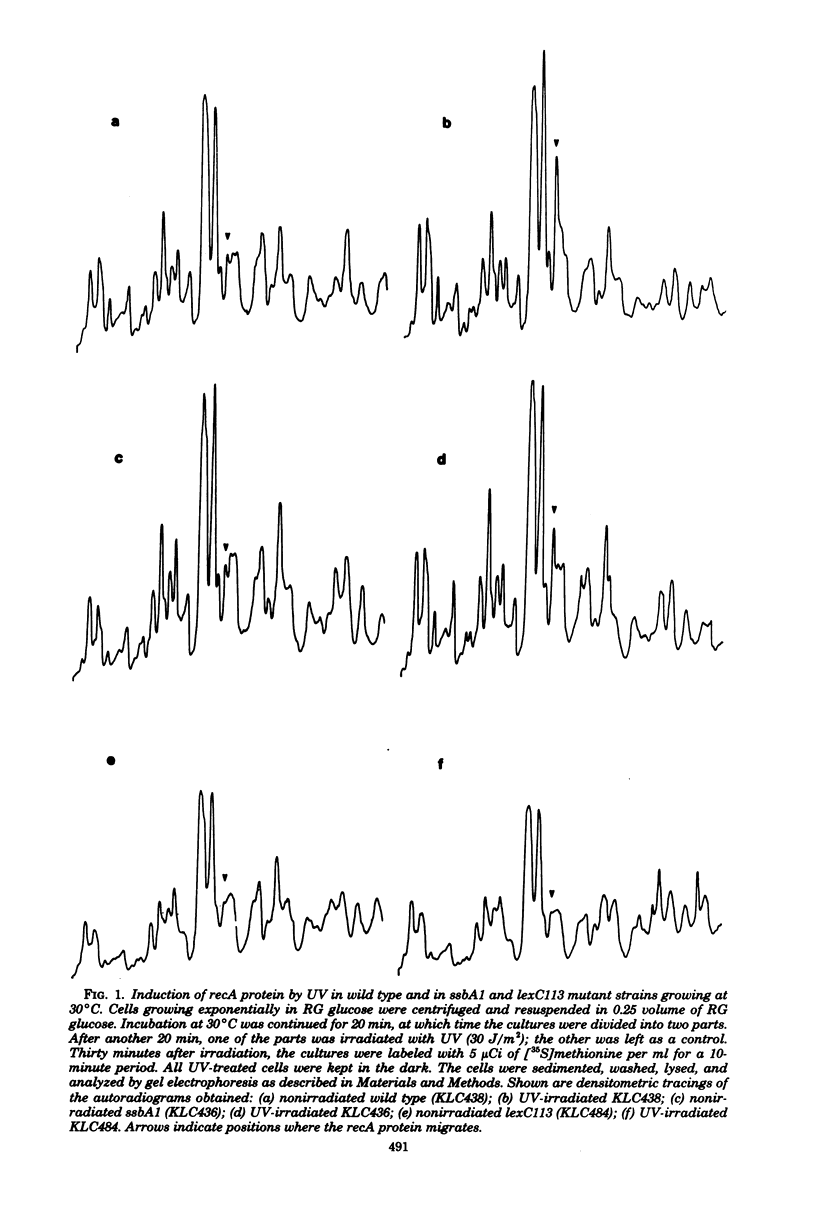
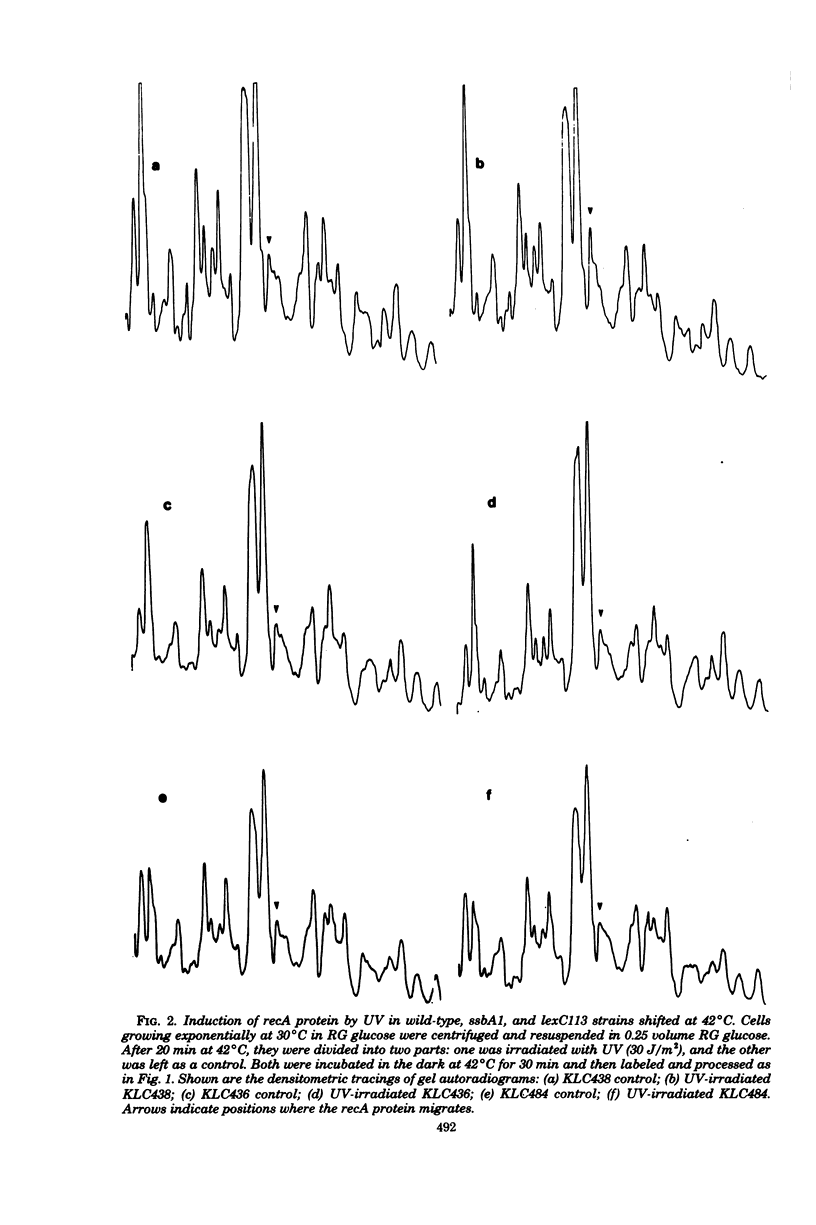
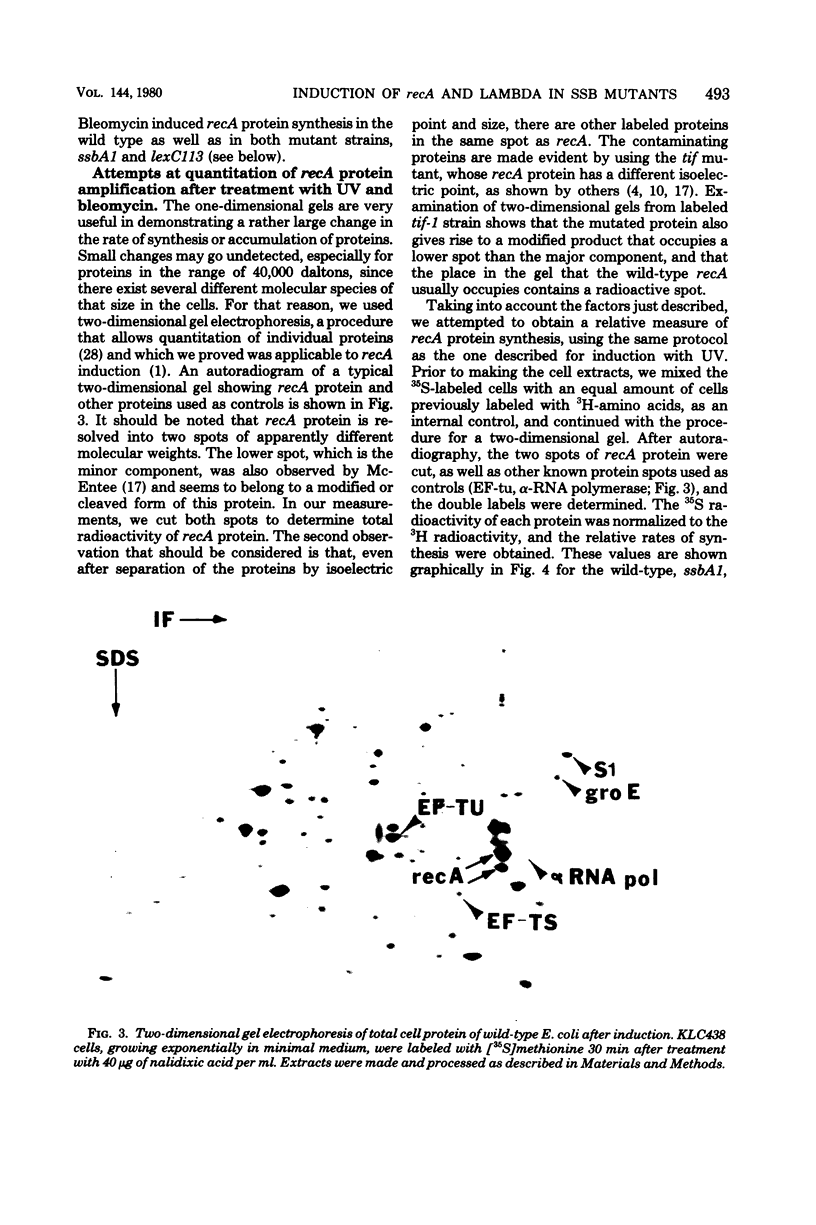
Abstract
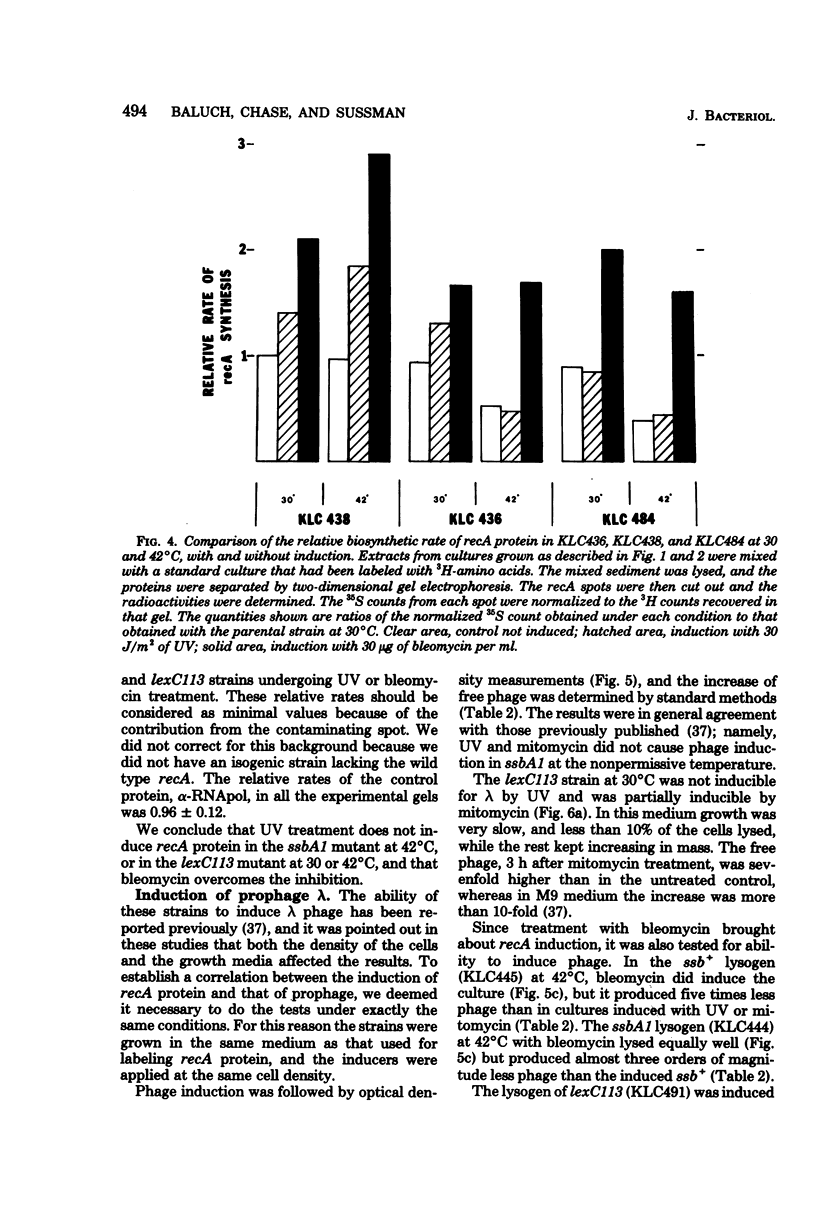
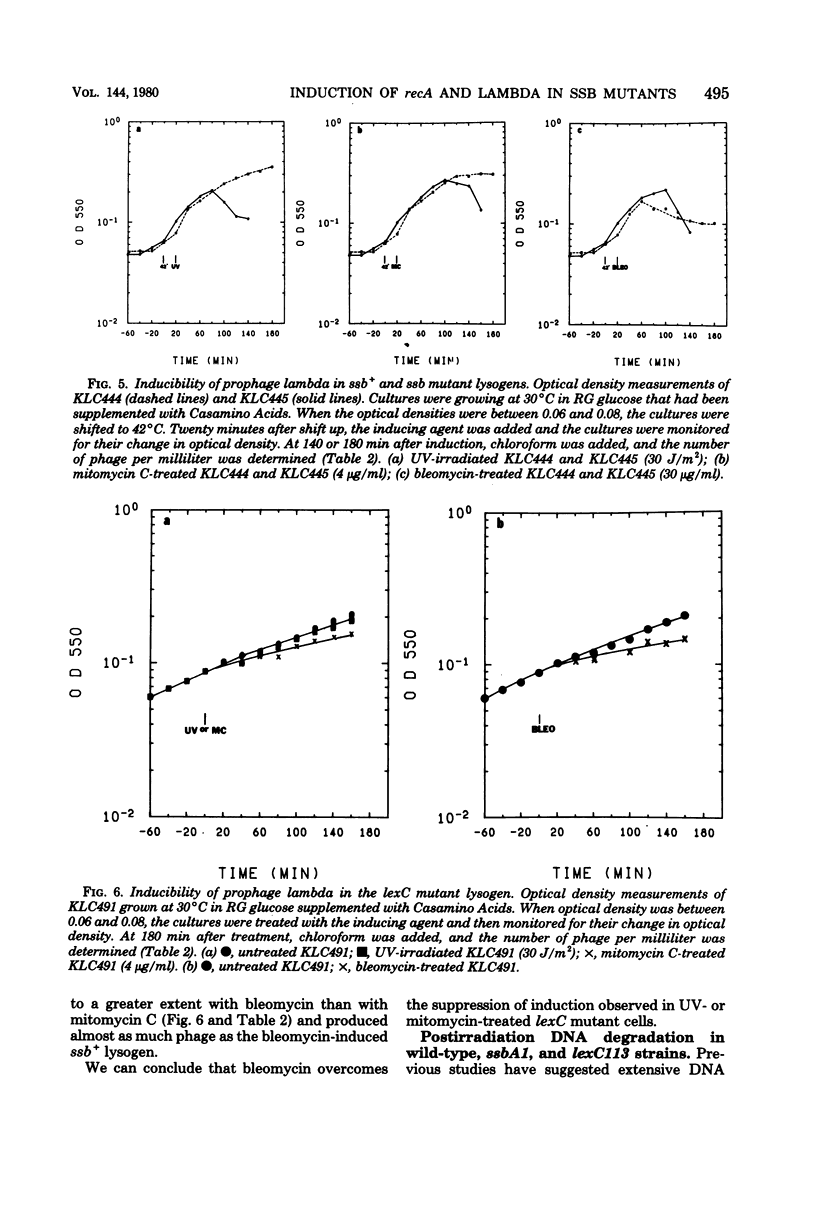
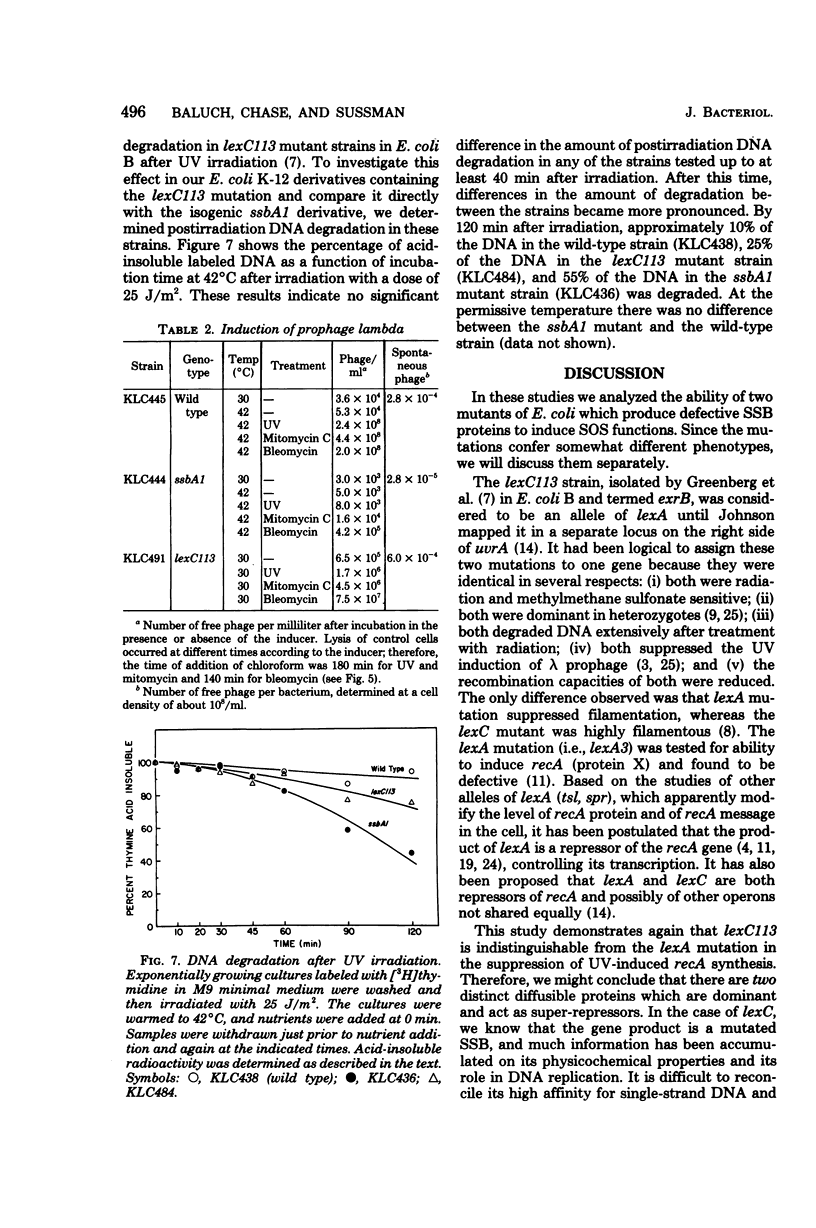
We investigated the capacity of Escherichia coli mutants defective in the single-strand deoxyribonucleic acid (DNA)-binding protein to amplify the synthesis of the recA protein, induce prophage lambda, and degrade their DNA after treatment with ultraviolet radiation, mitomycin C, or bleomycin. The thermosensitive ssbA1 strain induced recA protein and lambda phage normally at 30 degrees C, but no induction was observed at 42 degrees C when ultraviolet radiation or mitomycin C was used. The lexC113 mutant did not amplify recA protein synthesis or induce phage lambda at either 30 or 42 degrees C with those agents. Bleomycin was able to elicit induction of recA and phage lambda in both mutants at any temperature. After induction with ultraviolet radiation at the elevated temperature, no DNA degradation was observed for 40 min, but at later times there was increased degradation in the lexC113 strain, compared with the wild type, and even greater degradation in the ssbA1 mutant. We discuss the role of single-strand DNA-binding protein in induction and the possibility that the lexC product may exert its influence on recA and lambda induction at the level of the single-strand DNA gap.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluch J., Sussman R., Resnick J. Induction of prophage lambda without amplification of recA protein. Mol Gen Genet. 1980;178(2):317–323. doi: 10.1007/BF00270478. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Masker W. E. Deoxyribonucleic acid repair in Escherichia coli mutants deficient in the 5'----3' exonuclease activity of deoxyribonucleic acid polymerase I and exonuclease VII. J Bacteriol. 1977 May;130(2):667–675. doi: 10.1128/jb.130.2.667-675.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J., Green M. H. Repression of induction by U.V. of lambda phage by exrA mutations in Escherichia coli. Genet Res. 1970 Feb;15(1):87–97. doi: 10.1017/s0016672300001397. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- Glassberg J., Meyer R. R., Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979 Oct;140(1):14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J., Berends L. J., Donch J., Green M. H. exrB: a malB-linked gene in Escherichia coli B involved in sensitivity to radiation and filament formation. Genet Res. 1974 Apr;23(2):175–184. doi: 10.1017/s0016672300014798. [DOI] [PubMed] [Google Scholar]
- Greenberg J., Donch J., Berends L. The dominance of exrB over exrB+ in heterodiploids of Escherichia coli. Genet Res. 1975 Feb;25(1):39–44. doi: 10.1017/s001667230001541x. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Johnson B. F. Genetic mapping of the lexC-113 mutation. Mol Gen Genet. 1977 Nov 29;157(1):91–97. doi: 10.1007/BF00268691. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Hanawalt P. C. Induction of protein X in Escherichia coli. Mol Gen Genet. 1977 Feb 15;150(3):237–248. doi: 10.1007/BF00268122. [DOI] [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. recA protein-catalyzed strand assimilation: stimulation by Escherichia coli single-stranded DNA-binding protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):857–861. doi: 10.1073/pnas.77.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Glassberg J., Kornberg A. An Escherichia coli mutant defective in single-strand binding protein is defective in DNA replication. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1702–1705. doi: 10.1073/pnas.76.4.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Glassberg J., Scott J. V., Kornberg A. A temperature-sensitive single-stranded DNA-binding protein from Escherichia coli. J Biol Chem. 1980 Apr 10;255(7):2897–2901. [PubMed] [Google Scholar]
- Molineux I. J., Gefter M. L. Properties of the Escherichia coli DNA-binding (unwinding) protein interaction with nucleolytic enzymes and DNA. J Mol Biol. 1975 Nov 15;98(4):811–825. doi: 10.1016/s0022-2836(75)80012-x. [DOI] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Yamazaki Z., Breter H. J., Zahn R. K. Action of bleomycin on DNA and RNA. Eur J Biochem. 1972 Dec 18;31(3):518–525. doi: 10.1111/j.1432-1033.1972.tb02560.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Reeh S., Pedersen S., Friesen J. D. Biosynthetic regulation of individual proteins in relA+ and relA strains of Escherichia coli during amino acid starvation. Mol Gen Genet. 1976 Dec 22;149(3):279–289. doi: 10.1007/BF00268529. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Cloning of uvrA, lexC and ssb genes of Escherichia coli. Biochem Biophys Res Commun. 1979 Sep 12;90(1):123–129. doi: 10.1016/0006-291x(79)91598-5. [DOI] [PubMed] [Google Scholar]
- Schuster H., Beyersmann D., Mikolajczyk M., Schlicht M. Prophage induction by high temperature in thermosensitive dna mutants lysogenic for bacteriophage lambda. J Virol. 1973 Jun;11(6):879–885. doi: 10.1128/jvi.11.6.879-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevastopoulos C. G., Wehr C. T., Glaser D. A. Large-scale automated isolation of Escherichia coli mutants with thermosensitive DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3485–3489. doi: 10.1073/pnas.74.8.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Oishi M. Early events and mechanisms in the induction of bacterial SOS functions: analysis of the phage repressor inactivation process in vivo. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1657–1661. doi: 10.1073/pnas.75.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman R., Resnick J., Calame K., Baluch J. Interaction of bacteriophage lambda repressor with nonoperator DNA containing single-strand gaps. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5817–5821. doi: 10.1073/pnas.75.12.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales L. D., Chase J. W., Murphy J. B. Effect of ssbA1 and lexC113 mutations on lambda prophage induction, bacteriophage growth, and cell survival. J Bacteriol. 1980 Aug;143(2):887–896. doi: 10.1128/jb.143.2.887-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]