Synopsis
Cardiomyopathy is a serious disorder of the heart muscle and, although rare, is a common cause of heart failure in children and the most common cause for heart transplantation in children older than 1 year of age. Funded by the National Heart Lung and Blood Institute since 1994, the Pediatric Cardiomyopathy Registry (PCMR) has followed more than 3500 North American children with cardiomyopathy. Early analyses determined estimates for the incidence of pediatric cardiomyopathy (1.13 cases per 100,000 children per year), risk factors for cardiomyopathy (age less than 1 year, male sex, black race, and living in New England as opposed to the Central Southwestern states), the prevalence of heart failure at diagnosis (6%–84% depending on cause), and 10-year survival (29%–94% depending on cause). More recent analyses explored cause-specific functional status, survival and transplant outcomes, and risk factors in greater detail. For many topics these analyses are based on the largest and best-documented samples of children with disease such as the muscular dystrophies, mitochondrial disorders, and Noonan’s syndrome. Data from the PCMR continue to provide valuable information that guides clinical management and the use of life-saving therapies, such as cardiac transplantation and approaches to treating heart failure, and that prepares children, their families, and their caregivers for dealing with this serious condition.
Keywords: Cardiomyopathy, Pediatrics, Heart Failure, Pediatric Cardiomyopathy Registry
Introduction
Cardiomyopathy is a serious disorder of the heart muscle and, although rare, is a common cause of heart failure in children, and it is also the most common cause of heart transplantation in children older than 1 year of age [1–4]. Although cardiomyopathy has various functional types, the vast majority of children with this diagnosis have either a dilated or a hypertrophic type, both of which are associated with abnormal cardiac structure and function and poor outcomes. The true incidence, prevalence, risk factors, causes, and natural history of the various types of pediatric cardiomyopathy were not known before the mid-1990’s.
Accurately estimating the incidence of this rare and heterogeneous disease required applying a rigorous recruitment strategy over a large geographical area to collect a sufficiently large and unbiased population-based sample. The varied and often prolonged clinical course of the disease also required regular, long-term follow up of these children to better document their diagnosis, treatment, clinical course, and outcomes. Thus, in 1994, the National Heart, Lung and Blood Institute (NHLBI) funded the Pediatric Cardiomyopathy Registry (PCMR), a large, multi-center observational study of primary and idiopathic cardiomyopathies in children. The PCMR was designed to study the epidemiology and clinical course of selected cardiomyopathies in children and adolescents as well as to promote the development of etiology-specific prevention and treatment strategies. Currently, data from more than 3500 children with cardiomyopathy have been collected in the PCMR database with annual follow-up continuing from enrollment until death, heart transplant, or loss-to-follow up.
Some of the aims of the PCMR have evolved over the past 15 years in response to registry findings and changing clinical challenges. The original aims were primarily epidemiological: to describe the incidence and presentation of cardiomyopathy in all patients as well as by functional types and within demographic subgroups. Adding a retrospective cohort of children strengthened the ability to describe clinical outcomes and predictors of such outcomes. In fact, this clinical focus was emphasized in the second funding cycle by including prospectively collected, parent-reported functional status data to better characterize the impact of cardiomyopathy on the daily lives of affected children and their families.
In the current funding cycle, study aims were expanded by collaborating with the Pediatric Heart Transplant Study Group to examine the effect of cardiac transplantation on the clinical course of cardiomyopathy, as well as to establish long-term changes in functional status and their relationship to clinical events and outcomes, including heart transplantation. Also, for the first time since the establishment of the Registry, blood and cardiac tissue specimens were collected to investigate the relationship of genetic and viral markers to clinical and functional outcomes.
The PCMR has helped establish reliable estimates of the incidence of cardiomyopathy in children and has provided unbiased assessments of typical clinical presentations and outcomes. It has led to refined descriptions of functional types of disease and even descriptions by cause through identifying risk factors for cardiac transplantation and death. It has also provided the most complete accounts of how cardiomyopathy is diagnosed and treated providing an evidence-based background on which to create diagnostic and treatment algorithms.
We review here the most important PCMR findings and describe current PCMR investigations, focusing especially on findings related to pediatric heart failure.
The Design and Operation of the PCMR
The design and implementation of the PCMR are detailed elsewhere [5]. In brief, children up to 18 years old diagnosed with cardiomyopathy at participating centers are eligible for inclusion if they meet specific quantitative echocardiographic criteria, if the pattern of cardiomyopathy conforms to a defined semi-quantitative pattern, or if the diagnosis is confirmed by tissue analysis (List 1). Each case of cardiomyopathy is then classified morphologically as dilated, hypertrophic, restrictive, mixed, or other. Children are excluded if they have specific secondary causes of myocardial abnormalities, including potential causes of myocardial hypertrophy, such as congenital heart disease and exposure to drugs known to cause cardiac hypertrophy (List 2).
List 1.
Inclusionary echocardiographic criteria for the Pediatric Cardiomyopathy Registry
| Measurements |
| • Left ventricular fractional shortening or ejection fraction >2 standard deviations below the normal mean for age. Left ventricular fractional shortening is acceptable in children with a normal ventricular configuration and without abnormal regional wall motion. Abnormal ejection fractions detected by echocardiography, radionuclide or contrast angiography, or MRI are acceptable alternatives but age-appropriate norms for the individual laboratory must be applied. |
| • Left ventricular posterior wall thickness at end-diastole >2 standard deviations above the normal mean for body-surface area. |
| • Left ventricular posterior wall thickness at end-systole >2 standard deviations below the normal mean for body-surface area. |
| • Left ventricular end-diastolic dimension or volume >2 standard deviations above the normal mean for body-surface area. Dimension data are acceptable under the conditions outlined for left ventricular fractional shortening above, and volume data may be derived from the imaging methods as above. |
| Patterns |
| • Localized ventricular hypertrophy: such as, septal thickness >1.5 × left ventricular posterior wall thickness with at least normal left ventricular posterior wall thickness, with or without dynamic outflow obstruction. |
| • Restrictive cardiomyopathy: one or both atria enlarged relative to the ventricles of normal or small size with evidence of impaired diastolic filling and in the absence of marked valvular heart disease. |
| • Contracted form of endocardial fibroelastosis: similar to restrictive cardiomyopathy plus an echo-dense endocardium. |
| • Ventricular dysplasia or Uhl's congenital anomaly: a very thin right ventricle with a dilated right atrium (usually better assessed by MRI than by echocardiography). |
| • Concentric hypertrophy in the absence of a hemodynamic cause: a single measurement of LV posterior wall thickness at end-diastole >2 standard deviations suffices. |
| • Left ventricular myocardial noncompaction: highly trabeculated spongiform left ventricle myocardium with multiple interstices. |
List 2.
Exclusionary criteria for the Pediatric Cardiomyopathy Registry
| • Endocrine disease known to cause heart muscle disease (including infants of diabetic mothers). |
| • A history of rheumatic fever. |
| • Toxic exposures known to cause heart muscle disease (e.g., anthracyclines, mediastinal radiation, iron overload, or heavy metal exposure). |
| • HIV infection or born to an HIV positive mother. |
| • Kawasaki disease. |
| • Congenital heart defects unassociated with malformation syndromes (e.g., valvar heart disease or congenital coronary artery malformations). |
| • Immunologic disease. |
| • Invasive cardiothoracic procedures or major surgery during the preceding month except those specifically related to cardiomyopathy including LVAD, ECMO and AICD placement. |
| • Uremia, active or chronic. |
| • Abnormal ventricular size or function that can be attributed to intense physical training or chronic anemia. |
| • Chronic arrhythmia unless there are studies documenting inclusion criteria prior to the onset of arrhythmia (except a patient with chronic arrhythmia, subsequently ablated, whose cardiomyopathy persists after two months is not to be excluded). |
| • Malignancy. |
| • Pulmonary parenchymal or vascular disease (e.g., cystic fibrosis, cor pulmonale, or pulmonary hypertension). |
| • Ischemic coronary vascular disease. |
| • Age less than 18 years. |
| • Association with drugs known to cause hypertrophy (e.g., growth hormone, corticosteroids or cocaine) |
| • Left ventricular assist device; extracorporeal membrane oxygenation; automatic implantable cardioverter defibrillator. |
The original PCMR design consisted of two cohorts. The first was a retrospective cohort of children who were diagnosed between January 1, 1990, and December 31, 1995, and identified by chart review from 39 tertiary care centers in the US and Canada. The purpose of this cohort was to identify potential predictors of outcome as well as diagnostic approaches. The second cohort was a population-based, prospective cohort of children diagnosed after January 1, 1996, by pediatric cardiologists at 98 pediatric cardiac centers in two geographically distinct regions of the US (New England-Connecticut, Maine, Massachusetts, New Hampshire, and Rhode Island—and the Central Southwestern— Arkansas, Oklahoma, and Texas). These geographic areas were selected because of the local referral patterns, which should identify essentially all incident cases of pediatric cardiomyopathy. The purpose of this cohort was to estimate accurately the incidence of cardiomyopathy in children. Standardized data collection in both regions was performed by an outreach team that regularly traveled to the participating centers to enroll new cases and to abstract data from medical records.
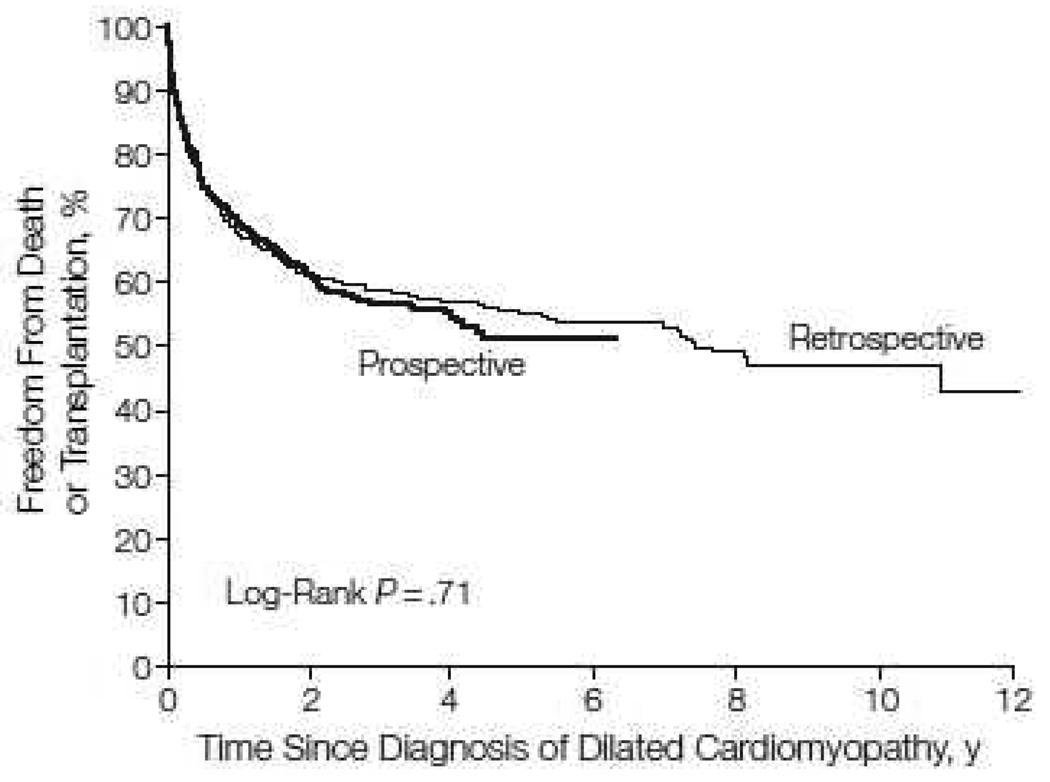
Collected data included demographic characteristics, quantitative echocardiographic measurements, a brief family history, vital and transplant status, and clinical findings. More detailed data were collected from the retrospective cohort. These data included a complete family history, qualitative echocardiographic studies (e.g., mitral regurgitation), electrocardiographic data, therapy, and hospitalizations. The clinical and echocardiographic characteristics and clinical outcomes were similar between cohorts (Fig. 1) [1]. Therefore, for most Registry analyses, with the exception of estimating incidence rate, the two cohorts are combined.
Figure 1.
Freedom from death or transplant for 491 children in the retrospective cohort and 935 children in the prospective cohort with pure dilated cardiomyopathy (P= 0.71). Data are from the Pediatric Cardiomyopathy Registry for the period between 1990 and 2002. [From Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006;296:1869, with permission.]
In the current award period (2005 to the present), 397 additional children from the 11 pediatric cardiology centers that provided the majority of PCMR cases were prospectively enrolled and followed. Data on these children are the most detailed and include medications and echocardiographic and other cardiac studies. Blood specimens were also obtained and tested for mutations in the G4.5 (taffazin) gene, which has been assumed to be associated with boys with Barth syndrome [6,7]. Also, biopsy or heart explant tissue was obtained from a subset of these children to determine the prevalence of viral causes of cardiomyopathy with polymerase chain reaction analysis.
An adjunct study to the PCMR is the NHLBI-funded the Pediatric Cardiomyopathy Specimen Repository (J. Towbin, principal investigator) that stores blood and tissue specimens from PCMR participants so that the genetic and viral causal associations with cardiomyopathy can be explored. Repository specimens (and a de-identified publicly available PCMR dataset) are made available to interested investigators on request.
The Incidence of Pediatric Cardiomyopathy
Between 1996 and 1999, 467 children with a new diagnosis of cardiomyopathy meeting PCMR criteria were identified in the two geographic regions described above. Completeness of case capture by the 18 pediatric cardiology centers in New England and the 20 centers in the Central Southwest was assessed in multiple ways. We estimate that fewer than 5 cases per year were missed [8]. The estimated annual incidence of pediatric cardiomyopathy in the United States based on these two regions is 1.13 cases per 100,000 children aged18 years or younger, a result similar to that reported for Finland and Australia [8, 9,10].
The annual incidence was significantly higher in infants less than 1 year of age (8.34 cases per 100,000, 95% confidence interval 7.21 to 9.61). The incidence was higher in boys than in girls (1.32 vs. 0.92 per 100,000 children, P<0.001), higher in blacks than in whites (1.47 vs. 1.06 per 100,000, P=0.02), and in New England than in the Central Southwest (1.44 cases vs. 0.98 per 100,000; P<0.001). The annual incidence of dilated cardiomyopathy was 0.58 cases per 100,000 children and of hypertrophic cardiomyopathy, 0.47 per 100,000 children. These variations in incidence by sex, race, and geographic region were found in both the dilated and hypertrophic functional subgroups. The incidence may be underestimated because children with sudden death as a presenting symptom may not have been identified: pathologists and medical examiners were not contacted in the original protocol. Children with asymptomatic left ventricular dysfunction would also not be identified until they sought medical evaluation; however, the PCMR definition of cardiomyopathy is based on clinically present disease.
Causes of Pediatric Cardiomyopathy
Examinations of more than 1400 children with dilated cardiomyopathy and more than 800 children with hypertrophic cardiomyopathy revealed that, for the most common types of cardiomyopathy, the vast majority of cases lack a known cause [5]. In the more than 1400 children with a newly diagnosed “pure” form of dilated cardiomyopathy, only 34% had a known cause: 16% of children with myocarditis, 9% with a neuromuscular disorder, 5% with familial cardiomyopathy, 4% with inborn errors of metabolism, and 1% with malformation syndrome. In total, 71% of children with dilated cardiomyopathy presented with congestive heart failure at diagnosis, and although the causes varied greatly, all groups presented with severely reduced left ventricular fractional shortening (Table 1). In the more than 800 children with newly diagnosed hypertrophic cardiomyopathy, only 26% had a known cause: 9% with malformation syndrome, 9% with inborn errors of metabolism, and 8% with a neuromuscular disorder [11]. Only 13% of children hypertrophic cardiomyopathy presented with congestive heart failure at diagnosis, although again, the causes varied greatly (Table 1).
Table 1.
Prevalence of Heart Failure and Left Ventricular Fractional Shortening Z-score at Diagnosis of Pediatric Cardiomyopathy, by Type and Cause of Cardiomyopathy. Data are from the Pediatric Cardiomyopathy Registry.
| Type of Cardiomyopathy, by Cause |
Heart Failure n |
Mean Left Ventricular Fractional Shortening Z-Score (95% CI) [Standard Deviation] |
|---|---|---|
| Dilated | ||
| Idiopathic | 74 | −9.62 (−11.42 to −7.16) |
| Myocarditis | 84 | −9.11 (−11.05 to −6.67) |
| Neuromuscular Disorders |
35 | −5.88 (−8.02 to −3.32) |
| Familial | 53 | −7.07 (−9.63 to −3.68) |
| Inborn Errors of Metabolism |
60 | −8.94 (−10.30 to −5.33) |
| Malformation Syndromes |
67 | −5.95 (−9.49 to −5.10) |
| Hypertrophic | ||
| Inborn Errors of Metabolism |
40.3 | −1.11 [5.65] |
| Malformation Syndromes |
23.4 | 5.42 [4.31] |
| Neuromuscular Disorders |
6.4 | 3.01 [3.40] |
| Infantile | 9.9 | 3.62 [5.15] |
Data from the Pediatric Cardiomyopathy Registry.
In a separate study of only the retrospective cohort, among 916 children with any type of cardiomyopathy, only one-third had a known cause for their cardiomyopathy at the time of diagnosis [12]. Patient demographics and presentation, including heart failure at presentation, family history, echocardiographic findings, laboratory testing, and biopsy, were analyzed for possible associations with specific causal diagnoses for each type of cardiomyopathy. Children with a family history of cardiomyopathy were more likely to have a causal diagnosis regardless of cardiomyopathy type, and children with either dilated or hypertrophic cardiomyopathy and a family history of sudden death or a genetic syndrome were more likely to have a known causal diagnosis.
For children with dilated cardiomyopathy, older age at diagnosis, smaller left ventricular dimensions, and a higher left ventricular fractional shortening were associated with a causal diagnosis. For children with hypertrophic cardiomyopathy, female sex, decreased height and weight for age, and increased left ventricular posterior wall thickness were also associated with a causal diagnosis. After adjusting for age at diagnosis, congestive heart failure, and geographic region and excluding cases with neuromuscular disease, familial isolated cardiomyopathy, and malformation syndromes, analyses found that children with hypertrophic cardiomyopathy who had metabolic blood and urine test results were more likely to have a causal diagnosis than were children without such test results (odds ratio, 4.15). In dilated cardiomyopathy patients, this same type of analysis identified endomyocardial biopsy and viral serology or culture as significant independent predictors of a causal diagnosis (odds ratios, 4.84 and 1.81, respectively).
Treatment of Pediatric Cardiomyopathy
Treatment at diagnosis for 350 children with idiopathic dilated cardiomyopathy diagnosed between 1990 and 1995 in the retrospective cohort was compared to that of similar children diagnosed between 2000 and 2006 in the prospective cohort [13]. Of the children from the retrospective cohort 43% were less than 1 year old, and 73% had heart failure at diagnosis. Within 1 month of diagnosis, 84% of those in the retrospective cohort were started on anti-heart-failure therapy (digoxin, a diuretic, or both), 66% were started on an angiotensin-converting enzyme inhibitor (ACE-I), and 4% were started on a beta-blocker. These proportions were similar for children in the prospective cohort, except that beta-blocker use increased to 18%. Predictors of both anti-heart-failure and ACE-I therapy were worsening left ventricular dilation and left ventricular fractional shortening. In addition, children with asymptomatic heart failure were frequently treated with anti-heart-failure therapy, and 47% were not started on ACE-I therapy. Such practice does not conform to current guidelines based on expert consensus, which recommend starting anti-heart-failure therapy only for symptomatic relief, whereas ACE-I therapy is recommended for nearly all heart failure children, regardless of symptoms [14].
Outcomes of Pediatric Cardiomyopathy
Analyses of the PCMR database have identified cause-specific outcomes and predictors of outcome for children with cardiomyopathy. The clinical outcomes examined were death and cardiac death (either death or heart transplantation).
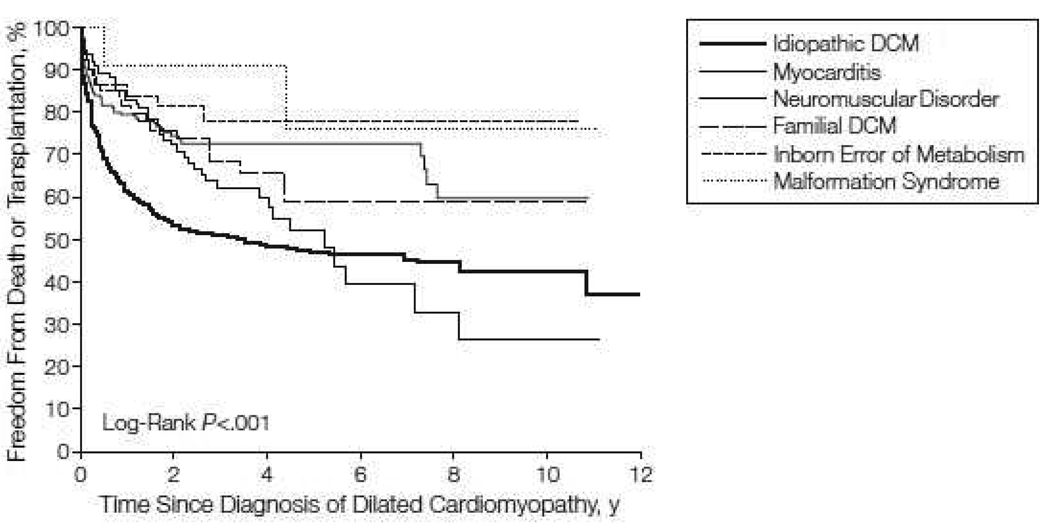
Of the more than 1400 cases of “pure” dilated cardiomyopathy, the 1- and 5-year rates of death or heart transplantation were 31% and 46% respectively. These rates varied greatly by the cause of disease (Fig. 2) [5]. Children aged 6 years or older were more likely to die or to undergo heart transplantation than were younger children (P<0.001). After excluding children with neuromuscular disease and inborn metabolic errors, Cox regression modeling showed that for children with idiopathic dilated cardiomyopathy (as opposed to cardiomyopathy with a known diagnosis), the presence of congestive heart failure at diagnosis and decreased left ventricular fractional shortening were significant predictors of the composite endpoint of death or heart transplantation. Thus, outcomes for children with dilated cardiomyopathy depend on cause, age at diagnosis, and heart failure at presentation. Most children do not have an identified cause for dilated cardiomyopathy, which limits the application of disease-specific therapy.
Figure 2.
Freedom from death or transplantation for 1423 children with pure dilated cardiomyopathy, by cause. Data are from the Pediatric Cardiomyopathy Registry for the period between 1990 and 2002. [From Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006;296:1873, with permission.]
An analysis of nutritional status in children with dilated cardiomyopathy showed that those diagnosed before 1 year of age were more likely to have growth retardation than were to older children with the same type of disease [15]. Cardiac dysfunction was associated with low height and body mass index, and low height was associated with increased risk of death.
Sudden death is less common in children with dilated cardiomyopathy than it is in adults with non-ischemic dilated cardiomyopathy, accounting for only 12% of deaths in these children enrolled in the PCMR. Heart failure and the use of anti-arrhythmic medications are associated with increased risk of sudden death. This knowledge should guide the use of automatic implantable cardiac defibrillators in these children.
Myocarditis accounts for 10%–20% of the cardiomyopathies in children. An analysis of PCMR data found no difference in outcomes between children diagnosed with biopsy and those diagnosed clinically. More than two-thirds of these children are alive and have not received a heart transplant 2 years after diagnosis, and left ventricular size returns to almost normal in nearly half of these children during the same time. However, dilation and decreased left ventricular fractional shortening at diagnosis are associated with increased risk of death or transplant.
A separate PCMR study compared children with the two most common types of muscular dystrophy, Duchenne (DMD) and Becker (BMD) [16]. All 128 children with DMD and 15 with BMD had dilated cardiomyopathy with roughly one-third of each group presenting with heart failure and both groups receiving similar treatment at diagnosis. Median follow up time was 3.3 years during which 47 DMD children died and 6 BMD children underwent heart transplant. Of the 47 deaths, 30 had a known cause and 20 of these were the result of heart failure. Children with BMD had a lower risk of heart transplant or death than did children with DMD.
Specific outcomes for children with mitochondrial disorders and cardiomyopathy were reported at the 5th World Congress of Pediatric Cardiology and Cardiac Surgery in 2009 [17]. Nearly half of children with a mitochondrial disorder and cardiomyopathy had dilated cardiomyopathy at diagnosis and these children had a 2-year mortality rate of 17%. In children with a mitochondrial disorder and hypertrophic cardiomyopathy, 2-year mortality rates were 36% and age less than 1 year at diagnosis was a significant risk factor for death.
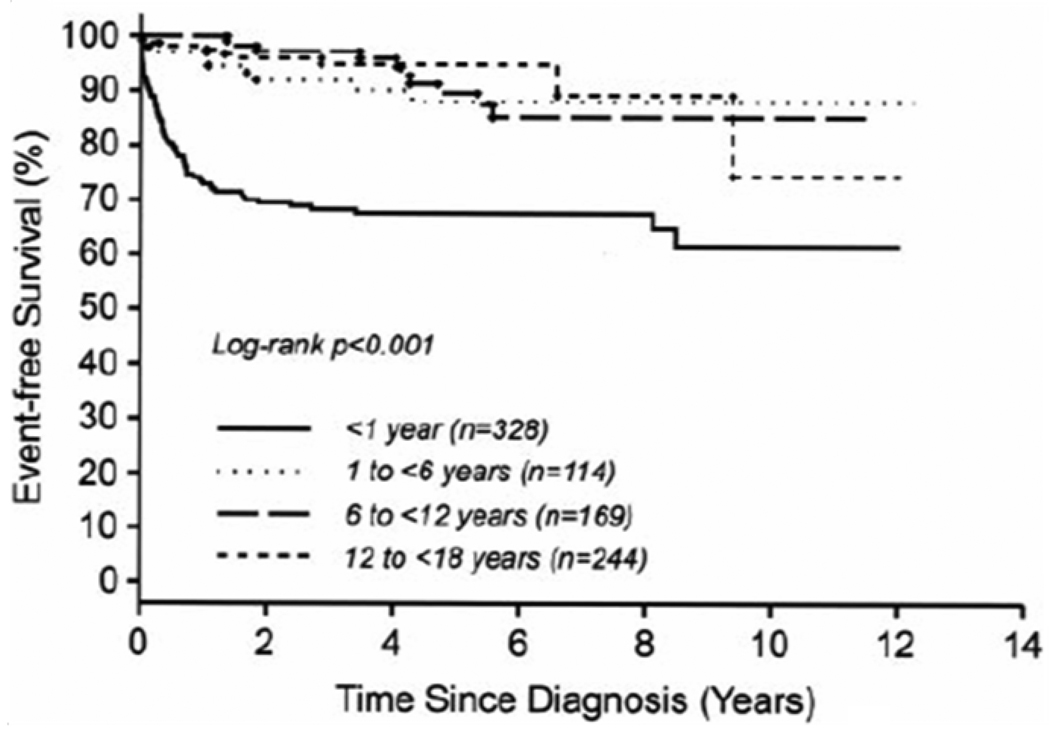
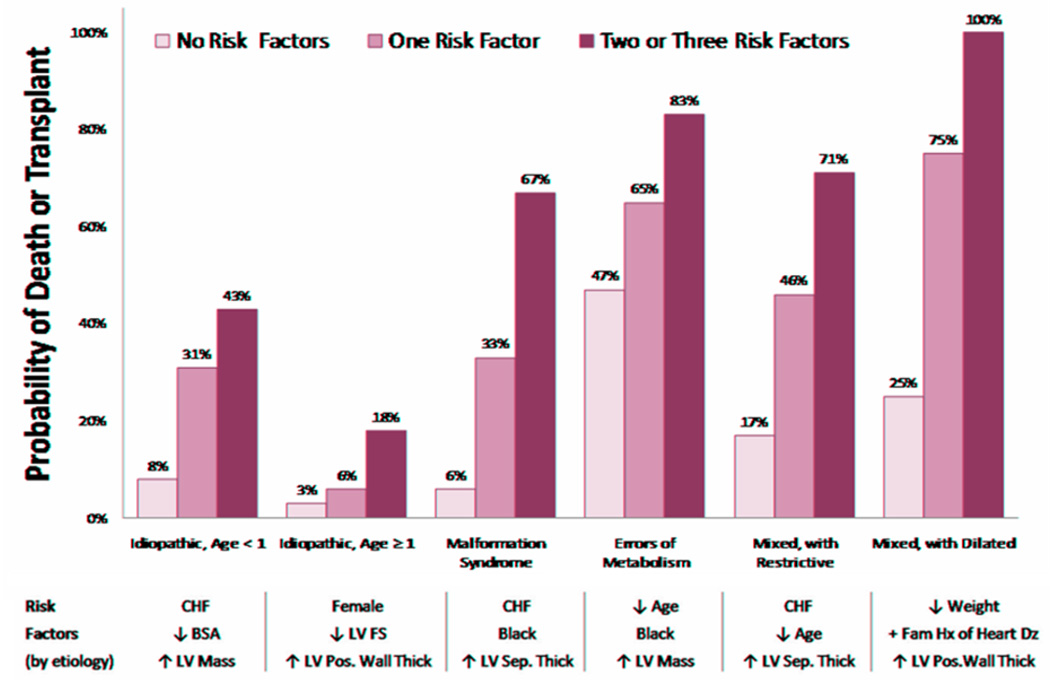
An examination of more than 800 cases of hypertrophic cardiomyopathy from the PCMR database showed that outcomes varied greatly by cause and age at diagnosis (Fig. 3) [11]. Children with hypertrophic cardiomyopathy associated with either an inborn error of metabolism or malformation syndrome, both of which present at a younger age, had low 5-year survival rates of 42% and 74%, respectively. Children with a neuromuscular disorder, which normally presents at an older age, and cardiomyopathy had a 5-year survival rate of 98%. Among children with idiopathic hypertrophic cardiomyopathy, 5-year survival was 94% for those diagnosed after 1 year of age but only 82% for those diagnosed before 1 year of age. In addition, the cause of death, when known, differed for children with idiopathic hypertrophic cardiomyopathy by age at diagnosis. Sudden death occurred in only 8 of 18 children diagnosed before 1 year of age but in all 8 of 8 children diagnosed after 1 year of age. Each of the cause-specific forms of pediatric hypertrophic cardiomyopathy has unique risk factors, and children with two or more of these risk factors are at significantly greater risk of death (Fig. 4) [18].
Figure 3.
Freedom from death or transplantation for 855 children with idiopathic hypertrophic cardiomyopathy, by age at diagnosis. Data are from the Pediatric Cardiomyopathy Registry for the period between 1990 and 2002. [From Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation 2007;115:777, with permission.]
Figure 4.
Risk of death or transplant in 882 children with hypertrophic cardiomyopathy, by number of cause-specific risk factors. Data are from the Pediatric Cardiomyopathy Registry for the period between 1990 and 2002. [From Lipshultz SE, Orav EJ, Wilkinson JD, Towbin JA, Messere J, Lowe AM, Sleeper LA, Clunie SK, Cox GF, Lurie PR, Hsu DT, Canter CE, Colan SD. A risk stratification analysis of predictors of death or transplant in children with hypertrophic cardiomyopathy. Circulation Supplement. 2008;118:4956.]
Children with Noonan syndrome have a 1-year survival rate of only 74% which is worse than that for other causes of hypertrophic cardiomyopathy [19]. Risk factors present at diagnosis included heart failure, decreased left ventricular fractional shortening, and increased septal thickness.
Left ventricular non-compaction occurs rarely in children comprising less than 5% of the cardiomyopathies recorded in PCMR [20]. This disease is most often diagnosed in infancy, and if left ventricular systolic function is preserved, 1- and 5-year outcomes are better than they are in other cardiomyopathies except for hypertrophic cardiomyopathy. However, decreased left ventricular fractional shortening predicts death or transplant.
Restrictive cardiomyopathy either isolated or in combination with hypertrophic cardiomyopathy, is also rare in children accounting for less than 5% of cardiomyopathies [21]. About one-third of cases present as a mixed restrictive-hypertrophic cardiomyopathy phenotype. The outcomes are the worst among the pediatric cardiomyopathies with only 20% of patients free from death or transplant 5 years after diagnosis. Younger age at presentation, heart failure, decreased left ventricular fractional shortening and increased left ventricular wall thickness are associated with poor outcomes.
Current Study Period Aims and Progress
The PCMR has continued to select and investigate research aims reflecting the most pressing clinical questions as evidenced by the three major aims of the most recent funding cycle (List 3). Aim 1, which was to merge the PCMR and Pediatric Heart Transplant Study Group (PHTS) databases, is now complete. This merger will be updated again in the final year of the current funding cycle. An analysis of the merged PCMR-PHTS database showed that marked left ventricular dilation, non-white race, and Medicaid or no insurance were associated with increased risk of death [22]. Another analysis found that in children with dilated cardiomyopathy, non-white race and older age were associated with poorer outcomes after heart transplantation. Although most children with myocarditis do not require heart transplantation, the post-transplantation outcomes for those that do are worse than for children with other types of dilated cardiomyopathy after heart transplantation [23]. Finally, children originally listed as Status 2 for heart transplantation had an unstable clinical course with about half being moved to Status 1 without 4 months of transplant listing [24].
List 3.
Specific Aims of the Current PCMR Funding Period: 2005 to 2010
| Aim 1 | To integrate the PCMR and the Pediatric Heart Transplant Study Group (PHTS) databases to examine whether and how cardiac transplantation modifies the clinical course of cardiomyopathy in children. |
| Aim 2 | To establish the longitudinal course of functional status in children with cardiomyopathy and to analyze the relationship of functional status to clinical events and outcomes. |
| Aim 3 | To investigate how genetic and viral markers of cardiomyopathy are associated with clinical and functional outcomes. |
The second and third aims were intended to improve our understanding of predictors of outcome for children with cardiomyopathy. Aim 2 sought to examine the long-term course of functional status in these children and how changes in functional status relate to clinically important events. Aim 3 was to investigate genetic and viral markers and their relationship to patient outcomes. With the ability to recruit and follow children over time, the PCMR offers the ideal framework for such projects which seek to examine the consequences of clinically relevant variables not suited to interventional study designs. To aid in these projects, 387 PCMR children were enrolled into a new prospective cohort and provided blood and tissue samples for analysis. The primary goal of this new study was to estimate the association between clinical outcomes and functional types of cardiomyopathy with physical and psychosocial functioning and genetic and viral status. The biologic specimens collected during the current protocol are tested for the G4.5 mutation to assess the prevalence of this mutation and the viral status in these patients [6, 7, 25].
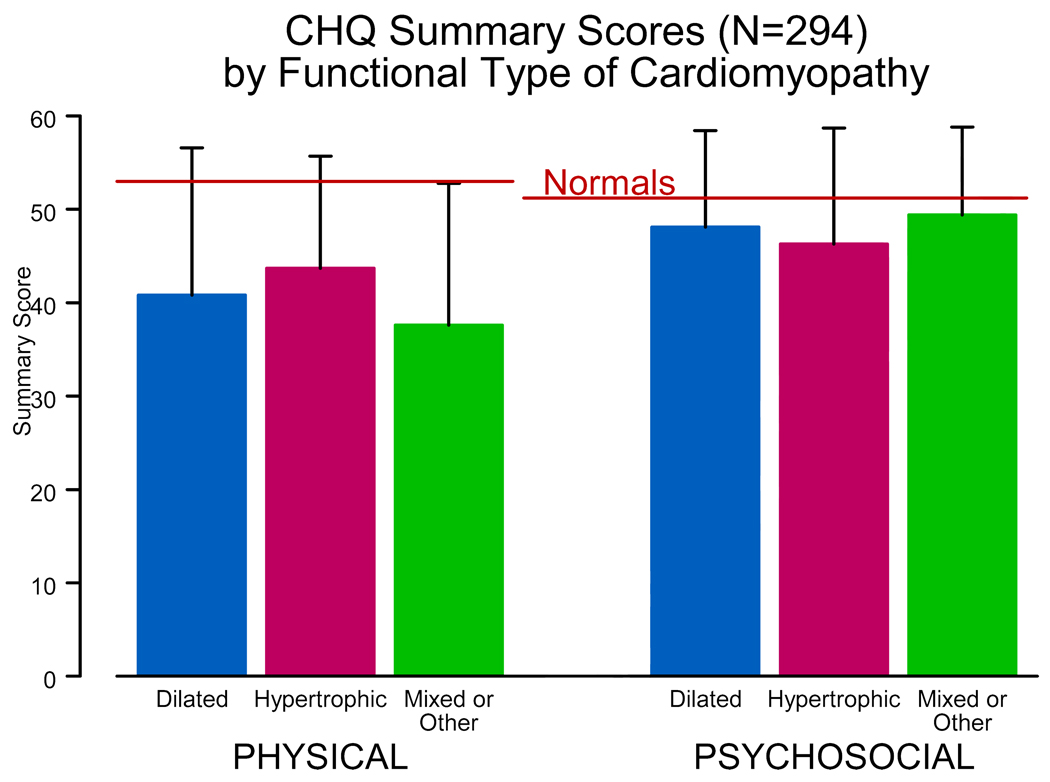
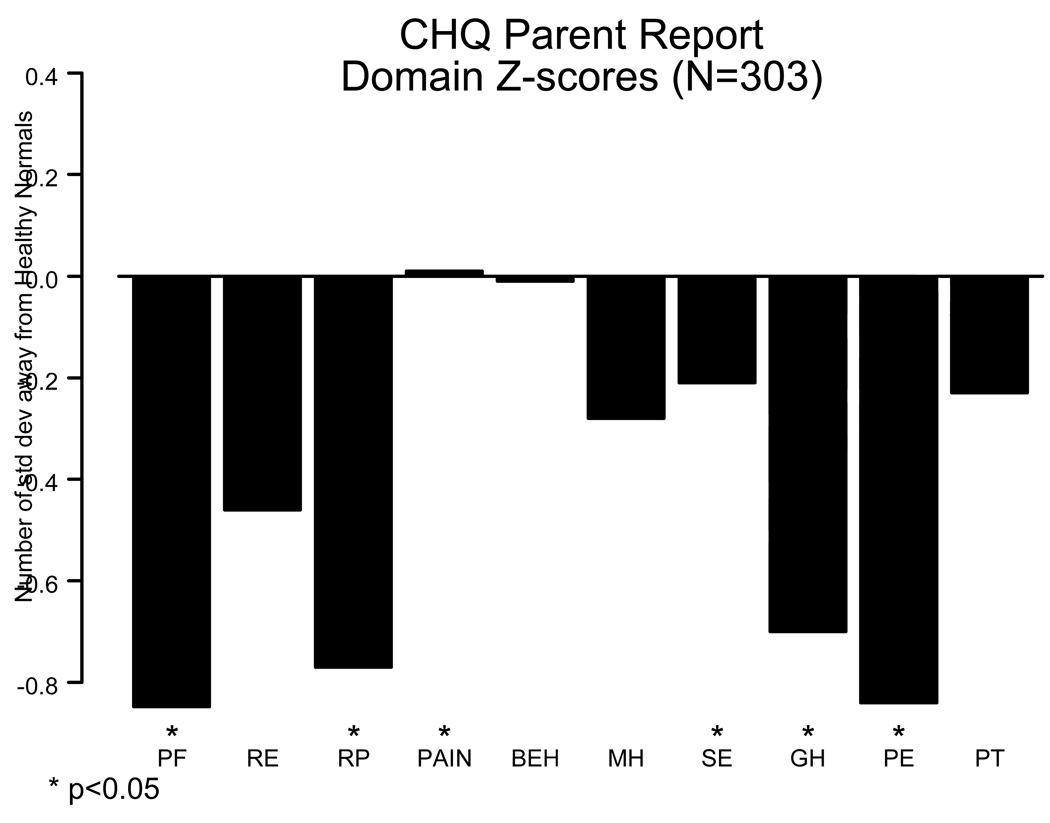
The PCMR has functional status data on more than 500 children with cardiomyopathy and more than half of these have data from more than one annual assessment. Functional status, although normal in many children with cardiomyopathy, is on average impaired with regard to both physical and, to a lesser degree, psychosocial functioning (Fig. 5 and Fig. 6) [26]. We found that higher physical functioning was associated with having married parents and higher educational level, whereas higher psychosocial functioning was associated with higher total household income. Using serial measures we found that functional status is positively associated with longer time since diagnosis, suggesting that many children improve over time. Physical functioning in children with dilated cardiomyopathy or hypertrophic cardiomyopathy was associated with increased left ventricular size and the left ventricular posterior wall thickness to end-diastolic dimension ratio, respectively. Finally, poorer functional status is a risk factor for later death or transplant in children with dilated cardiomyopathy and mixed or other types of cardiomyopathy, but not hypertrophic cardiomyopathy.
Figure 5.
Physical and psychosocial function, as measured by the Child Health Questionnaire, of 294 children with cardiomyopathy, by functional type. Data are from the Pediatric Cardiomyopathy Registry for the period between 1990 and 2002. [From Sleeper LA, Towbin JA, Colan SD, et al. Functional Status is Impaired and Correlated with Clinical Status in Pediatric Cardiomyopathy [abstract]. In: Proc 5th World Cong Pediatr Cardiol and Cardiac Surg 2009;5:134.]
Figure 6.
Child Health Questionnaire domain mean z scores for 303 children with cardiomyopathy in the Pediatric Cardiomyopathy Registry. Scores for the physical domains, general health, self-esteem, and parental impact-emotional domains were significantly below the average for healthy children. Scores for mental health and behavior did not differ significantly from those of healthy children. (PF = physical functioning; RE = role/social limits-emotional; RP = role/social limits-physical; PAIN= bodily pain; BEH = behavior; MH = mental health; SE = self-esteem; GH = general health perception; PE = parental impact-emotional; PT= parental impact-time). Data are from the Pediatric Cardiomyopathy Registry for the period between 1990 and 2002. [From Sleeper LA, Towbin JA, Colan SD, et al. Functional Status is Impaired and Correlated with Clinical Status in Pediatric Cardiomyopathy [abstract]. In: Proc 5th World Cong Pediatr Cardiol and Cardiac Surg 2009;5:134.]
Preliminary analysis of the G 4.5 mutation in 160 children showed that more than 20% had gene variants and that, unexpectedly, these were similar for both boys and girls (Table 2) [27]. Polymerase chain reaction analyses of myocardial tissue samples from 44 children contributing to the Pediatric Cardiomyopathy Specimen Repository, revealed 2 were positive for Epstein-Barr Virus (1 with dilated cardiomyopathy and 1 with hypertrophic cardiomyopathy), and 6 were positive for parvovirus (4 with idiopathic disease and 2 with myocarditis). Tests for adenovirus, cytomegalovirus, and enterovirus, among others, were negative.
Table 2.
G4.5 Gene Variants Found in 37 of 158 Children Enrolled in the Pediatric Cardiomyopathy Registry*
| Sex | n/N (%) | Hemizygous SNP, n/N (%) |
Intronic Substitution, SNP, n/N (%) |
Missense Substitution, Unclassified, n/N (%) |
Hemizygous Mutation*, n/N (%) |
|---|---|---|---|---|---|
| Boys | 2 7/110 (25)† |
22/27 (81) | 24/27 (89) | 3/27 (11) | 2/27 (7) |
| Girls | 10/48 (22)† |
3/10 (30) | 10/10 (100) | 0/10 (0) | 0/10 (0) |
The number of children with G4.5 gene variants as a proportion of all children with the same diagnosis were: 9 of 45 (20%) children with pure hypertrophic cardiomyopathy; 19 of 79 (24%) with pure dilated cardiomyopathy; 3 of 10 (30%) with pure restrictive cardiomyopathy; 4 of 22 (18%) with other or mixed forms of cardiomyopathy; and 2 of 2 with unknown forms.
Causes of cardiomyopathy in boys: idiopathic disease (n = 6); Barth syndrome or probably myocarditis (n = 2 each); Cori disease, Noonan syndrome, or familial dilated cardiomyopathy (n = 1 each); causes in girls: idiopathic disease (n = 6); familial hypertrophic cardiomyopathy or confirmed myocarditis (n = 2 each). Children with Barth syndrome patients each had two variants, denoted here as hemizygous mutations (hemizygous SNPs were not counted). [From Towbin JA, Sleeper L, Jefferies JL, et al. Genetic and viral genome analysis of childhood cardiomyopathy: the PCMR/PCSR experience [Abstract]. In: J Am Coll Cardiol 2010;55;A43.E409.
Submitted for oral presentation at the 2010 America College of Cardiology Annual Scientific Sessions, Orlando, FL.]
International Conference on Pediatric Cardiomyopathy
In January 2007, PCMR investigators organized the first International Workshop on Idiopathic and Primary Pediatric Cardiomyopathies. Co-sponsored by the Children’s Cardiomyopathy Foundation and NHLBI, more than 50 researchers, young investigators, and NHLBI staff attended the 2-day conference. The results of the conference were published in three issues of the journal, Progress in Pediatric Cardiology [28–62]. In addition to the conference results, two additional special articles were included in these issues that addressed ethical issues in the care of children with cardiomyopathy and the importance of a comprehensive, multidisciplinary approach to this care [63,64]. A follow-up conference is currently scheduled for May 2010.
PCMR: Future Directions
By continuously collecting follow-up data from children enrolled in the PCMR, the description of the clinical course of pediatric cardiomyopathy will be made more complete. These data will also allow for risk factors to be examined in more detail, and their long-term utility in diagnosis, prognosis and care to be determined. This type of registry data and their usefulness in guiding clinical decision making is increasingly appreciated by research methodologists and is being made more useful with advances in analytic and statistical theory [65]. The PCMR investigators have proposed using the PCMR to identify the genetic causes of pediatric cardiomyopathy and the usefulness of cardiac biomarkers in the evaluation of these children. The ultimate goal is to identify cause-specific treatment and clinical approaches for these children.
Conclusions
Currently in its 15th year of funding by the NHLBI, the PCMR contains clinically important information on more than 3500 cases of pediatric cardiomyopathy. Important contributions to date include refined estimates of the incidence and outcomes of pediatric cardiomyopathy, the identification of risk factors and predictors of outcomes for children with several cause-specific forms of cardiomyopathy, the identification of the factors associated with making a causal diagnosis of pediatric cardiomyopathy, and a description of the clinical care being provided to children with dilated cardiomyopathy. The most recent funding period for the PCMR is nearing a successful completion and analyses are already beginning to produce results. As increased follow-up information is acquired and linked with information from blood and tissue specimens, PCMR data are likely to become only more valuable over time.
Acknowledgements
The work of the PCMR would not be possible without the collaboration of many physicians and other health professionals, scientists, and research staff from the United States and Canada. Special acknowledgement should be given to our current and former PCMR Study group: Jane Messere, RN; Stephanie Ware, MD, PhD; John Lynn Jefferies, MD, MPH; Linda Addonizio, MD; Beth Kaufman, MD; Melanie Everitt, MD; Elfriede Pahl, MD; Paul Kantor, MBBCh; Paulo Rusconi, MD; Robert E. Shaddy, MD; and Paul R. Lurie, MD.
We would also like to acknowledge Mrs. Lisa Yue and the Children’s Cardiomyopathy Foundation for their continuing support of the PCMR.
And finally, we would like to express our most sincere gratitude to the children with cardiomyopathy and their families whose participation has made the PCMR possible.
Funding Support: This work was supported by the National Heart Lung and Blood Institute (HL53392) and the Children’s Cardiomyopathy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 2.Webber SA. New-onset heart failure in children in the absence of structural congenital heart disease. Circulation. 2008;117:11–12. doi: 10.1161/CIRCULATIONAHA.107.747469. [DOI] [PubMed] [Google Scholar]
- 3.Andrews RE, Fenton MJ, Ridout DA, Burch M. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United Kingdom and Ireland. Circulation. 2008;117:79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 4.Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol. 2008;31:388–391. doi: 10.1002/clc.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139:S86–S95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 6.Bione S, D'Adamo P, Maestrini E, et al. A novel X-linked gene, G4.5, is responsible for Barth syndrome. Nat Genet. 1996;12:385–389. doi: 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- 7.Schlame M, Towbin JA, Heerdt PM, et al. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann Neurol. 2002;51:634–637. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- 8.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 9.Arola A, Jokinen E, Ruuskanen O, et al. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am J Epidemiol. 1997;146:385–393. doi: 10.1093/oxfordjournals.aje.a009291. [DOI] [PubMed] [Google Scholar]
- 10.Nugent AW, Daubeney PEF, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 11.Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115:773–781. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 12.Cox GF, Sleeper LA, Lowe AM, et al. Factors associated with establishing a causal diagnosis for children with cardiomyopathy. Pediatrics. 2006;118:1519–1531. doi: 10.1542/peds.2006-0163. [DOI] [PubMed] [Google Scholar]
- 13.Harmon WG, Sleeper LA, Cuniberti L, M, et al. Treating children with idiopathic dilated cardiomyopathy (from the Pediatric Cardiomyopathy Registry) Am J Cardiol. 2009;104:281–286. doi: 10.1016/j.amjcard.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal D, Chrisant MR, Edens E, et al. International Society for Heart and Lung Transplantation: practice guidelines for the management of heart failure in children. J Heart Lung Transplant. 2004;23:1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Miller TL, John Orav E, Wilkinson JD, Sleeper LA, Towbin JA, Colan SD, Hsu DT, Canter CE, Webber SA, Lipshultz SE. Accepted for Oral Presentation at the 2009 American Heart Association Scientific Sessions. Orlando, FL: 2009. Nov 18, Nutrional status associated with cardiac outcomes and mortality in children with idiopathic dilated cardiomyopathy [abstract] [Google Scholar]
- 16.Connuck DM, Sleeper LA, Colan SD, et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2008;155:998–1005. doi: 10.1016/j.ahj.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox GF, Colan SD, Towbin JA, et al. Mitochondrial disorders: characteristics and outcomes from the pediatric cardiomyopathy registry [abstract] In: Proceedings of the 5th World Congress of Paediatric Cardiology and Cardiac Surgery. 2009;5:134. [Google Scholar]
- 18.Lipshultz SE, Orav EJ, Wilkinson JD, et al. A risk stratification analysis of predictors of death or transplant in children with hypertrophic cardiomyopathy. In: Circulation Supplement. 2008;118:4956. [Google Scholar]
- 19.Wilkinson JD, Lowe AM, Salbert BA, et al. Outcomes in children with Noonan syndrome and cardiomyopathy [abstract] In: Circulation Supplement. 2007;116 II-513. [Google Scholar]
- 20.Jefferies JL, Colan SD, Sleeper LA, Towbin JA, Pahl E, Kantor PF, Everitt MD, Webber SA, Kaufman BD, Lamour JM, Canter CE, Hsu DT, Addonizio LJ, Lipshultz SE. Accepted for Oral Presentation at the 2009 American Heart Association Scientific Sessions. Orlando, FL: 2009. Nov 16, Outcome and risk stratification for children with left ventricular noncompaction: findings from the pediatric cardiomyopathy registry [abstract] [Google Scholar]
- 21.Webber S, Lipshultz SE, Sleeper LA, et al. Phenotypic heterogeneity and outcomes of restrictive cardiomyopathy in childhood: a report from the NHLBI pediatric cardiomyopathy registry [abstract] Circulation Supplement. 2008;118:6071. [Google Scholar]
- 22.Singh TP, Gauvreau K, Thiagarajan R, et al. Racial and ethnic differences in mortality in children awaiting heart transplant in the United States. Am J Transplantat. 2009;9:1–8. doi: 10.1111/j.1600-6143.2009.02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietra BA, Kantor PF, Bartlett HL, et al. Clinical factors associated with UNOS status and outcome after listing for heart transplant in children with dilated cardiomyopathy [abstract] In: Circulation Supplement. 2007;116 II-565. [Google Scholar]
- 24.Larsen R, Naftel DC, Rosenthal DN, et al. The impact of heart failure severity at time of listing for cardiac transplantation on short and long term stability with pediatric cardiomyopathy [abstract] In: Circulation Supplement. 2008;118:4953. [Google Scholar]
- 25.Kelley RI, Cheatham JP, Clark BJ, et al. X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J Pediatr. 1991;119:738–747. doi: 10.1016/s0022-3476(05)80289-6. [DOI] [PubMed] [Google Scholar]
- 26.Sleeper LA, Towbin JA, Colan SD, et al. Functional status is impaired and correlated with clinical status in pediatric cardiomyopathy [abstract] In: Proc 5th World Cong Pediatr Cardiol and Cardiac Surg. 2009;5:134. [Google Scholar]
- 27.Towbin JA, Sleeper L, Jefferies JL, et al. Genetic and viral genome analysis of childhood cardiomyopathy: the PCMR/PCSR experience [Abstract] In: J Am Coll Cardiol. 2010;55:A43. E409. [Google Scholar]
- 28.Lipshultz SE, Colan SD, Towbin JA, Wilkinson JD. Introduction for "idiopathic and primary cardiomyopathy in children". Progress in Pediatric Cardiology. 2007;23(1–2):3. doi: 10.1016/j.ppedcard.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colan SD. Classification of the cardiomyopathies. Prog Pediatr Cardiol. 2007;23(1–2):5–15. [Google Scholar]
- 30.Weintraub RG, Nugent AW, Daubeney PEF. Pediatric cardiomyopathy: the Australian experience. Prog Pediatr Cardiol. 2007;23(1–2):17–24. [Google Scholar]
- 31.Alvarez JA, Wilkinson JD, Lipshultz SE. Outcome predictors for pediatric dilated cardiomyopathy: a systematic review. Prog Pediatr Cardiol. 2007;23(1–2):25–32. doi: 10.1016/j.ppedcard.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung WK. Predictive genetic testing for cardiomyopathies. Prog Pediatr Cardiol. 2007;23(1–2):33–38. [Google Scholar]
- 33.Kishnani PS, Burns Wechsler S, Li JS. Enzyme-deficiency metabolic cardiomyopathies and the role of enzyme replacement therapy. Prog Pediatr Cardiol. 2007;23(1–2):39–48. [Google Scholar]
- 34.Rodrigues CO, Shehadeh LA, Webster KA, Bishopric NH. Myocyte deficiency as a target in the treatment of cardiomyopathy. Prog Pediatr Cardiol. 2007;23(1–2):49–59. [Google Scholar]
- 35.Jefferies JL. Novel medical therapies for pediatric heart failure. Prog Pediatr Cardiol. 2007;23(1–2):61–66. [Google Scholar]
- 36.Canter CE, Kantor PF. Heart transplant for pediatric cardiomyopathy. Prog Pediatr Cardiol Cardiology. 2007;23(1–2):67–72. [Google Scholar]
- 37.Hsu DT. Age-related factors in child heart transplants. Prog Pediatr Cardiol. 2007;23(1–2):73–79. [Google Scholar]
- 38.Lipshultz SE, Colan SD, Towbin JA, Wilkinson JD. Idiopathic and primary cardiomyopathies in children. Prog Pediatr Cardiol. 2007;24(1):1. doi: 10.1016/j.ppedcard.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mestroni L, Miyamoto SD, Taylor MRG. Genetics of dilated cardiomyopathy conduction disease. Prog Pediatr Cardiol. 2007;24(1):3–13. [Google Scholar]
- 40.Cox GF. Diagnostic approaches to pediatric cardiomyopathy of metabolic genetic etiologies and their relation to therapy. Prog Pediatr Cardiol. 2007;24(1):15–25. doi: 10.1016/j.ppedcard.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheikh F, Chen J. Mouse models for cardiomyopathy research. Prog Pediatr Cardiol. 2007;24(1):27–34. [Google Scholar]
- 42.Dellefave LM, McNally EM. Cardiomyopathy in neuromuscular disorders. ProProg Pediatr Cardiol. 2007;24(1):35–46. [Google Scholar]
- 43.Cooper LT., Jr Giant cell myocarditis in children. Prog Pediatr Cardiol. 2007;24(1):47–49. doi: 10.1016/j.ppedcard.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman BD, Shaddy RE. Beta-adrenergic receptor blockade and pediatric dilated cardiomyopathy. Prog Pediatr Cardiol. 2007;24(1):51–57. [Google Scholar]
- 45.Miller TL, Neri D, Extein J, Somarriba G, Strickman-Stein N. Nutrition in pediatric cardiomyopathy. Prog Pediatr Cardiol. 2007;24(1):59–71. doi: 10.1016/j.ppedcard.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcalai R, Arad M, Depreux F, Wang L, Seidman JG, Seidman CE. Hypertrophy, electrical abnormalities, autophagic vacuoles accumulation and cardiac fibrosis in LAMP2 cardiomyopathy mouse model. Prog Pediatr Cardiol. 2007;24(1):73–74. [Google Scholar]
- 47.Joshi VA, Roberts AE, Kucherlapati RS. Noonan syndrome associated congenital hypertrophic cardiomyopathy and the role of sarcomere gene mutations. Prog Pediatr Cardiol. 2007;24(1):75–76. [Google Scholar]
- 48.Rossano JW, Dreyer WJ, Kim JJ, et al. Pre-transplant serum creatinine predicts long-term outcome in pediatric heart transplant patients. Prog Pediatr Cardiol. 2007;24(1):77–78. [Google Scholar]
- 49.Taylor MRG. When echocardiogram screening "is not enough". Prog Pediatr Cardiol. 2007;24(1):79–80. [Google Scholar]
- 50.Ratnasamy C, Kinnamon DD, Lipshultz SE, Rusconi PG. Associations between neurohormonal and inflammatory activation and heart failure in children. Prog Pediatr Cardiol. 2007;24(1):81–82. doi: 10.1016/j.ahj.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Lipshultz SE, Colan SD, Towbin JA, Wilkinson JD. Introduction for "idiopathic and primary cardiomyopathy in children". Prog Pediatr Cardiol. 2008;25(1):1. doi: 10.1016/j.ppedcard.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Towbin JA. Molecular mechanisms of pediatric cardiomyopathies and new targeted therapies. Prog Pediatr Cardiol. 2008;25(3):3–21. [Google Scholar]
- 53.Lipshultz SE, Wilkinson JD. Epidemiological and outcomes research in children with pediatric cardiomyopathy. Prog Pediatr Cardiol. 2008;25(3):23–25. doi: 10.1016/j.ppedcard.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colan SD. Clinical issues in the pediatric hypertrophic cardiomyopathies. Prog Pediatr Cardiol. 2008;25(3):27–29. doi: 10.1016/j.ppedcard.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson JD, Sleeper LA, Alvarez JA, Bublik N, Lipshultz SE. The Pediatric Cardiomyopathy Registry: 1995–2007. Prog Pediatr Cardiol. 2008;25(3):31–36. doi: 10.1016/j.ppedcard.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young K, Hare JM. Stem cells in cardiopulmonary development: implications for novel approaches to therapy for pediatric cardiopulmonary disease. Prog Pediatr Cardiol. 2008;25(3):37–49. [Google Scholar]
- 57.Negro A, Dodge-Kafka K, Kapiloff MS. Signalosomes as therapeutic targets. Progress Prog Pediatr Cardiol. 2008;25(3):51–56. doi: 10.1016/j.ppedcard.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menon SC, Olson TM, Michels V. Genetics of familial dilated cardiomyopathy. Progress Prog Pediatr Cardiol. 2008;25(3):57–67. [Google Scholar]
- 59.Hill KD, Rizwan H, Exil VJ. Pediatric cardiomyopathies related to fatty acid metabolism. Prog Pediatr Cardiol. 2008;25(3):69–78. [Google Scholar]
- 60.Fisher SD, Pearson GD. Peripartum cardiomyopathy: an update. Prog Pediatr Cardiol. 2008;25(3):79–84. [Google Scholar]
- 61.Webber SA. Primary restrictive cardiomyopathy in childhood. Prog Pediatr Cardiol. 2008;25(3):85–90. [Google Scholar]
- 62.Somarriba G, Extein J, Miller TL. Exercise rehabilitation in pediatric cardiomyopathy. Prog Pediatr Cardiol. 2008;25(3):91–102. doi: 10.1016/j.ppedcard.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokol KC, Armstrong FD, Rosenkranz ER, et al. Ethical issues in children with cardiomyopathy: making sense of ethical challenges in the clinical setting. Prog Pediatr Cardiol. 2007;23(1–2):81–87. [Google Scholar]
- 64.Bublik N, Alvarez JA, Lipshultz SE. Pediatric cardiomyopathy as a chronic disease: a look at comprehensive care programs. Prog Pediatr Cardiol. 2008;25:103–111. doi: 10.1016/j.ppedcard.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dreyer NA, Garner S. Registries for robust evidence. JAMA. 2009;302:790–791. doi: 10.1001/jama.2009.1092. [DOI] [PubMed] [Google Scholar]