Abstract
Upon illumination visual arrestin translocates from photoreceptor cell bodies to rhodopsin and membrane-rich photosensory compartments - vertebrate outer segments or invertebrate rhabdomeres - where it quenches activated rhodopsin. Both the mechanism and function of arrestin translocation are unresolved and controversial. In dark-adapted photoreceptors of the fruitfly Drosophila, confocal immunocytochemistry shows arrestin (Arr2) associated with distributed photoreceptor endomembranes. Immunocytochemistry and live imaging of GFP-tagged Arr2 demonstrate rapid reversible translocation to stimulated rhabdomeres in stoichiometric proportion to rhodopsin photoisomerization. Translocation is very rapid in normal photoreceptors (time constant <10 s), and can also be resolved in the time course of electroretinogram recordings. Genetic elimination of key phototransduction proteins, including phospholipase C (PLC), Gq and the light-sensitive Ca2+ permeable TRP channels, slows translocation by 10-100 fold. Our results indicate that Arr2 translocation in Drosophila photoreceptors is driven by diffusion, but profoundly accelerated by phototransduction and Ca2+ influx.
Keywords: phospholipase C, endomembrane, metarhodopsin, TRP channels, GFP, NINAC, retina, diffusion, protein translocation
Introduction
Photoreceptors employ multiple strategies to accommodate the enormous range of light in the natural environment. In addition to well-described “classical” mechanisms of light adaptation (Pugh et al., 1999; Fain et al., 2001), it is now recognised that several phototransduction proteins undergo massive subcellular translocation in response to light. Although molecular details may differ, this strategy is conserved from flies to mammals. For example, visual arrestin is predominantly cytoplasmic in dark-adapted photoreceptors of both flies and mammals, but on illumination translocates to photosensory membrane rich compartments, where it quenches ongoing photosignaling by binding to activated rhodopsin (Broekhuyse et al., 1985; Kiselev et al., 2000; Peterson et al., 2003; Calvert et al., 2006; Slepak and Hurley, 2008). Despite intensive investigation, both the mechanism and function of such translocation are unresolved, with two conflicting models dominating the discussion: translocation by motor proteins (McGinnis et al., 2002; Peterson et al., 2003; Lee and Montell, 2004; Reidel et al., 2008) and translocation by diffusion (Peet et al., 2004; Nair et al., 2005; Calvert et al., 2006; Slepak and Hurley, 2008). Further unresolved is whether phototransduction participates in arrestin translocation, with evidence supporting (Strissel et al., 2006), and challenging (Mendez et al., 2003; Elsaesser et al., 2010) a role.
Drosophila phototransduction, a phospholipase C (PLC) mediated Ca2+ influx cascade, is initiated by photoisomerization of rhodopsin (R) to metarhodopsin (M), which is inactivated by binding to the dominant arrestin isoform Arr2 (Dolph et al., 1993). Unlike vertebrate rhodopsin, which requires C-terminal phosphorylation by G-protein-coupled-receptor kinase, Arr2 binding to M does not require phosphorylation (Vinos et al., 1997; Alloway et al., 2000; Kiselev et al., 2000). In dark-adapted fly photoreceptors most Arr2 localizes to the cell body, but on illumination translocates to the rhabdomere (Alloway et al., 2000; Lee et al., 2003; Satoh and Ready, 2005), a densely packed column of photosensory apical microvilli loaded with rhodopsin (Rh1 in the R1-6 photoreceptors; ~1000 copies per microvillus) and proteins of the phototransduction cascade (reviewed in Wang and Montell, 2007; Hardie and Postma, 2008; Katz and Minke, 2009). A current, influential model for Arr2 translocation in Drosophila proposes that a myosin III motor (NINAC) powers Arr2 translocation by delivery of PIP3-enriched vesicles to the rhabdomere (Lee et al., 2003; Lee and Montell, 2004), but this was challenged by a conflicting report of normal Arr2 translocation in ninaC null mutants (Satoh and Ready, 2005). An alternative hypothesis, proposed for vertebrate rods, is that light transforms the photosensory compartment into a ‘light sink’, a diffusional trap that concentrates Arr2 via high-affinity binding to activated metarhodopsin (Mangini et al., 1994; Nair et al., 2005). Such a passive diffusional model predicts that arrestin translocation should be driven by stoichiometric binding to activated M.
To study arrestin translocation in Drosophila photoreceptors, we used genetics, in vivo imaging of GFP-tagged arrestin, electrophysiology and confocal immunolocalization to investigate light-induced redistribution of Arr2. To investigate stoichiometry we exploited the bistable, photointerconvertible pigment system of Drosophila, in which the number of M molecules can be accurately and reversibly controlled by the wavelength of illumination, with short wavelength (blue) light favouring R to M photoisomerization and long wavelength (e.g. orange) light photoreconverting M to R (see Figure 3A, reviewed in Hillman et al., 1983; Stavenga, 1996). Our results support a diffusion model for Drosophila by showing that translocation is rapidly and reversibly driven by stoichiometric binding to M without requirement for NINAC. We also show that, whilst not being absolutely required, phototransduction profoundly accelerates arrestin translocation, suggesting a novel regulatory role for PLC-mediated Ca2+ influx.
Figure 3. Spectral dependence of Arr2 translocation.
(A) Spectral properties of the Rh1 pigment system; normalized photosensitivities of rhodopsin (R, λmax = 480 nm) and metarhodopsin (M, λmax = 570 nm) are plotted using nomograms (Govardovskii et al., 2000). The photoequilibrium function fM(λ), derived by dividing the R photosensitivity spectrum by the M spectrum (magenta), closely fits available data (magenta symbols, our own data, mean, n = 3; closed symbols from Minke and Kirschfeld, 1979; open symbols, Belusic et al., 2010) . (B) Normalized fluorescence Fnorm = (F-Fmin)/(Fmax-Fmin) 50 ms after onset of blue test flash as a function of wavelength of prior photoequilibrating stimulus. Black plot: “control” data from flies expressing one copy of Rh1-Arr2-GFP (mean ± SEM, n = 12). Inset shows representative traces measured 10 s after termination of 20 s photoequilibrating stimuli of different wavelengths. Green plot, data from Rh1-Arr2GFP; ninaE/+ heterozygotes containing only ~60% Rh1 (n = 5). Blue symbols, dotted line: normalized fraction of endogenous Arr2 immunofluorescence in rhabdomere in wild type (w1118) measured from images as in C (mean ± SEM of 12 ommatidia from 4 flies at each wavelength). (C) Confocal images of Arr2 immunofluorescence in wildtype (w1118) eyes dark-dissected immediately following illumination at indicated wavelengths. Localization after 560 nm light was indistinguishable from dark-reared flies (c.f. Figure 1), but the fraction of Arr2 in rhabdomeres increased with shorter wavelengths. Scale bar: 5 μm. (D) Control and ninaE/+ heterozygote data from panel (B) replotted as a function of fM transformed using the fM(λ) function from panel A. (E) Control data set from D replotted in terms of number of Arr2 and M molecules per microvillus, assuming there are 2.7 × more Rh1 molecules than available Arr2 molecules in the cell, and that 25% of Arr2 is in the rhabdomere in the dark (first responder pool). The straight line represents 1:1 stoichiometry.
Results
Light concentrates Arr2 in rhabdomeres
In order to study the dark-adapted localization of Arr2, flies were dark-reared and dark-dissected and fixed under infrared illumination. As previously described, in the dark, approximately 20-30% of immunopositive Arr2 staining localizes to the rhabdomere (Alloway et al., 2000; Lee et al., 2003; Satoh and Ready, 2005), constituting a ‘first-responder’ pool for M inactivation (Figure 1A). The remainder (~ 70-80%) localizes to the cell body cytoplasm, a reserve that can be drawn upon when M out-titrates the rhabdomeric first responders.
Figure 1. Light concentrates Arr2 in rhabdomeres.
(A) Left: Arr2 in control (dark-reared) ommatidium immunolocalizes to both cell body and rhabdomeres (r). Also indicated: rhabdomere terminal web (rtw); nucleus (n); inter-rhabdomere space (irs). Pigment cells surrounding ommatidia lack Arr2. In eyes dissected after 10 min exposure to 470 nm (blue) light, cytoplasmic Arr2 has translocated to rhabdomeres. Translocation was completely reversed by orange (560 nm) exposure following blue light (blue->orange). Orange light did not redistribute Arr2 (n = 2-4 flies). (B) Arr2 in ommatidia of dark-reared wild-type (w1118) flies colocalized with the endomembrane marker HDEL (n = 4 flies). Arr2 and HDEL immunopositive crescents highlight the SRC. Bright rings of HDEL immunofluorescence between ommatidia highlight perinuclear ER of pigment cells, which do not express Arr2. Merged image (right) shows extensive colocalization (yellow). (C) In dark-adapted photoreceptors, Arr2 did not colocalize with microtubules (MT) stained with anti-tubulin; MT are largely excluded from cytoplasm. Projection image from 5 pictures taken at 0.5 μm intervals (n = 2 flies). Scale bar: 2 μm.
As previously reported (Alloway et al., 2000; Kiselev et al., 2000; Lee et al., 2003; Satoh and Ready, 2005), blue light dramatically redistributes Arr2. In eyes dark-dissected immediately following ten minutes of saturating blue illumination, Arr2 was concentrated in the rhabdomeres, and the adjacent apical membrane, the stalk, which contains rhodopsin at levels approximately 5% that of the rhabdomere (Arikawa and Matsushita, 1994). Bright Arr2 crescents at the rhabdomere base are not dissimilar to rhabdomere base crescents sometimes highlighted by anti-Rh1 immunostaining (Chinchore et al., 2009) and like them may indicate reduced antibody access to the core of the dense microvillar field (Figure S1A). Residual weak Arr2 staining was detected in cytoplasmic puncta and at the plasma membrane (Figure 1A). Exposure of blue-illuminated eyes to orange light reversed Arr2 translocation (Figure 1A), showing that a continuing presence of M is required for Arr2 concentration in rhabdomeres. Orange light alone, although evoking a near maximal electrical response in photoreceptors (e.g. Figure 4A), did not cause detectable Arr2 translocation (Figure 1A). The ‚first-responder’ pool of Arr2 resident in dark-adapted rhabdomeres presumably suffices to quench the limited M produced (see also Figures 3 and 4). When M is not photoreconverted, Arr2 remains rhabdomeric, even in the dark, for more than 12 hours, after which it begins to be removed by endocytosis; photoreceptors return to normal by three days (Figure S1B). Translocation of Arr2 in response to blue, but not orange light would be consistent with binding to high-affinity M generated in the apical, rhabdomeric membrane.
Figure 4. Electrophysiological correlates of Arr2 translocation.
(A) Electroretinogram (ERG) responses to 2 s photoequilibrating stimuli of different wavelengths in white-eyed flies (w1118). M was fully photoreconverted to R one minute before each stimulus by an ultrabright orange LED. At 520 nm there was a PDA that failed to recover. Between ca. 535 and 525 nm the recovery time courses became progressively longer. (B, C) Averaged data (mean ± SEM n = 7) plotted against wavelength (B) and fM (C): PDA, normalized to maximal PDA (at λ <515 nm) measured 40 s after the stimulus; t½ is the time to 50% recovery of the ERG. The normalized extent of translocation measured from Arr2-GFP fluorescence is replotted from Figure 3. (D) Responses to 2 s photoequilibrating 530 nm flashes expected to induce ~50% translocation (see panel B). 1st flashes, delivered 1 min after long wavelength illumination had fully reconverted M to R, showed a slow recovery phase as in panel A (2 example traces superimposed); responses to subsequent identical “2nd” flashes without an intervening reconverting stimulus (green) showed accelerated decay, similar to responses to 550 nm light (orange trace) that induced negligible translocation. Inset: bargraph showing time to 50% decay (t½: mean ± SEM, n = 9). (E) Responses to brief (4 ms) 560 nm flashes which by themselves caused negligible (<1%) R>M or M>R conversion, before (DA) or after (530 Ad, green) translocation induced by photoequilibrating 530 nm illumination. Bar graph, showing t½ for decay, indicates that translocation induced no significant difference in response kinetics under these conditions (mean ± SEM n= 4).
Arr2 in dark-adapted cytoplasm co-localized strongly, but not exclusively, with the HDEL endomembrane marker (Figure 1B) and the photoreceptor ER chaperone, NinaA (Figure S1C), in a textured cytoplasmic network. Notably, Arr2, HDEL and NinaA all highlighted the subrhabdomeric cisternae (SRC), a specialized smooth endoplasmic reticulum of anastomosing tubules applied to the base of the rhabdomere (MatsumotoSuzuki et al., 1989; Vihtelic et al., 1993). An Arr2-poor band, corresponding to the actin-rich cytoplasm of the rhabdomere terminal web, typically sets the SRC apart from cytoplasm generally. Arr2 is largely absent from the nucleus. Distinct from vertebrate rod visual arrestin (Nair et al., 2005), Arr2 in dark-adapted photoreceptors did not localize to microtubules (Figure 1C). The identity of putative endomembrane binding target(s) will be the subject of future investigation; candidates include phosphoinositide components of the lipid bilayer, and/or the NINAC myosin III, both of which have been reported to interact, directly or indirectly, with Arr2 (Lee et al., 2003; Lee and Montell, 2004; Liu et al., 2008).
In vivo imaging of Arr2-GFP translocation
To track arrestin movements in vivo, we expressed GFP-tagged Arr2 in photoreceptors R1-R6. Arr2-GFP appeared to function normally because it rescued the response deactivation defect in arr2 mutants and showed a similar immunolocalization pattern to endogenous Arr2. Overall Arr2 expression in our transgenic lines was similar to wild-type (Figures S1 and S2). We monitored Arr2-GFP concentration in the rhabdomeres in real time by imaging rhabdomere fluorescence in intact flies from the deep pseudopupil (DPP: Franceschini et al., 1981), a virtual image that sums rhabdomere fluorescence from approximately 20-50 ommatidia with the optics used. The blue excitation light simultaneously served as the stimulus for translocation and was sufficiently bright to convert the majority of R to M within ~100 ms (see Experimental Procedures). In eyes pre-illuminated with long wavelength (≥ 560 nm) light and then dark-adapted, the DPP showed weak, but obvious GFP-fluorescence in the first image captured by a 100 ms blue flash (Figure 2A-C; Movie S1). In subsequent images repeated at 1 Hz, DPP fluorescence rapidly brightened 2-3 fold (2.52 ± 0.10; mean ± SEM, n = 19) with an approximately single exponential time course with time constant of 6.8 s in flies dark-adapted for 1 minute and 11.3 s when dark-adapted for 10 minutes (Figure 2C). Optical neutralization of corneal lenses allows the distal tips of individual rhabdomeres to be visualized in live flies (Franceschini and Kirschfeld, 1971) and these likewise brightened rapidly in response to blue light, confirming the rapid translocation from cell body to rhabdomere at the single cell level (Figure S3 and Movie S2). For some experiments it was more convenient to measure DPP fluorescence using a photomultiplier (PMT), which also allows much faster sampling rates. The first increase in fluorescence indicative of translocation was detected within ~50 ms and the overall time course was similar to that determined using imaging (Figure 2D).
Figure 2. In vivo imaging of Arr2-GFP translocation.
(A) Frames at indicated times from a time-lapse movie (100 ms exposure, 1 s-1) of DPP fluorescence in transgenic flies expressing Arr2-GFP in outer photoreceptors, R1-R6 (see Movie S1). Non-expressing R7 forms the dark ‘keyhole’ at the DPP center. The first DPP image (t = 0 s) in orange- and then dark-adapted (10 min) eyes shows weak fluorescence, brightening rapidly in response to blue imaging flashes. (B) Using the DPP as region of interest, average pixel intensity, F (background subtracted, raw signal, arbitrary units), increased rapidly ca 2.5 fold, data fitted with single exponential function, time constant 12.5 s. (C) Averaged time course, normalized (Fnorm) between Fmax and Fmin (i.e F at time = 60s and 0 s) from similar measurements from flies dark adapted for 10 min (right curve, mean ± SEM, n = 19 flies) or 1 minute (left curve, n = 23), data fitted with single exponentials with time constants 6.8 and 11.3 s respectively. (D) Continuous recording of normalized DPP fluorescence sampled at 500 Hz by a photomultiplier after 1 minute dark adaptation. Inset on expanded timebase; transient (arrow) reflects R>M photoisomerization. (E) Two flash experiment to determine time course of reverse translocation. Starting with Arr2-GFP fully translocated following blue excitation (B), a brief (500 ms) ultrabright orange (Or) LED stimulus photoreconverted M to R; Arr2-GFP fluorescence was measured (by PMT) after varying delays (8, 6, 4, 3, 2 s). For trace marked “0” there was no reconverting Or stimulus. (F) Averaged data from traces as in E (mean ± SEM n = 8): fluorescence returned rapidly to the dark-adapted state, fitted by a single exponential with time constant 2.4 s. Data in A-C obtained using w1118; Rh1Gal4, UAS-Arr-2GFP,arr23/TM3 heterozygotes; D-F using w1118; Rh1-Arr2-GFP/CyO.
Because the blue light used for measuring Arr2-GFP fluorescence rapidly converts R to M, the return of Arr2-GFP to cytoplasm after reconversion of M to R with long wavelength light could not be observed directly. In order to measure the time course of this reverse translocation, we used a two flash paradigm. Starting with a fly pre-exposed to blue light to induce maximum translocation, M was rapidly photoreconverted to R with a brief (500 ms) intense long wavelength stimulus and the extent of translocation was then probed by PMT measurements at varying intervals with a blue test flash. The reduction in DPP fluorescence indicative of reverse translocation was even more rapid than movement into the rhabdomere, and could be fitted with a single exponential time constant of 2.4 s (Figure 2E,F). Overall, these results are fully consistent with results from confocal immunocytochemistry, whilst revealing the surprisingly rapid kinetics of translocation in vivo and in real time.
Arr2 translocates in proportion to light-activated metarhodopsin
Ideally, a passive diffusional model in which translocation is driven by binding of M to arrestin predicts a stoichiometric 1:1 relationship between the number of Arr2 molecules translocated and the number of photoisomerized M molecules in the rhabdomere. The bistable, photointerconvertible pigment system of invertebrates provides an excellent opportunity to measure this relationship in Drosophila. In particular, the fraction of M molecules (fM) can be rapidly and accurately controlled by illumination with defined spectral content according to the photoequilibrium spectrum (Figure 3A, Minke and Kirschfeld, 1979; Belusic et al., 2010) and can always be reset to effective zero (100% R) by long wavelength illumination. Although well established, we measured the photoequilibrium spectrum, fM(λ), with our equipment by measuring M fluorescence in wild-type (w1118) flies after photoequilibration to different wavelengths, and obtained essentially identical results to published data (Figure 3A).
We estimated the extent of translocation as a function of fM in vivo by measuring DPP fluorescence from flies expressing one copy of Rh1-Arr2-GFP, which have levels of Rh1 and Arr2 indistinguishable from wild type flies (Figure S2). We used photoequilibrating illumination at different wavelengths to vary fM and then measured DPP fluorescence immediately (50-100 ms) after the onset of a blue test flash, before any additional translocation had time to occur (Figure 3B). Measurements made following prolonged (20 s) exposure to wavelengths of 550 nm (fM = 0.09) or longer showed minimal increase in DPP fluorescence above the basal, dark-adapted signal (e.g. 10 min dark adaptation after 570 nm), even though such illumination causes a saturating electrical response in the photoreceptors (e.g. Figure 4A). Significant translocation (increase in DPP fluorescence) was first detected following photoequilibration with 545 nm light (fM = 0.12), and then increased steeply with decreasing wavelength, saturating at wavelengths of ~515 nm (fM = 0.41). The extent of translocation could be rapidly (< 30 s), repeatedly and stably adjusted to the same level by any given wavelength indefinitely (many hours), and irrespective of whether the direction of movement was into or out of the rhabdomere (Figure 3B). When the results were replotted as function of fM, using the photoequilibrium spectrum, they yielded a sigmoidal function that was approximately linear over much of the operational range (Figure 3D), indicating that the number of translocated Arr2 molecules is proportional to the number of M molecules generated.
We also explored the spectral dependence of translocation of endogenous Arr2 by immunolocalization in “wild-type” white-eyed flies dissected and fixed under infrared illumination after 10 min illumination at different wavelengths. The results showed a very similar spectral dependence with no detectable translocation in flies exposed to 560 nm light, but a progressively greater fraction of Arr2 localizing to the rhabdomeres at shorter wavelengths (Figure 3B,C).
The slope of the translocation vs fM plot ([Arr2]/fM) represents the ratio between translocated Arr2 and M molecules. Because the data were normalized, the stoichiometry can be simply derived by dividing the slope (3.8 ± 0.1 n =12 for the linear region of the control data set in Figure 3D) by the ratio between total Rh1 and Arr2 available for translocation (e.g. a 1:1 stoichiometry would result if this Rh1/Arr2 ratio were also 3.8:1). The ratio between Rh1 and total available Arr2 is generally accepted as between ~3 or 4:1 on the basis of the amount of R to M conversion required to out-titrate Arr2 leading to persistent activation of the transduction cascade and a “prolonged depolarizing after-potential”, or PDA (Minke and Kirschfeld, 1979; Dolph et al., 1993; Belusic et al., 2010). This is also in reasonable agreement with direct biochemical estimates: (e.g. see Johnson and Pak, 1986; Matsumoto et al., 1994). We have now refined the estimate of this ratio at ca 2.7:1 using measurements of the wavelength dependence of PDA generation and the formalism of Belusic et al 2010 (see Figures 4 and S2). However, assuming ~ 25% of Arr2 is already resident in the rhabdomere in the dark, only ~75% of Arr2 is available for translocation, so that the ratio between Rh1 and Arr2 available for translocation would be 2.7/0.75 or 3.6:1. This is very close to the slope of the translocation vs fM plot (3.8), and therefore strongly indicative of a 1:1 stoichiometry.
As a further test of stoichiometry, we performed a gene dosage experiment, using flies heterozygous for a null mutation of the Rh1 gene (ninaE/+), where the total number of Rh1 molecules was reduced to ~60% of wild-type levels (Figure S2). As predicted by a stoichiometric relationship between translocated Arr2 and M, there was a pronounced shift in the spectral dependence, and the limiting slope of the fM vs translocation plots (2.03 ± 0.03; n = 7), was reduced by a similar amount (Figure 3D).
To illustrate the stoichometric relationship between M and translocated Arr2, Figure 3E replots the data in terms of numbers of Arr2 molecules per microvillus (using the generally accepted value of 1000 Rh1 molecules per microvillus) as a function of the number of M molecules assuming an Rh1/Arr2 ratio of 2.7:1 in the cell as a whole (Figure S2), and that 25% of the Arr2 molecules are in the rhabdomere in the dark. Using these independently estimated parameters, significant translocation begins to occur as the first responder pool becomes exhausted, and thereafter there is a near 1:1 relationship between translocated Arr2 and M until saturation when total Arr2 becomes out-titrated by M. This implication of 1:1 stoichiometry strongly supports a diffusional basis for translocation driven by high affinity 1:1 binding of Arr2 to M.
For technical reasons, our measurements were made using monochromatic light in flies lacking the dense red-transparent screening pigment. To ask whether sufficient metarhodopsin to drive translocation is generated under natural conditions we immunolocalized Arr2 in true, red-eyed, wild-type flies (Canton S). The results showed that some translocation was apparent even in room light and very substantial, if not full translocation had clearly occurred under sunlight conditions (Figure S3C).
Functional correlates of arrestin translocation
The rapid time course and strict spectral dependence of Arr2 translocation leads to very specific predictions for electrophysiological responses. In flies, stimuli that convert more than ~35% of R to the M state out-titrate total Arr2, thereby inducing a PDA, which persists for several hours in the dark, but which rapidly returns to baseline once M is photoreconverted to R by long wavelength illumination (e.g. Minke and Kirschfeld, 1979; Dolph et al., 1993; Belusic et al., 2010). The first responder pool of Arr2 present in the rhabdomere in dark-adapted cells (~25%) should suffice to rapidly inactivate M created by stimuli that convert up to ~10% of Rh1 into the M state. But with stimuli photoisomerizing between ~10 and ~35% of the visual pigment we predicted that complete response inactivation could only be achieved by Arr2 translocating from the cytoplasm, and should therefore increasingly reflect the time course of translocation. To test this we measured electroretinogram (ERG) responses to intense photoequilibrating 2-3 s flashes of different wavelengths, photoreconverting M to R between flashes by a long wavelength stimulus. Responses to wavelengths of 540 nm or longer all inactivated with a similar relatively rapid time course (t1/2 ~ 3 s). As is well documented, short wavelength (<520 nm) flashes that converted >35% of R to M resulted in a full PDA that failed to inactivate (e.g. Minke and Kirschfeld, 1979). Strikingly however, in a tightly defined spectral window (535-525 nm), corresponding to the range where translocation occurs, response decay times increased steeply, reaching t1/2 values of 7-8 s at wavelengths around 530 nm, closely approximating the time course of translocation measured using Arr2-GFP. The significance of these results can be further appreciated when the PDA (measured 40 s after stimulus), the extent of translocation (measured by Arr2-GFP) and t1/2 of ERG decay are plotted together as a function of wavelength or fM (Figure 4B,C). As is well known, the PDA develops with a steep dependence on fM or wavelength (Minke and Kirschfeld, 1979; Belusic et al., 2010), first becoming pronounced at wavelengths shorter than ~525 nm (fM > 0.3). Translocation, with a dynamic range between ~545 and 515 nm (fM = 0.12-0.4), effectively prevents the development of a full PDA after the first-responder pool is exhausted, but at the expense of a slower response inactivation reflecting the time course of translocation.
These results indicate that for photoequilibrating stimuli, which create M in excess of the first responder pool of Arr2, response inactivation is limited by the time course of Arr2 translocation. We next asked whether, following such translocation, inactivation to a repeated identical stimulus (which would now, however, no longer further alter fM) might be accelerated. To test this we delivered repeated photoequilibrating flashes at a critical wavelength around 530 nm, which generates at most a small PDA, but activates substantial translocation (Figure 4D). As before, the response to a first (“priming”) 530 nm flash following a long wavelength stimulus (to reset fM to ~0) decayed with a t1/2 of ~7.5 s reflecting the time course of Arr2 translocation. A second identical flash (but without prior long wavelength photoreconversion), although generating an initial response of similar amplitude and waveform, now recovered much more rapidly, with a decay time course similar to control responses elicited by longer wavelengths (≥ 550 nm), which induced no translocation.
This result shows that translocated Arr2 can function effectively to accelerate response inactivation to a given photoequilibrating stimulus. However, our results indicate that translocated Arr2 remains bound to stably formed M, begging the question of how this Arr2 can inactivate newly formed M molecules. The explanation is probably two-fold: firstly, as long as the total Arr2 pool in the cell has not been exhausted, there will still be a pool of “first responder” Arr2 molecules in the rhabdomere, not bound to M, and in equilibrium with residual cytosolic Arr2. In addition, the second photoequilibrating 530 nm flash, whilst isomerizing further R molecules to M, must also reisomerize an equal number of M molecules to R (because fM must remain the same at the end of the second flash). These release their bound Arr2 making it available for binding to newly formed M. The kinetics of the release of Arr2 from R after photoreconversion from M have not previously been measured; this result implies that it is rapid and does not limit response inactivation measured under these conditions.
A related, and perhaps more pertinent question in functional terms, is whether responses to dimmer, more physiologically relevant stimuli, which do not substantially alter fM, are accelerated following translocation, as suggested, for example, by Lee et al. (2003). To test this we delivered brief orange test flashes of sufficiently low quantal content that they had negligible (<1%) effect on fM before and after inducing substantial translocation with a 530 nm photoequilibrating stimulus. In marked contrast, there was now no detectable acceleration of response inactivation following translocation (Figure 4E).
In summary, we have found clear electrophysiological correlates of arrestin translocation. Specifically, when stimuli are of sufficient intensity and spectral composition to out-titrate the first responder pool of immediately available arrestin, responses terminate slowly, reflecting the time course of translocation. However, following translocation, responses to the same stimulus again terminate rapidly. This suggests that translocation has the role of adapting arrestin levels in the rhabdomere to the prevailing fM level determined by the ambient illumination. However, with the caveat that the ERG is a rather crude measure of response kinetics, translocation per se does not appear to accelerate response inactivation to modest and physiologically more relevant stimuli that do not substantially alter fM.
Phototransduction mutants slow, but do not eliminate, Arr2 translocation
A recent study, based on immunolocalization of Arr2 in transduction mutants, concluded that the phototransduction cascade had no impact on arrestin translocation in Drosophila (Elsaesser et al., 2010). We re-examined this important question using our more sensitive in vivo approach. Firstly, we measured Arr2-GFP translocation in norpAP24 mutants, which lack the effector enzyme phospholipase C and have no electrophysiological response to light. Strikingly, although an increase in DPP fluorescence, indicative of translocation, was still detected in norpA mutants, it was greatly slowed with a distinct delay of 5-10 s and a time course that could be fitted with two time constants of ~90 and 1100 s (Figure 5A-C). Furthermore, overall the fluorescence increased by only a factor of 1.8 even after 30 minutes illumination, compared to the ~2.5-fold increase measured in wild-type controls within 1 minute.
Figure 5. Arr2 translocation is slowed in transduction mutants.
(A) Translocation timecourses determined by imaging DPP fluorescence of norpAP24, trp301 and ninaCP235 mutants expressing Arr2-GFP; data normalized to Fmin (i.e. F at time zero). Both speed and relative increase were greatly reduced in norpA and trp. In ninaCP235 the time course was similar to wild-type, but the overall increase enhanced. Traces averaged from measurements in 4 (norpA), 9 (trp, ninaC) and 19 (wild-type) flies dark-adapted for 10 min after orange pre-illumination. norpA data fitted by sum of two exponentials (t1 = 91 s, t2= 1107 s). (B) Arr2-GFP fluorescence now fully normalized between Fmin and Fmax (or F at 300 s in norpA and trp) to compare time courses on a faster time scale. The time course in ninaC was similar to that in wild-type flies (data from panel A), but much slower in norpA (averaged from n = 5 further flies recorded on faster time base). In trp (n = 9) translocation was initially as fast as in wt but after ~5 s slowed dramatically. (C) Left, overall fluorescence increase (Fmax/Fmin) after 60 s (wt and ninaC) and after 3 minutes (1) and 30 min (2) for norpA and 5 min for trp; right, time constant of exponentials fitted to data (wt and ninaC fitted with one exponential, norpA with two time constants; trp with one time constant, ignoring the first 5 seconds). * p < 0.01; ** p< 10-5 2-tailed t-test with respect to wt. (D) Arr2 immunostaining from wild-type and norpA retinae dark-dissected following 2 min blue light illumination, and after 2 min blue light followed by 1 hr in dark. In norpA, cytoplasmic Arr2 persisted abnormally at 2 min, but was rhabdomeric by 1 hr. Scale bar: 5 ±m. (E) Fraction of total Arr2 immunofluorescence in the rhabdomere (frhab), in flies fixed in the dark or after blue illumination. Compared to wild-type all mutants except ninaC showed significantly less Arr2 in the rhabdomere 3 min after blue light. Levels in trpl;trp and norpA were also significantly lower than in dgq or trp. Any differences in dark-adapted values amongst the various mutants did not reach statistical significance. Wild-type and norpA data are also shown from flies fixed 2 min after blue illumination. Mean ± SEM, n = 4-8 flies for each genotype, analysed using ImageJ (* p < 0.05; ** p< 0.0005 ).
Next we explored translocation of Arr2-GFP expressed in trp mutants, which lack the major light-sensitive and highly Ca2+ permeable TRP channels. In trp mutants the electrical response to bright light (now mediated by the still moderately Ca2+ permeable TRPL channels) is initially near normal, but decays to baseline within seconds, following which the photoreceptors become completely refractory to further stimulation until allowed to recover in the dark (see Figure 6A, Cosens and Manning, 1969; Hardie and Minke, 1992; Hardie et al., 2001). Translocation in trp mutants closely mirrored this electrophysiological phenotype: DPP fluorescence initially increased rapidly with a similar time course to that observed in wild-type flies, but slowed dramatically after a few seconds and then proceeded over a time course similar to that in norpA mutants (Figure 5A-C).
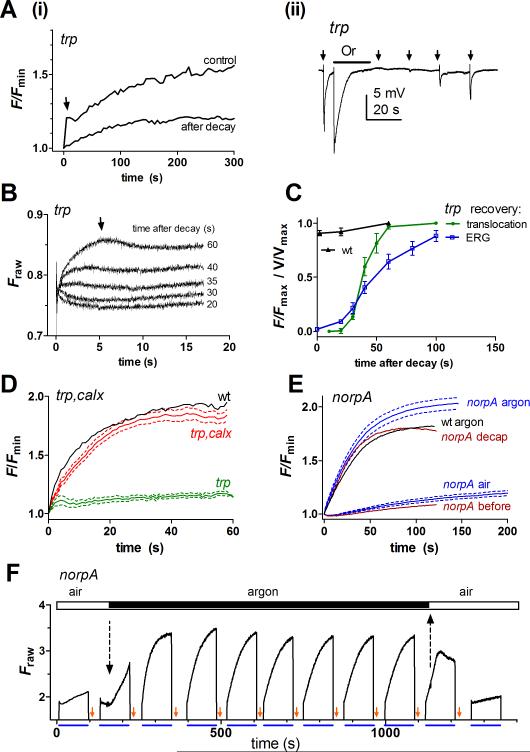
Figure 6. Ca2+ influx mediates acceleration of translocation.
(A) (i) translocation in a trp mutant (monitored by imaging Arr2-GFP) measured after 1 min (control), or only 15 s in the dark after “trp decay” induced by 20 s orange illumination. The initial fast phase (arrow) was absent in measurements made 15 s after decay. (ii) ERG recording from trp mutant; 20 s orange illumination (Or) induced a full “trp” decay and rendered the eye temporarily refractory to brief (100 ms) test flashes (arrows). (B) Translocation on a faster timescale (PMT measurements of Arr2-GFP fluorescence) in a trp mutant measured after varying times in dark following “trp decay” induced by 20 s orange LED. The fast phase of translocation (arrow) began to recover after >30 s in the dark. (C) Normalized time-course of recovery of fast phase translocation in trp mutants measured from the level of Arr-2GFP fluorescence 10s after onset of blue light (green plot, from traces as in B, mean ± SEM n = 5). Blue plot: time course of recovery of the response to test flashes in the ERG (n = 5). In otherwise wild-type flies (black, n = 4), at least 90% full, rapid translocation was observed immediately (ca 1.5 s) after the orange light was turned off. (D) Translocation measured from imaging Arr2-GFP fluorescence in calxA, trp343 double mutants (red, mean ± SEM, n = 6 flies) was similar to wild-type (black) and clearly rescued compared to trp343 mutants (green, n = 4 flies). (E) Averaged translocation time courses in norpA mutants (blue; mean ± SEM, n = 5; PMT measurements), measured in air and argon from the same flies. Red traces: norpA mutant (in air) before and after (decap) severing the neck (representative, n = 6). Black trace: Arr2-GFP translocation measured in wild-type fly in argon (n = 2). (F) Continuous (PMT) recording of Arr2-GFP fluorescence in norpA. Following each 90 s exposure to blue excitation, M was reisomerized to R by a photoequilibrating orange flash (small arrows). The second run started in air, but after ~10 s, argon was streamed over the fly. Within seconds an increase in fluorescence indicates acceleration of translocation (dotted arrow). Subsequently robust, rapid and reversible translocation was observed for at least 15 min under continuous anoxia. On return to air (upward dotted arrow), translocation stalled, and again only very slow, partial translocation was observed.
We also measured translocation of Arr2-GFP in ninaCP235 mutants lacking the NINAC myosin III, which has previously been proposed as a motor protein required for Arr2 translocation (Lee and Montell, 2004). However, we found that the time course of Arr2-GFP translocation in both forward and reverse (not shown) directions was very similar to that in wild type, whilst the overall extent was in fact significantly increased (Figure 5A-C). We did find that the spectral dependence of translocation in ninaC mutants was significantly shifted, requiring substantially shorter wavelengths (greater fM values) to achieve the equivalent extent of translocation (Figure S3B). This is to be expected though, because ninaC mutants are well known to have substantially reduced levels of Rh1 (Matsumoto et al., 1987; Hofstee et al., 1996) and hence require greater fM values to achieve the same number of M molecules.
Finally, we investigated translocation of endogenous Arr2 in wild type and mutant photoreceptors using confocal immunofluorescence microscopy (Figure 5D). Dark-adapted norpA mutant showed textured endomembrane localization similar to wild-type. By contrast, following 2 min blue light exposure in norpA mutants, although Arr2 was enhanced to some extent at the rhabdomere base and stalk, compared to wild-type retinae, it was now abnormally retained in cell body cytoplasm (Figure 5D). However, after 1 hr in the dark following 2 min blue light exposure, Arr2 in norpA mutants again became highly concentrated in the rhabdomere as occurs in wild type. Photoreceptors deficient in other components of the transduction cascade, including the heterotrimeric Gq protein (dgq1), TRP channels (trp301) and double mutants lacking both TRP and TRPL channels (trpl; trp343), showed a similar pattern, with normal dark-adapted Arr2 cytoplasmic localization, but abnormally high levels of residual cytoplasmic Arr2, remaining at 3 min post exposure (Figure 5E; Figure S4). However, ninaCP235 null mutants were quantitatively indistinguishable from wild-type (see also Satoh and Ready, 2005).
These results indicate that, whilst not absolutely required, phototransduction greatly accelerates translocation, downstream of PLC and activation of the light-sensitive channels.
Acceleration of translocation is mediated by Ca2+ influx
norpA and trpl;trp mutants, which have no electrophysiological response to light, showed the greatest defects in translocation, whilst defects in dgq1 and trp mutants, although highly significant, were less marked. Because there is a substantial residual light response and Ca2+ influx in the latter mutants (Scott et al., 1995; Hardie, 1996) we hypothesized that the acceleration might be mediated by Ca2+ influx, possibly by promoting Arr2 release from putative binding targets in the cell body.
To test this, we first asked whether the residual translocation observed in trp mutants depended upon the initial Ca2+ influx before the response decays to baseline. If so, we predicted that even the initial fast phase of translocation should be prevented if the photoreceptors were first rendered refractory by prolonged illumination (using long wavelengths to maintain low fM). In confirmation, measurements made in trp within ~20 s of intense (photoequilibrating) orange illumination now showed no sign of the initial fast increase in fluorescence. However, after a recovery period of 30-60 s in the dark the fast phase again became increasingly prominent (Figure 6A-C). Parallel ERG recordings confirmed that the orange illumination was sufficient to induce full trp decay, and that recovery of the light response coincided with the recovery of the rapid phase of translocation (Figures 6A, C).
Secondly, if impaired translocation in trp mutants is due to insufficient Ca2+, we reasoned that the phenotype might be rescued by genetic elimination of Na+/Ca2+ exchanger activity (encoded by the calx gene), which is the dominant mechanism for Ca2+ extrusion in the photoreceptors (Wang et al., 2005). In striking confirmation, both the extent and time course of translocation of Arr2-GFP expressed in calxA,trp343 double mutants were now restored close to wild-type (Fig. 6 D).
Finally we asked whether the slow translocation phenotype in norpA mutants could be rescued by activation of the Ca2+ permeable light-sensitive channels. This can be readily and reversibly achieved in intact flies by anoxia, which, even in norpA mutants, rapidly leads to spontaneous activation of the Ca2+ permeable TRP channels (Agam et al., 2000), probably due to failure of ATP dependent kinases such as DAG kinase and PIP kinase (Hardie et al., 2003). To achieve anoxic conditions, intact norpA mutants expressing Arr2-GFP were mounted for PMT measurements of DPP fluorescence and exposed to a stream of argon gas. Invariably, shortly after the onset of anoxia, norpA mutants developed robust and accelerated Arr2-GFP translocation in response to blue excitation, which, as usual, could be rapidly reversed following photoreisomerization with long wavelengths (Figure 6E,F). On return to air, however, translocation was once again profoundly slowed. A similar rescue of rapid translocation in both norpA could be achieved by the simple (albeit fatal) expedient of severing the neck, which provides the tracheal oxygen supply to the head. The time course of translocation (time constant 31.2 ± 5.8 n = 5) during anoxia was not fully accelerated to the wild-type situation. However, it was indistinguishable from the time course in wild-type flies exposed to argon (30.8 ± 3.5 n = 3; dotted trace on Figure 6E). This could be because the TRP current activated during anoxia is less than during a saturating light response; however, we do not exclude the possibility that additional factors generated during phototransduction may also contribute to the acceleration of translocation.
Overall these results indicate that Ca2+ influx, whilst not able to trigger Arr2 translocation without formation of M, is an essential mediator of the acceleration translocation.
Discussion
We have exploited the optics of the fly compound eye to measure dynamics of GFP-tagged arrestin translocation in intact animals. In combination with the photointerconvertible bistable (R/M) pigment system this has allowed us to resolve the unexpectedly rapid kinetics of arrestin translocation in vivo and to demonstrate proportionality between translocated arrestin and photoisomerised visual pigment with a stoichiometry equal or close to 1:1. We also found direct electrophysiological correlates of translocation and showed that the rate of translocation is profoundly slowed in a range of phototransduction mutants, but not in mutants of the putative motor protein, NINAC. Although we do not exclude the possibility that GFP-tagging Arr2 might subtly affect its properties, Arr2-GFP rescued the arr2 response deactivation phenotype, and co-localized with endogenous Arr2. Furthermore all our major conclusions were independently confirmed by immunolocalization of endogenous Arr2 in wild-type and mutant flies.
These results suggest a regulated, diffusion driven two-sink model for Arr2 translocation in Drosophila photoreceptors (Figure 7). In dark-adapted photoreceptors, approximately 25% of total Arr2 resides in rhabdomeres. We propose that this distribution is dictated by a distributed, low-affinity endomembrane partner for Arr2 in dark-adapted cytoplasm balanced by a, possibly similar, low-affinity target in the microvilli, such as phosphoinositides (Lee et al., 2003) and/or NINAC (Liu et al., 2008). Assuming total Arr2 is ~35% the level of rhodopsin, this first-responder pool suffices to rapidly quench M as long as fM remains below ~0.1. Upon illumination M increases according to the spectral and quantal content of illumination. Coupled with high affinity 1:1 binding of M with Arr2, diffusion then automatically drives Arr2 into the rhabdomere. At first sight the rapid time course of translocation might seem to question a passive diffusional model. But in fact, this is probably the only way this could be achieved, because molecular motors would likely be incapable of such rapid bulk transport of up to ~107 Arr2 molecules (e.g. Calvert et al., 2006; Slepak and Hurley, 2008). The conspicuously fast time course compared to vertebrate rods, where translocation takes place over minutes (Peterson et al., 2003; Elias et al., 2004; Strissel et al., 2006), probably reflects the different anatomies of the respective photoreceptors. In rods, longitudinal diffusion must occur over many tens of μm between the outer and inner segments via the narrow connecting cilium, and a diffusional model predicts equilibration with a half-time of 3.5 minutes assuming a diffusion coefficient (D) for arrestin of 1.4 μm2 s-1 (Calvert et al., 2006). In flies, rhodopsin-rich rhabdomere membrane is broadly exposed to cell cytoplasm across the rhabdomere base and Arr2 translocation is radial, with an average distance (d) to be travelled of 2 - 2.5 μm (radius of the cell body). Assuming the same diffusion coefficient (but ignoring geometrical constraints such as the narrow microvillar neck), this would represent a diffusion time (td = d2/2D) of only 2-3 seconds. The ca 4-fold faster time constant determined for reverse translocation would also be consistent with diffusion, as now the Arr2 only needs to move about half this distance in order to exit the rhabdomere (radius ~0.8 μm).
Figure 7. A regulated two-sink model for Drosophila Arr2 translocation.
Left: Dark-adapted rhabdomeres and adjacent stalk membrane contain R (blue). Cytoplasmic Arr2 (green) associates with ER/endomembrane, a distributed, low affinity 'dark sink'. ~25% of total Arr2 (“first responder” pool) also localizes to the rhabdomere, presumably due to a similar low affinity sink in the microvilli, e.g. binding to phosphoinositides (Lee et al., 2003) or NINAC (Liu et al., 2008). Middle: when the fraction of M (fM) lies between ca. 0.1 and 0.35 (M<Arr2), high affinity, photoactivated M (red) has bound all first responder Arr2 and recruited additional Arr2 from photoreceptor cytoplasm, initiating translocation and quenching signalling. Increased cytosolic Ca2+ downstream of phototransduction is hypothesized to promote Arr2 endomembrane dissociation, facilitating its diffusive search for high affinity M. Right: Stimuli generating M in excess of Arr2 (fM> 0.35) result in binding and translocation of virtually all Arr2, but still leave unquenched M, generating a PDA.
Such a two-sink model resembles the interaction-restricted diffusion model proposed to underlie translocation in vertebrate rods, where microtubules constitute the ‘dark sink’ and activated, phosphorylated rhodopsin in outer segments the ‘light sink’ (Nair et al., 2005; Slepak and Hurley, 2008). However, others still favour a motor-based mechanism, particularly for reverse translocation, which, in contrast to Drosophila, has distinctly slower kinetics than forward translocation in vertebrate rods (e.g. Reidel et al., 2008).
If or how phototransduction impacts arrestin translocation in vertebrate rod outer segments, where light causes a decrease in cytosolic Ca2+, also remains controversial (Mendez et al., 2003; Nair et al., 2005; Strissel et al., 2006; Slepak and Hurley, 2008). Although light dependent release from the cytosolic sink has been proposed (Calvert et al., 2006; Strissel et al., 2006), the “superstoichiometry” that underpinned this hypothesis has recently been questioned (Slepak and Hurley, 2008). Intriguingly, a recent report suggested that translocation in Xenopus rods is initiated via a G-protein coupled PLC cascade (Orisme et al., 2010). We show here that, contrary to a recent report (Elsaesser et al., 2010), translocation in Drosophila is profoundly slowed in various phototransduction mutants, but unimpaired in null mutants of myosin III (ninaC). Our evidence strongly suggests that translocation is normally accelerated by Ca2+ influx via the light-sensitive TRP and TRPL channels, and the next challenge will be to identify the underlying mechanism(s). We envisage at least two, not mutually exclusive, possibilities. Firstly Ca2+ might reduce the affinity of Arr2 for its putative endomembrane binding partner(s). Secondly, Ca2+ might “gate” diffusional access of Arr2 to the microvilli, the lumen of which is connected to the rest of the cell body by a narrow (ca. 20-30 nm) neck. Either or both of these novel mechanisms would add to the already rich repertoire of targets regulated by PLC-mediated Ca2+ influx pathways.
Experimental procedures
Fly stocks
Flies were reared on standard cornmeal, glucose, agar and yeast based medium at 20-25 °C in a dark incubator. Fly stocks were crossed into a white (w1118) background to eliminate compound eye screening pigments, except weak eye pigmentation from the mini-white marker gene in Rh1Gal4 and UAS-Arr2-GFP. For some experiments a cn,bw background was used to eliminate even this weak pigmentation. Dynamics of translocation were similar in either case (Figure S3). Rh1-Gal4 flies were a kind gift from Dr Chihiro Hama; norpAp24, trp301, trpl302;trp343, calxA, and ninaCp235 from Drs. William Pak and Craig Montell; dgq1 and arr23 from Dr. Charles Zuker. Experiments with transduction mutants were performed on young (< 2 days) adults to minimise any effects of retinal degeneration. On a wild-type background, the age of the fly (up to at least two weeks) did not influence results. GFP imaging was performed using flies expressing one copy of Arr2-GFP driven by the rhodopsin (Rh1) promoter, either in tandem (Rh1-Arr2-GFP) or using UAS-Arr2-GFP driven by Rh1Gal4. For some experiments Rh1Gal4 and UAS-Arr2-GFP were recombined with arr23 mutation to generate Rh1Gal4, UAS-Arr2-GFP,arr23 /TM3 flies. The total Arr2 expression level, estimated by Western analysis, was ~80% of wild-type in this arr2/+ heterozygote background. For measurements of spectral dependence of translocation flies expressing Rh1-Arr2GFP on a wild-type Arr2 background were used as these had essentially wild type levels of total Arr2 and Rh1 (Figure S2). For immunohistochemical experiments, w1118 flies were reared at 20 °C in dark, and dark-dissected using infrared illumination and infrared sensitive eyepieces. Light illumination experiments were performed as described previously (Satoh and Ready, 2005).
Construction of transgenic flies expressing C-terminal tagged Arr2-GFP
The entire Arr2 coding region was inserted into either the pUAST-GFP or pCaSpeR vector (gift from Dr J. O'Tousa), permitting Gal4 and/or Rh1-regulated expression. Constructs were transformed into Drosophila and insertion strains containing a single copy of each transgene were generated by standard methods (outsourced to Bestgene, Chino Hills, CA).
Antibody formation and indirect immunohistochemistry
Chicken anti-Arr2 antibody was made against C-terminal peptide, HRNVKGYYQD (Pacific Immunology). This antibody recognizes a single band around 48 kD, the position marked by Rabbit anti-Arr2 (Dolph et al., 1993). Fixation and staining methods are described in (Satoh and Ready, 2005). Primary antisera were chicken anti-Arr2 antibody (1:100), rabbit anti-Arr2 (1:100) (gift from Dr. Rama Ranganathan), alpha-tubulin (1/50) (Serotec), HDEL (1:100) (Santa Cruz Biotech. Inc) and NinaA (1:100) (gift from Dr. Charles Zuker). For quantitation of Arr2 localization, the total fluorescence (area × intensity) in cell body and rhabdomeres, were separately measured using ImageJ, including any signal in the stalk and crescent at base in the rhabdomere signal. A background, measured from several random regions of surrounding glia was subtracted and rhabdomere signal (frhab in Figure 5E) expressed as fraction of total (cell body plus rhabdomere).
Live GFP imaging
To image DPP fluorescence, flies expressing Arr2-GFP in photoreceptors R1-R6 were glued to coverslips using a drop of clear nail polish, mounted on a Nikon Eclipse TE200 microscope and observed with a 10x/0.30 NA Nikon Plan Fluor objective. To image fluorescence in individual rhabdomeres, flies were glued with eyes apposed to coverslips and imaged through the coverslip using a 100X/1.3 NA Nikon Plan Fluor oil immersion objective. Metamorph v. 6.3 controlled epifluorescence illumination from a 100 W Hg lamp via a Sutter Lambda 10-2 filter changer. Time-lapse movies were acquired by capturing images (100 ms exposures, 1 Hz) on a Photometrics CoolSnap HQ CCD camera. To correct for DPP movement, movie stacks were aligned in ImageJ using an image stabiliser plugin (http://bigwww.epfl.ch/thevenaz/turboreg/) and background was subtracted using the ImageJ ‘rolling ball’ algorithm with a 50 pixel radius. The DPP or rhabdomere tip was outlined as a region of interest for average intensity measurement.
For higher time resolution, the DPP was cropped via a rectangular diaphragm and sampled at up to 500 Hz using a photomultiplier (Cairn Research Ltd) collecting fluorescence excited by a blue (470 nm peak) ultrabright LED observed through a Nikon Plan Fluor 20x/0.5 NA Fluor objective, a 515 nm dichroic filter and OG515 long pass filter using a Nikon TE300 microscope. This set-up was also used for the imaging experiments of Figure 6D, using continuous blue LED illumination and a Scion Firewire camera. Photoequilibria to different wavelengths were achieved by light from a 75 W Xe arc passed through a monochromator (Photon Technology International) via the front port of the microscope. The photoequilibrium spectrum, fM(λ), was measured as described by Belusic et al 2010 using M fluorescence (600 nm excitation, emission > 695 nm), and was well fitted by a theoretical function calculated by dividing visual pigment nomograms (Govardovskii et al., 2000) assuming λmax of 480 and 570 nm for the R and M states, respectively, and peak photosensitivity of the M state 1.6 times that of R (Fig. 3A). The amount of light required to achieve effective photoequilibrium in any experiment was determined empirically by increasing duration until saturation of M fluorescence, translocation (or its reversal) was reached.
Electrophysiology
Intact white-eyed flies (w1118) were immobilized with low melting point wax in truncated Eppendorf tips. Electroretinograms were recorded using ~10 MΩ glass microelectrodes filled with Ringer (140 mM NaCl, 5 KCl, 1.5 CaCl2, 4 MgCl2) inserted into the retina. A similar indifferent electrode was inserted into the head capsule. Signals were amplified by a DAM60 DC amplifier (WPI) and sampled and analysed using pClamp 8.0 software. Light was delivered via the same 75W Xe arc lamp and monochromator used to determine the spectral dependence of translocation. When required, M was quantitatively reconverted to R by saturating (photoequilibrating) exposure (5 s) of full intensity light from an ultrabright orange LED (Thorlabs). Again, the amount of light required to achieve photoequilibrium was determined empirically by increasing duration until saturation (e.g. of PDA or its reversal) was reached.
Supplementary Material
Acknowledgements
We are grateful to Dr. R. Ranganathan (Rabbit anti-Arr2) and Dr. C.S. Zuker (Rabbit anti-NinaA) for reagents and fly stocks. This work was supported by NIH grant NEI10306 (DFR). BBSRC BB/G006865/1 (RCH). AKS was supported by Naito Foundation (25-040920), Novartis Foundation (20-120), and KAKENHI (21687005 and 21113510). Live cell imaging was supported by NSF-MEU grant 0217552-IBN (PI, Dr. K. Robinson). We are indebted to Drs Ken Robinson and Doekele Stavenga for critical comments on earlier versions of the MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agam K, von Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, Minke B. Metabolic stress reversibly activates the Drosophila light- sensitive channels TRP and TRPL in vivo. J. Neurosci. 2000;20:5748–5755. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsinarrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. doi: 10.1016/s0896-6273(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Matsushita A. Immunogold localization of opsin and actin in Drosophila photoreceptors that undergo active rhabdomere morphogenesis. Zool. Soc. Japan. 1994;11:391–398. [Google Scholar]
- Belusic G, Pirih P, Stavenga DG. Photoreceptor responses of fruitflies with normal and reduced arrestin content studied by simultaneous measurements of visual pigment fluorescence and ERG. J. Comp. Physiol. A. 2010;196:23–35. doi: 10.1007/s00359-009-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse RM, Tolhuizen EF, Janssen AP, Winkens HJ. Light induced shift and binding of S-antigen in retinal rods. Curr. Eye Res. 1985;4:613–618. doi: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr., Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Chinchore Y, Mitra A, Dolph PJ. Accumulation of rhodopsin in late endosomes triggers photoreceptor cell degeneration. PLoS Genet. 2009;5:e1000377. doi: 10.1371/journal.pgen.1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260:1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol. Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- Elsaesser R, Kalra D, Li R, Montell C. Light-induced translocation of Drosophila visual Arrestin2 depends on Rac2. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4740–4745. doi: 10.1073/pnas.0906386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in Vertebrate Photoreceptors. Physiol. Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Franceschini N, Kirschfeld K. Etude optique in vivo des elements photorecepteurs dans l'oeil compose de Drosophila. Kybernetik. 1971;9:159–182. doi: 10.1007/BF00270828. [DOI] [PubMed] [Google Scholar]
- Franceschini N, Kirschfeld K, Minke B. Fluorescence of photoreceptor cells observed in vivo. Science. 1981;213:1264–1267. doi: 10.1126/science.7268434. [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis. Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Hardie RC. INDO-1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors. J. Neurosci. 1996;16:2924–2933. doi: 10.1523/JNEUROSCI.16-09-02924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Chyb S, Raghu P. Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by the diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J. Biol. Chem. 2003;278:18851–18858. doi: 10.1074/jbc.M300310200. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Postma M. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In: Albright TD, Masland R, Basbaum K, Westheimer Shepher, editors. The Senses - a comprehensive reference Vision. Vol. 1. Academic Press; Oxford: 2008. pp. 77–130. [Google Scholar]
- Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–159. doi: 10.1016/s0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- Hillman P, Hochstein S, Minke B. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol. Rev. 1983;63:668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- Hofstee CA, Henderson S, Hardie RC, Stavenga DG. Differential effects of NINACproteins (p132 and p174) on light-activated currents and pupil mechanism in Drosophila photoreceptors. Vis. Neurosci. 1996;13:897–906. doi: 10.1017/s0952523800009147. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Pak WL. Electrophysiological study of Drosophila rhodopsin mutants. J. Gen. Physiol. 1986;88:651–673. doi: 10.1085/jgp.88.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci. 2009;3:2. doi: 10.3389/neuro.03.002.2009. doi:10.3389/neuro.3303.3002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron. 2000;28:139–152. doi: 10.1016/s0896-6273(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Montell C. Light-dependent translocation of visual arrestin regulated by the NINAC myosin III. Neuron. 2004;43:95–103. doi: 10.1016/j.neuron.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Liu CH, Satoh AK, Postma M, Huang J, Ready DF, Hardie RC. Ca2+-dependent metarhodopsin inactivation mediated by Calmodulin and NINAC myosin III. Neuron. 2008;59:778–789. doi: 10.1016/j.neuron.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangini NJ, Garner GL, Okajima TI, Donoso LA, Pepperberg DR. Effect of hydroxylamine on the subcellular distribution of arrestin (S-antigen) in rod photoreceptors. Vis. Neurosci. 1994;11:561–568. doi: 10.1017/s0952523800002467. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Isono K, Pye Q, Pak WL. Gene encoding cytoskeletal proteins in Drosophila rhabdomeres. Proc. Natl. Acad. Sci. U.S.A. 1987;84:985–989. doi: 10.1073/pnas.84.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Kurien BT, Takagi Y, Kahn ES, Kinumi T, Komori N, Yamada T, Hayashi F, Isono K, Pak WL, et al. Phosrestin I undergoes the earliest light-induced phosphorylation by a calcium/calmodulin-dependent protein kinase in Drosophila photoreceptors. Neuron. 1994;12:997–1010. doi: 10.1016/0896-6273(94)90309-3. [DOI] [PubMed] [Google Scholar]
- MatsumotoSuzuki E, Hirosawa K, Hotta Y. Structure of the subrhabdomeric cisternae in the photoreceptor cells of Drosophila melanogaster. J. Neurocytol. 1989;18:87–93. doi: 10.1007/BF01188427. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Matsumoto B, Whelan JP, Cao W. Cytoskeleton participation in subcellular trafficking of signal transduction proteins in rod photoreceptor cells. J. Neurosci. Res. 2002;67:290–297. doi: 10.1002/jnr.10120. [DOI] [PubMed] [Google Scholar]
- Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of Rhodopsin phosphorylation and transducin signaling. J. Neurosci. 2003;23:3124–3129. doi: 10.1523/JNEUROSCI.23-08-03124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Kirschfeld K. The contribution of a sensitizing pigment to the photosensitivity spectra of fly rhodopsin and metarhodopsin. J. Gen. Physiol. 1979;73:517–540. doi: 10.1085/jgp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisme W, Li J, Goldmann T, Bolch S, Wolfrum U, Smith WC. Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cell Signal. 2010;22:447–456. doi: 10.1016/j.cellsig.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet JA, Bragin A, Calvert PD, Nikonov SS, Mani S, Zhao X, Besharse JC, Pierce EA, Knox BE, Pugh EN., Jr. Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J. Cell. Sci. 2004;117:3049–3059. doi: 10.1242/jcs.01167. [DOI] [PubMed] [Google Scholar]
- Peterson JJ, Tam BM, Moritz OL, Shelamer CL, Dugger DR, McDowell JH, Hargrave PA, Papermaster DS, Smith WC. Arrestin migrates in photoreceptors in response to light: a study of arrestin localization using an arrestin-GFP fusion protein in transgenic frogs. Exp. Eye Res. 2003;76:553–563. doi: 10.1016/s0014-4835(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr., Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr. Opin. Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- Reidel B, Goldmann T, Giessl A, Wolfrum U. The translocation of signaling molecules in dark adapting mammalian rod photoreceptor cells is dependent on the cytoskeleton. Cell Motil. Cytoskeleton. 2008;65:785–800. doi: 10.1002/cm.20300. [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gq a protein function in vivo: Genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- Slepak VZ, Hurley JB. Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: interaction-restricted diffusion. IUBMB Life. 2008;60:2–9. doi: 10.1002/iub.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG. Insect retinal pigments: Spectral characteristics and physiological functions. Prog. Retinal Eye Res. 1996;15:231–259. [Google Scholar]
- Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J. Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS, Goebl M, Milligan S, O'Tousa JE, Hyde DR. Localization of Drosophila retinal degeneration B, a membrane- associated phosphatidylinositol transfer protein. J. Cell. Biol. 1993;122:1013–1022. doi: 10.1083/jcb.122.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinos J, Jalink K, Hardy RW, Britt SG, Zuker CS. A G protein-coupled receptor phosphatase required for rhodopsin function. Science. 1997;277:687–690. doi: 10.1126/science.277.5326.687. [DOI] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pfluegers Archiv. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C. Light activation, adaptation, and cell survival Functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–378. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.