95% CI); I2% |
Diagnostic OR (95%CI); I2% |
Test for Interaction P-value | |
|---|---|---|---|---|---|---|
| SFSR | ||||||
| Study Design | ||||||
| Prospective (n= 7) 5, 6, 10, 29-31, 33 | 88 (84-92); 76 |
54 (52-56); 98 |
1.93 (1.46-2.55); 96 |
0.22 (0.11-0.47); 79 |
8.82 (3.5-22); 81 |
0.055 |
| Retrospective (n= 2) 7, 35 | 81 (74-87); 75 |
33 (30-36); 0 |
1.24 (1.07-1.45); 69 |
0.51 (0.23-1.13); 68 |
2.45 (0.96-6.21); 68 |
|
| Outcome Period | ||||||
| 7 days (n=5) 6, 7, 10, 31, 33 | 86 (83-90); 82 |
51 (49-53); 97 |
1.80 (1.35-2.40); 97 |
0.23 (0.11-0.50); 86 |
7.94 (3.04-21); 87 |
0.868 |
| > 7 days (n=4) 5, 29, 30, 35 | 90 (84-94); 65 |
47 (45-50); 99 |
1.75 (1.10-2.78); 98 |
0.27 (0.10-0.71); 76 |
6.92 (1.89-25.4); 82 |
|
| ECG Definition | ||||||
| SFSR (n= 5) 5, 6, 31, 33, 35 | 90 (85-93); 83 |
56 (54-58); 97 |
2.04 (1.48-2.82); 96 |
0.16 (0.05-0.50); 85 |
13.3 (3.60-48.0); 84 |
0.197 |
| Other than SFSR (n= 3) 7, 10, 29 | 84 (78-89); 81 |
42 (40-45); 98 |
1.46 (1.19-1.79); 90 |
0.36 (0.17-0.77); 72 |
4.51 (1.64-12.4); 76 |
|
| ECG determination | ||||||
| Evaluating Physicians (n= 3) 5, 6, 10 | 95 (90-97); 56 |
60 (57-62); 96 |
2.24 (1.57-3.21); 96 |
0.09 (0.02-0.38); 77 |
25.5 (4.41-148); 82 |
0.053 |
| Other (e.g. researcher, cardiologist) (n= 5) 7, 29, 31, 33, 35 | 82 (77-86); 74 |
45 (43-47); 98 |
1.53 (1.28-1.81); 88 |
0.42 (0.27-0.64); 49 |
4.03 (2.15-7.55); 61 |
|
| Patients | ||||||
| Unstable (n= 5) 6, 7, 30, 31, 33 | 83 (78-87); 80 |
46 (44-48); 98 |
1.63 (1.12-2.37); 97 |
0.35 (0.16-0.64); 84 |
4.86 (1.69-13.9); |
0.689 |
| Stable (n= 4) 5, 10, 29, 35 | 89 (83-94); 77 |
53 (51-56); 98 |
1.80 (1.13-2.85); 97 |
0.27 (0.09-0.81); 82 |
6.9 (1.78-26.8); 82 |
|
| OESIL risk score | ||||||
| Outcome Period | ||||||
| 12 months (n= 1) 32 | 100 (89-100); NA |
22 (17-28); NA |
1.26 (1.16-1.36); NA |
0.07 (0.00-1.13); NA |
17 (1-293); NA |
0.477 |
| < 12 months (n= 2) 29, 30 | 92 (83-97); NA |
34 (31-37); NA |
1.51 (0.71-3.19); NA |
0.27 (0.12-0.64); NA |
5.25 (1.10-24.9); NA |
|
CI, confidence interval; LR, likelihood ratio; NA, incalculable for less than three studies; OR odds ratio; p-values values were obtained from DOR.
Meta-analysis
Meta-analysis of each of the 5 included clinical decision rules is shown in Table 5. The subgroup analyses of studies evaluating the SFSR (1 derivation and 7 validations) and the OESIL risk score (1 derivation and 2 validations) are shown in Table 6. Sensitivity analysis conducted using a bivariate random effect model gave results consistent with the original model and study conclusions seemed robust to the choice of model (for example, bivariate estimates of the diagnostic accuracy of the SFSR show a sensitivity of 87% (95%CI 79-92) and specificity of 48% (95%CI 38-59).
Limitations and strengths of the review
There were a number of studies that were omitted from our meta-analysis due to incomplete prognostic accuracy data. This increases the risk of selection bias and may have affected our summary estimates of prognostic performance.
A key limitation of this meta-analysis at the level of the outcome is the diversity of clinical and methodological aspects across studies. In this review we pooled all studies from the same clinical decision rule regardless of study design, duration of follow-up, or rigor of predictor variable assessment. Pooling across studies with a high degree of clinical and statistical heterogeneity decreases the quality of the synthesized evidence. To identify potential sources of heterogeneity, we examined sub-groups to look for homogeneous populations and conducted meta-regression analysis to assess whether differences in study characteristics explain variation in findings.
At the study level, there were several limitations. The absence of an appropriate reference test or “gold standard” made the final diagnosis provided to a syncope patient difficult to confirm and subject to variability.2 This combined with the differing syncope definitions used across studies could lead to over or underestimation of the prognostic accuracy of the included clinical decision rules.
Forty four percent of the derived rules16, 32, 38, 39 did not report blinding outcome assessors, representing a potential source of observation bias. The use of clinical decision rules in different populations or subgroups than those originally intended could have resulted in spectrum bias.
Our review has several strengths. The meta-analyses reported here combine data across studies from the same clinical decision rule in order to estimate their prognostic accuracy with greater precision than is possible in a single study. We took steps to minimize the potential of publication and selection bias by conducting a protocol-driven review which included an exhaustive search (including extensive author contact) and explicit methodology for study selection, data extraction and analysis.
Discussion
Summary of findings
To our knowledge this is the first systematic review and meta-analysis to evaluate the methodological quality and prognostic accuracy of clinical prediction guides for ED patients with syncope. Overall this review included a limited number of medium size studies with differing degrees of methodological quality. Although the small number of included studies likely limited our ability to detect meaningful differences on subgroup analysis, differences in study design and ECG interpretation may account for the variable prognostic performance of the SFSR when validated in different practice settings.
Interpretation of results
Quality of the clinical decision rules and risk scores at the individual study level was limited, primarily because study findings have not been externally validated. Only the SFSR and the OESIL risk score have been validated in more than one practice setting (level 2 evidence) and could therefore be considered for use in clinical practice.
Eight of the nine studies had methodological weaknesses in the derivation phase. Deficiencies in outcome measure assessment (blinding) increased the risk of observation bias. Knowing that a patient had an adverse outcome, for example, may influence retrospective predictor variable assessment.21 Only 2 studies assessed the interrater reliability of the predictor variables. Demonstration of inter-rater reliability is important in the derivation of clinical prediction rules, as it determines which variables are sufficiently reliable to consider for incorporation in the rule.
Quinn and his colleagues validated the San Francisco Syncope Rule (SFSR) in a single-center, prospective sample of 791 patients.6 The prognostic performance in the derivation phase was quite promising. However, when Sun et al. externally validated the SFSR, rule performance was suboptimal. 10 In the original SFSR syncope was defined as transient loss of consciousness with return to baseline neurological function, and abnormal ECG was defined as any rhythm other than sinus or any new changes. Sun et al defined syncope as transient loss of consciousness and abnormal ECG as any rhythm other than sinus, any bundle branch block, left axis deviation, mono-or biventricular hypertrophy, any abnormal conduction interval except for first degree atrioventricular block, any Q, ST, or T-wave change consistent with ischemia (acute or chronic), or isolated, nonspecific ST or T-wave abnormalities. Altering the description of the patient population and the definition of important predictor variables could have resulted in differences in patient selection or misclassification of participants and therefore discordant results upon validation. To assess the accuracy of a clinical prediction rule, validation studies should correctly apply the rule itself.21
Other studies have validated the SFSR and OESIL risk score using different study designs and different individuals who interpreted the ECG (e.g. researcher, cardiologist, or the EP caring for the patient). Prospective validation by clinicians using the rule is important to ensure that the CDR works when applied in the real-world clinical setting. 23 It determines how the rule is been used in practice, and its effect on patient care and outcome. 42 Statistical validation decreases the probability that the CDR reflects associations that are due primarily to chance; however it does not address factors such as rule application feasibility and implementation, which can compromise its prognostic performance. 23 Subgroup analysis of SFSR studies revealed that studies with prospective designs outperformed those with retrospective designs. It is also surprising that studies in which the EP caring for the patient interpreted the ECG outperformed those in which the researcher or cardiologist interpreted the ECG. It is possible that additional information available to the clinician impacted subjective ECG interpretation. In addition, although there were no statistically significant differences in prognostic accuracy between SFSR studies based on these elements, the small number of studies included in the review limited our ability to detect meaningful differences. Thus, it is still possible that differences in ECG interpretation may account for the variable performance of the SFSR.
Implications for practice and research
We identified 2 clinical decision rules for ED syncope patients that were sufficiently developed to consider for use in practice (level 2 evidence): the OESIL risk score32 and the San Francisco Syncope Rule.6 The San Francisco Syncope Rule represents one of the first clinical decision rules in syncope derived according to published methodological standards and the only one that predicts short-term outcomes. It was prospectively derived in 684 patients by Quinn et al in 2004.5 Adverse outcomes, which included a large spectrum of clinical events, diagnoses and in hospital interventions, were assessed within 7 days of the initial ED visit. The SFSR has been externally validated in several settings with variable prognostic performance. The prevalence of adverse outcomes in patients classified as SFSR negative ranged from 2%5 to 36%31 across studies. In our meta-analysis the pooled sensitivity and specificity estimates for the SFSR were lower than in the original study and showed considerable inconsistency. We did not find a statistically significant explanation for the variability of these results. However, study design (prospective versus retrospective) and ECG determination (evaluating physician versus researcher or cardiologist) approached statistical significance. Given the small number of studies included in the review and the limitations of interaction testing, we likely had insufficient power to detect potentially meaningful differences in rule performance due to these factors.27
In 2003, Colivicchi and colleagues derived and validated the OESIL risk score.32 They found that an abnormal ECG result, a history of cardiovascular disease, lack of prodrome, and age older than 65 years predicted all deaths at one year in the 2 cohorts. Subsequent validations were not able to reproduce their results. The prevalence of adverse outcomes in patients classified as “low risk” by the OESIL risk score (score of 0-1) ranged from 5%30 to 13%.29 When pooled, the OESIL risk score showed substantial inconsistency across studies which could not be explained by differences in outcome period.
All the clinical prediction guides included in the review need further development before they can be routinely used in clinical practice. Future clinical decision rules for syncope should carefully define the patient population selected, clearly define a clinically important definition of abnormal ECG, and adhere to current methodological standards for clinical prediction rules.
Conclusion
The methodological quality and prognostic accuracy of current clinical decision rules for syncope is limited, and the prognostic performance of the SFSR and OESIL risk score varied between studies. Although the small number of included studies likely limited our ability to detect meaningful differences on subgroup analysis, differences in study design and ECG interpretation may account for the variable prognostic performance of the SFSR when validated in different practice settings.
Table 4. Metaanalysis of each of the 5 included clinical decision rules.
| SFSR | |||||
|---|---|---|---|---|---|
| Sensitivity % (95%CI) |
Specificity % (95%CI) |
LR Positive (95%CI) |
LR Negative (95%CI) |
DOR (95%CI) |
|
| Quinn et al 2004 6 | 96 (89-99) | 62 (58-66) | 2.53 (2.27-2.83) | 0.06 (0.02-0.19) | 41.3 (13.9-132) |
| Quinn et al 2006 5 | 98 (90-100) | 66 (62-70) | 2.89 (2.56-3.27) | 0.03 (0.00-0.20) | 101 (13.9-738) |
| Sun et al 2007 10 | 89 (78-96) | 42 (36-48) | 1.54 (1.35-1.76) | 0.25 (0.12-0.55) | 6 (2-15) |
| Cosgriff et al 2007 33 | 90 (55-100) | 57 (45-68) | 2.09 (1.51-2.90) | 0.18 (0.03-1.14) | 11.9 (1.44-98.6) |
| Birnbaum et al 2008 31 | 74 (61-84) | 57 (53-61) | 1.73 (1.45-2.06) | 0.46 (0.30-0.70) | 4 (2.1-6.83) |
| Schladenhaufen et al 2008 7 | 77 (67-85) | 34 (30-38) | 1.15 (1.02-1.31) | 0.70 (0.48-1.02) | 2 (1-3) |
| Thiruganasambandamoorthy et al 2009 35 | 90 (78-97) | 33 (28-38) | 1.34 (1.19-1.50) | 0.31 (0.13-0.72) | 4.3 (2.64-11.1) |
| Dipaola et al (in press) 29 | 81 (61-93) | 63 (58-67) | 2.18 (1.75-2.72) | 0.31 (0.14-0.67) | 7.1 (2.65-19.3) |
| Reed et al 2010 30 | 85 (70-94) | 24 (21-28) | 1.12 (0.98-1.29) | 0.62 (0.29-1.31) | 1.8 (0.75-4.45) |
| Pooled Results; I2% | 86 (83-89); 76 | 49 (48-51); 98 | 1.74 (1.36-2.24); 96 | 0.28 (0.16-0.50); 81 | 6 (3-14): 83 |
| OESIL Risk Score | |||||
| Colivicci et al 2003 32 (derivation) | 100 (89-100) | 22 (17-28) | 1.26 (1.16-1.36) | 0.07 (0.00-1.13) | 17 (1-293) |
| Dipaola et al (in press) 29 | 88 (70-98) | 59 (54-64) | 2.16 (1.81-2.58) | 0.20 (0.07-0.57) | 11 (3-37) |
| Reed et al 2010 30 | 95 (83-99) | 11 (8-14) | 1.06 (0.98-1.15) | 0.47 (0.12-1.87) | 2 (1-10) |
| Pooled Results; I2% | 95 (88-98); 59 | 31 (29-34) 99 | 1.41 (1.03-1.92); 97 | 0.24 (0.11-0.54); 0 | 6 (2-22); 40 |
| ROSE | |||||
| Reed et al 2010 30 (derivation) | 93 (80-97) | 74 (73-74) | 3.53 (2.97-3.78) | 0.10 (0.035-0.266) | 34.8 (11.1-108) |
| Reed et al 2010 30 (validation) | 87 (74-94) | 66 (64-67) | 2.52 (2.07-2.78) | 0.20 (0.085-0.406) | 12.9 (5.1-32.6) |
| Pooled Results | 90 (81-95); NA | 70 (67-72); NA | 2.98 (2.14-4.15); NA | 0.15 (0.079-0.293); NA | 20 (7.8-51.4); NA |
| Boston Syncope Rule | |||||
| Grossman et al 2007 34 | 97 (93-100) | 62 (56-69) | 2.57 (2.2-3.1) | 0.05 (0.01-0.16) | 54 (14-205) |
| Pooled Results | OS | OS | OS | OS | OS |
| Syncope Risk Score | |||||
| Sun et al 2009 36 | 88 (82-93) | 32 (30%-34) | 1.3 (1.2-1.4) | 0.36 (0.24-0.54) | 4 (2-6) |
| Pooled Results | OS | OS | OS | OS | OS |
CI, confidence interval; LR, likelihood ratio; NA, incalculable for less than three studies; OS, only one study; OR odds ratio
Acknowledgments
The project described was supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Appendix 1. Medline search strategy
OVID MEDLINE 1950- Nov Week 3 2009
exp Syncope/
(faint$3 or presyncop* or unconscious* or (drop adj attack$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
1 or 2
emergency service, hospital/ or emergency medical services/ or triage/
(emergen$ adj3 (center$ or centre$ or unit$1 or room$1 or department$1 or service or physician$ or medicine or care or ward$1)).mp.
3 and (4 or 5) 658
limit 6 to “clinical prediction guides (optimized)”
exp *Syncope/di and (4 or 5)
.mp. = mp=title, original title, abstract, name of substance word, subject heading word
/ = Medical Subject Heading term/controlled vocabulary
$ or * = wild card truncation, $3 = up to three words adjacent
Adj = adjacent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009): The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) European heart journal. 2009 Aug 27; doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huff JS, Decker WW, Quinn JV, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with syncope. Journal of Emergency Nursing. 2007;33(6):e1–e17. doi: 10.1016/j.jen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Alshekhlee A, Shen WK, Mackall J, et al. Incidence and mortality rates of syncope in the United States. The American journal of medicine. 2009 Feb;122(2):181–188. doi: 10.1016/j.amjmed.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Blanc JJ, L'Her C, Gosselin G, et al. Prospective evaluation of an educational programme for physicians involved in the management of syncope. Europace. 2005;7(4):400–406. doi: 10.1016/j.eupc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Quinn J, McDermott D, Stiell I, et al. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med. 2006 May;47(5):448–454. doi: 10.1016/j.annemergmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Quinn JV, Stiell IG, McDermott DA, et al. Derivation of the San Francisco Syncope Rule to Predict Patients with Short-Term Serious Outcomes. Annals of Emergency Medicine. 2004;43(2):224–232. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 7.Schladenhaufen R, Feilinger S, Pollack M, et al. Application of San Francisco Syncope Rule in elderly ED patients. Am J Emerg Med. 2008 Sep;26(7):773–778. doi: 10.1016/j.ajem.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Sun BC, Emond JA, Camargo CA., Jr Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Academic Emergency Medicine. 2004;11(10):1029–1034. doi: 10.1197/j.aem.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Lappin R, Quinn J. Syncope. Blackwell Publishing Ltd.; 2009. [Google Scholar]
- 10.Sun BC, Mangione CM, Merchant G, et al. External Validation of the San Francisco Syncope Rule. Annals of Emergency Medicine. 2007;49(4):420–427.e424. doi: 10.1016/j.annemergmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Hori S, Nakamura I, et al. Long-term survival of Japanese patients transported to an emergency department because of syncope. Annals of Emergency Medicine. 2004 Sep;44(3):215–221. doi: 10.1016/j.annemergmed.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Smars PA, Decker WW, Shen WK. Syncope evaluation in the emergency department. Current Opinion in Cardiology. 2007;22(1):44–48. doi: 10.1097/HCO.0b013e32801173d7. [DOI] [PubMed] [Google Scholar]
- 13.Ammirati F, Colivicchi F, Santini M. Diagnosing syncope in clinical practice. Implementation of a simplified diagnostic algorithm in a multicentre prospective trial - the OESIL 2 study (Osservatorio Epidemiologico della Sincope nel Lazio) European heart journal. 2000 Jun;21(11):935–940. doi: 10.1053/euhj.1999.1910. [DOI] [PubMed] [Google Scholar]
- 14.Brignole M, Menozzi C, Bartoletti A, et al. A new management of syncope: prospective systematic guideline-based evaluation of patients referred urgently to general hospitals. European heart journal. 2006 Jan;27(1):76–82. doi: 10.1093/eurheartj/ehi647. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor WN. Evaluation and outcome of patients with syncope. Medicine. 1990 May;69(3):160–175. doi: 10.1097/00005792-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Sarasin FP, Hanusa BH, Perneger T, et al. A risk score to predict arrhythmias in patients with unexplained syncope. Acad Emerg Med. 2003 Dec;10(12):1312–1317. doi: 10.1111/j.1553-2712.2003.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 17.Maisel WH. Specialized syncope evaluation. Circulation. 2004 Dec 14;110(24):3621–3623. doi: 10.1161/01.CIR.0000151358.06578.57. [DOI] [PubMed] [Google Scholar]
- 18.Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992 Apr;21(4):384–390. doi: 10.1016/s0196-0644(05)82656-3. [DOI] [PubMed] [Google Scholar]
- 19.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thrombosis and haemostasis. 2000 Mar;83(3):416–420. [PubMed] [Google Scholar]
- 20.Antes G, von Elm E. The PRISMA Statement - what should be reported about systematic reviews? Deutsche medizinische Wochenschrift (1946) 2009 Aug;134(33):1619. doi: 10.1055/s-0029-1233989. [DOI] [PubMed] [Google Scholar]
- 21.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999 Apr;33(4):437–447. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
- 22.Thijs RD, Benditt DG, Mathias CJ, et al. Unconscious confusion--a literature search for definitions of syncope and related disorders. Clin Auton Res. 2005 Feb;15(1):35–39. doi: 10.1007/s10286-005-0226-2. [DOI] [PubMed] [Google Scholar]
- 23.McGinn TG, Guyatt GH, Wyer PC, et al. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. Jama. 2000 Jul 5;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC medical research methodology. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. Journal of clinical epidemiology. 2003 Nov;56(11):1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ (Clinical research ed. 2003 Jan 25;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Statistics in medicine. 2008 Feb 28;27(5):687–697. doi: 10.1002/sim.2992. [DOI] [PubMed] [Google Scholar]
- 29.Dipaola F, C G, Perego F, Borella M, Galli A, Cantoni G, Barbic F, Casella F, Duca PG, Furlan R. San Francisco Syncope Rule, Osservatorio Epidemiologico sulla Sincope nel Lazio risk score, and clinical judgment in the assesment of short-term outcome of syncope. American Journal of Emergency Medicine. 2010 doi: 10.1016/j.ajem.2008.12.039. (accepted)(XX):XXX-XXX. [DOI] [PubMed] [Google Scholar]
- 30.Reed MJ, Newby DE, Coull AJ, et al. The ROSE (risk stratification of syncope in the emergency department) study. Journal of the American College of Cardiology. 2010 Feb 23;55(8):713–721. doi: 10.1016/j.jacc.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Birnbaum A, Esses D, Bijur P, et al. Failure to validate the San Francisco Syncope Rule in an independent emergency department population. Ann Emerg Med. 2008 Aug;52(2):151–159. doi: 10.1016/j.annemergmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Colivicchi F, Ammirati F, Melina D, et al. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: The OESIL risk score. European heart journal. 2003;24(9):811–819. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 33.Cosgriff TM, Kelly AM, Kerr D. External validation of the San Francisco Syncope Rule in the Australian context. Cjem. 2007 May;9(3):157–161. doi: 10.1017/s1481803500014986. [DOI] [PubMed] [Google Scholar]
- 34.Grossman SA, Fischer C, Lipsitz LA, et al. Predicting Adverse Outcomes in Syncope. Journal of Emergency Medicine. 2007;33(3):233–239. doi: 10.1016/j.jemermed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiruganasambandamoorthy V, Hess EP, Alreesi A, et al. External Validation of the San Francisco Syncope Rule in the Canadian Setting. Ann Emerg Med. 2009 Nov 25; doi: 10.1016/j.annemergmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Sun BC, Derose SF, Liang LJ, et al. Predictors of 30-Day Serious Events in Older Patients With Syncope. 2009 Ann Emerg Med 18;Sep 18; doi: 10.1016/j.annemergmed.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgeson S, Linzer M, Griffith JL, et al. Acute cardiac ischemia in patients with syncope: importance of the initial electrocardiogram. J Gen Intern Med. 1992 Jul-Aug;7(4):379–386. doi: 10.1007/BF02599151. [DOI] [PubMed] [Google Scholar]
- 38.Martin TP, Hanusa BH, Kapoor WN. Risk stratification of patients with syncope. Ann Emerg Med. 1997 Apr;29(4):459–466. doi: 10.1016/s0196-0644(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 39.Costantino G, Perego F, Dipaola F, et al. Short- and Long-Term Prognosis of Syncope, Risk Factors, and Role of Hospital Admission. Results From the STePS (Short-Term Prognosis of Syncope) Study. Journal of the American College of Cardiology. 2008;51(3):276–283. doi: 10.1016/j.jacc.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 40.Hing R, Harris R. Relative utility of serum troponin and the OESIL score in syncope. Emerg Med Australas. 2005 Feb;17(1):31–38. doi: 10.1111/j.1742-6731.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 41.Reed MJ, Newby DE, Coull AJ, et al. The Risk stratification Of Syncope in the Emergency department (ROSE) pilot study: a comparison of existing syncope guidelines. Emerg Med J. 2007 Apr;24(4):270–275. doi: 10.1136/emj.2006.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. Jama. 1997 Feb 12;277(6):488–494. [PubMed] [Google Scholar]