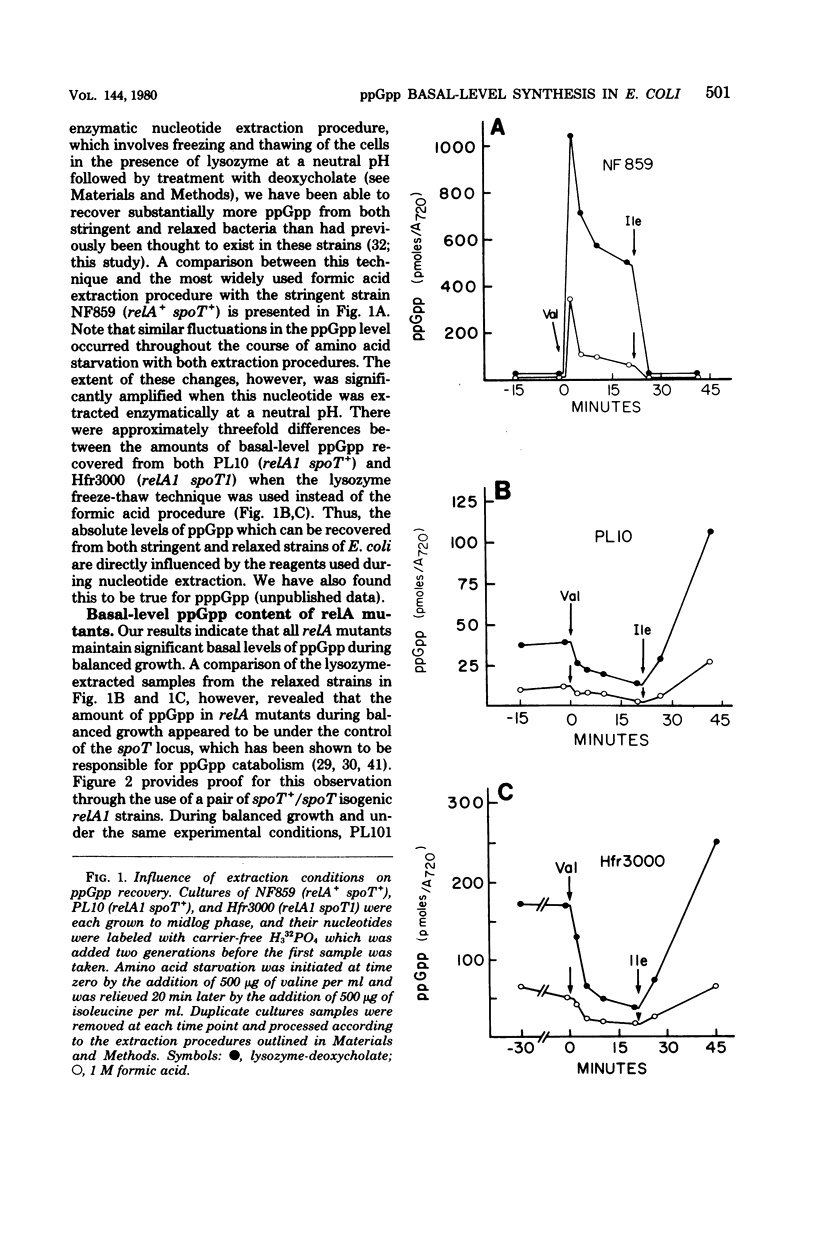
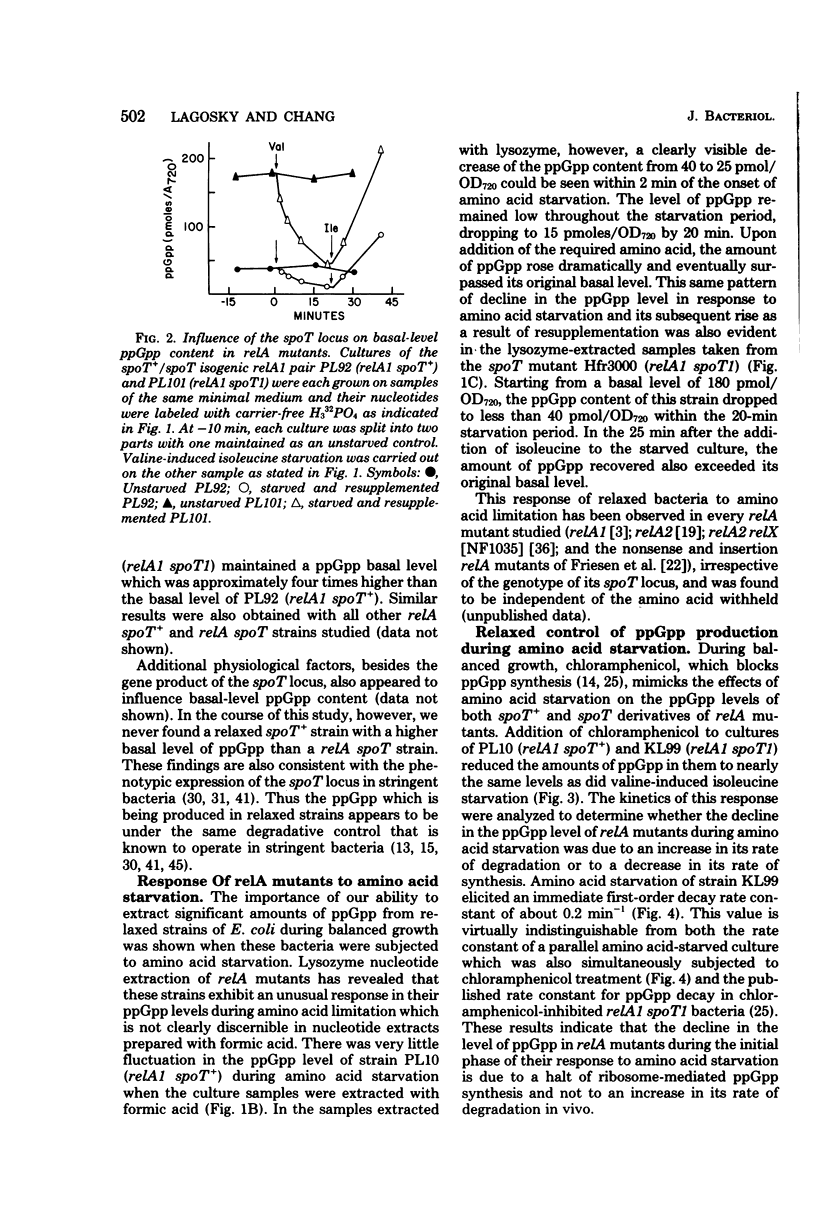
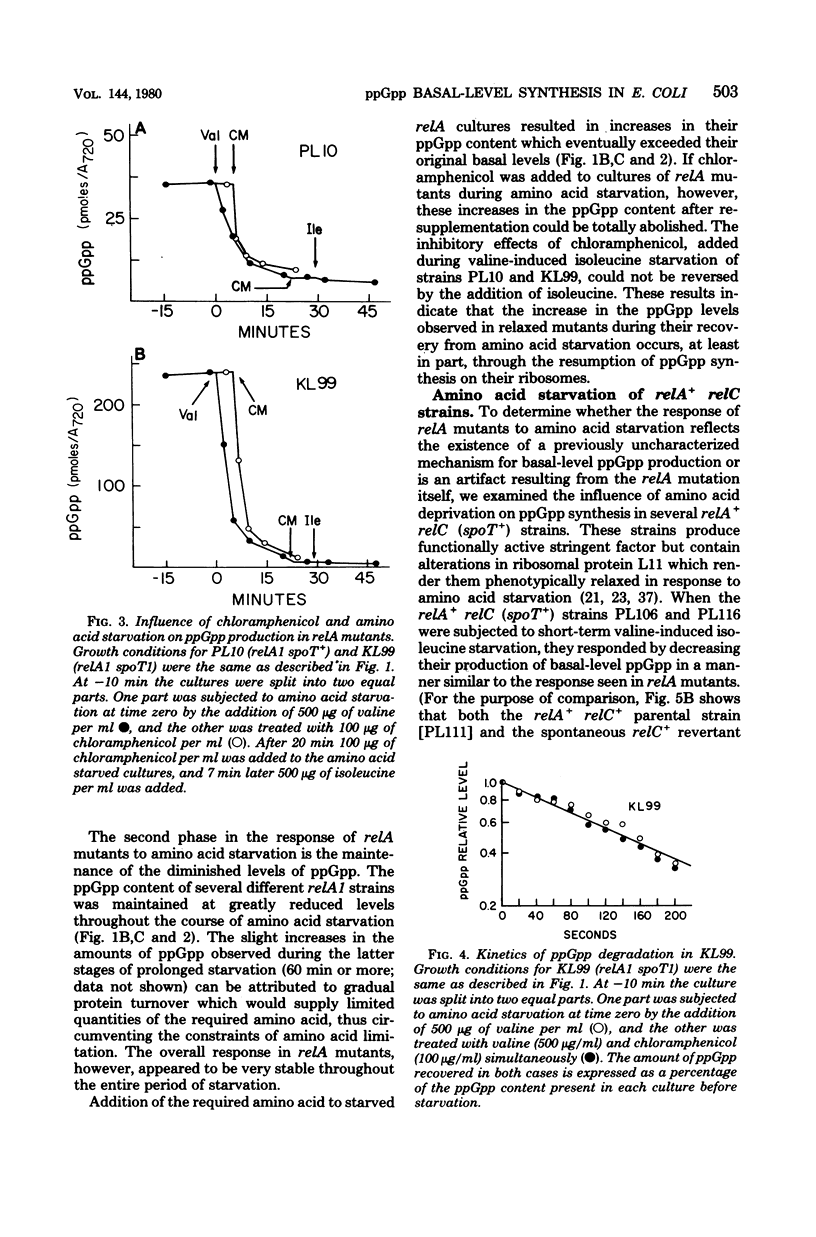
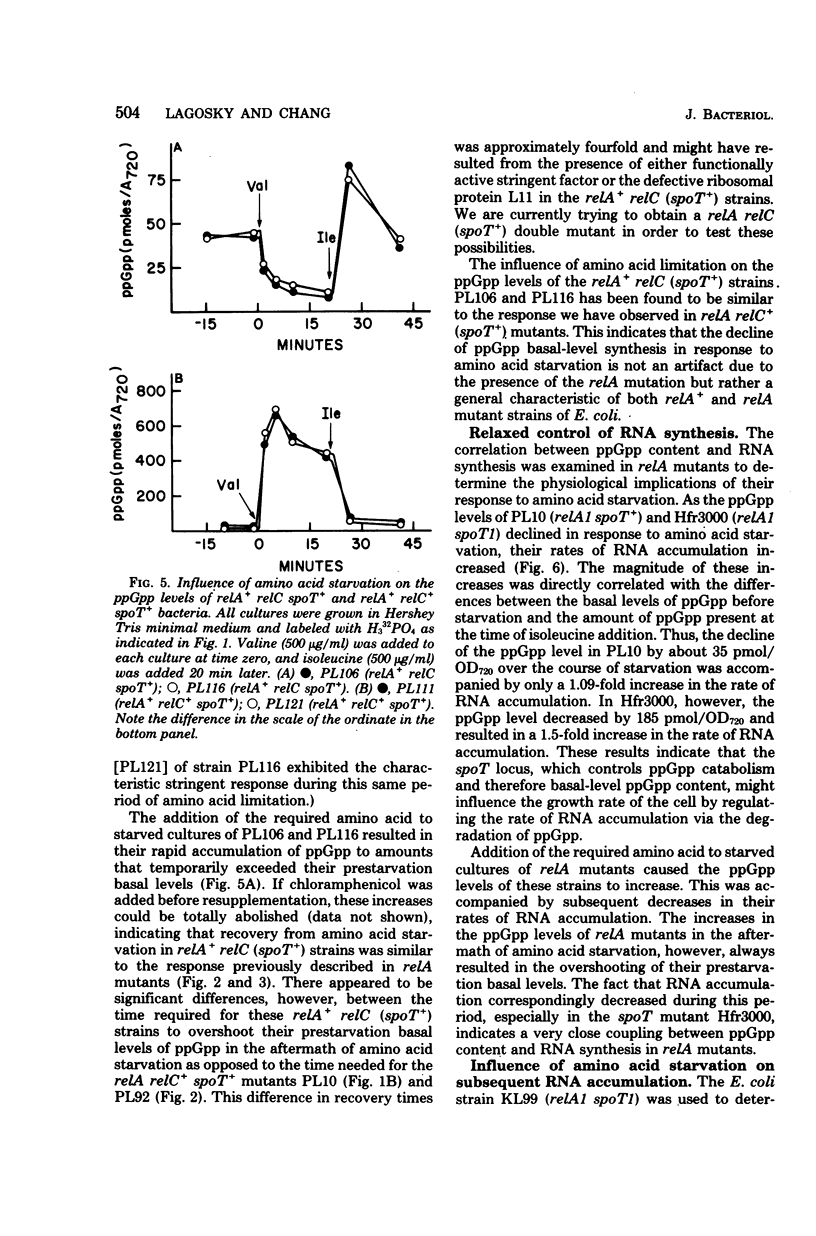
Abstract
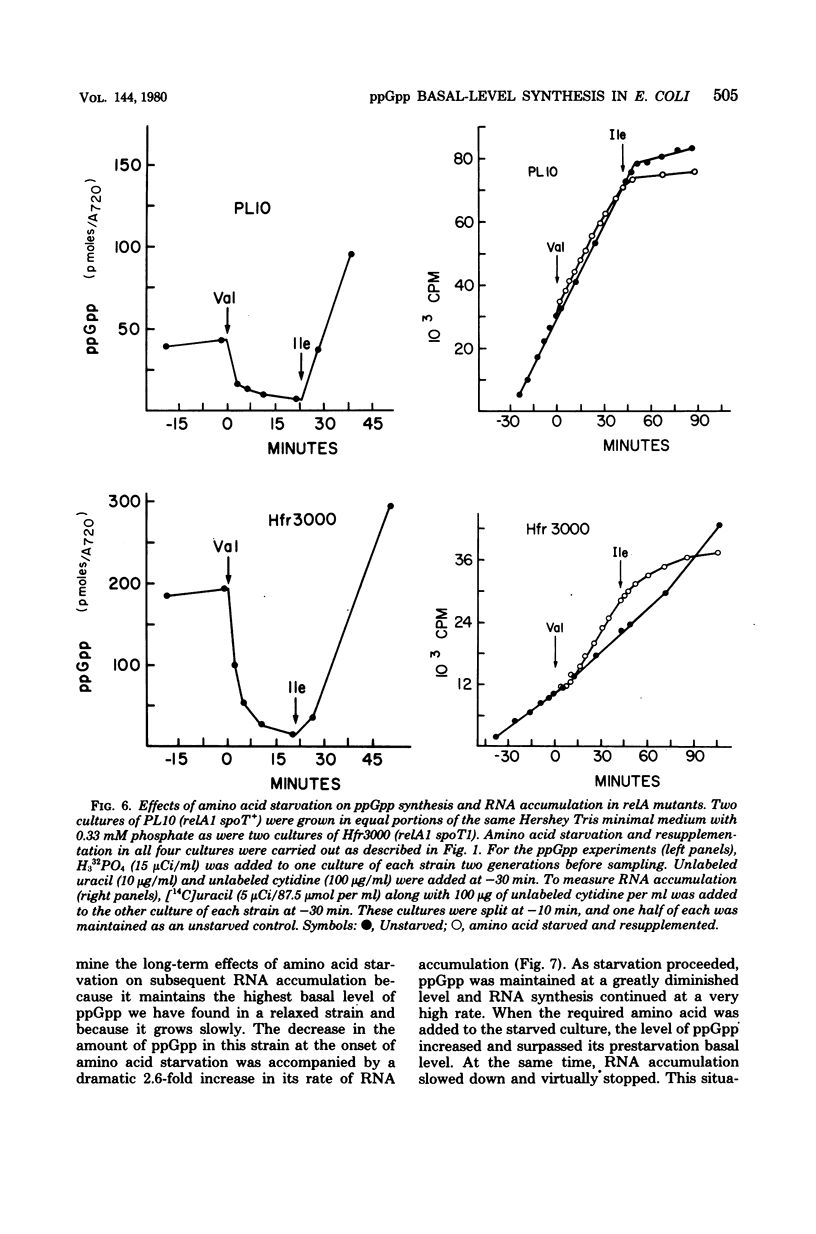
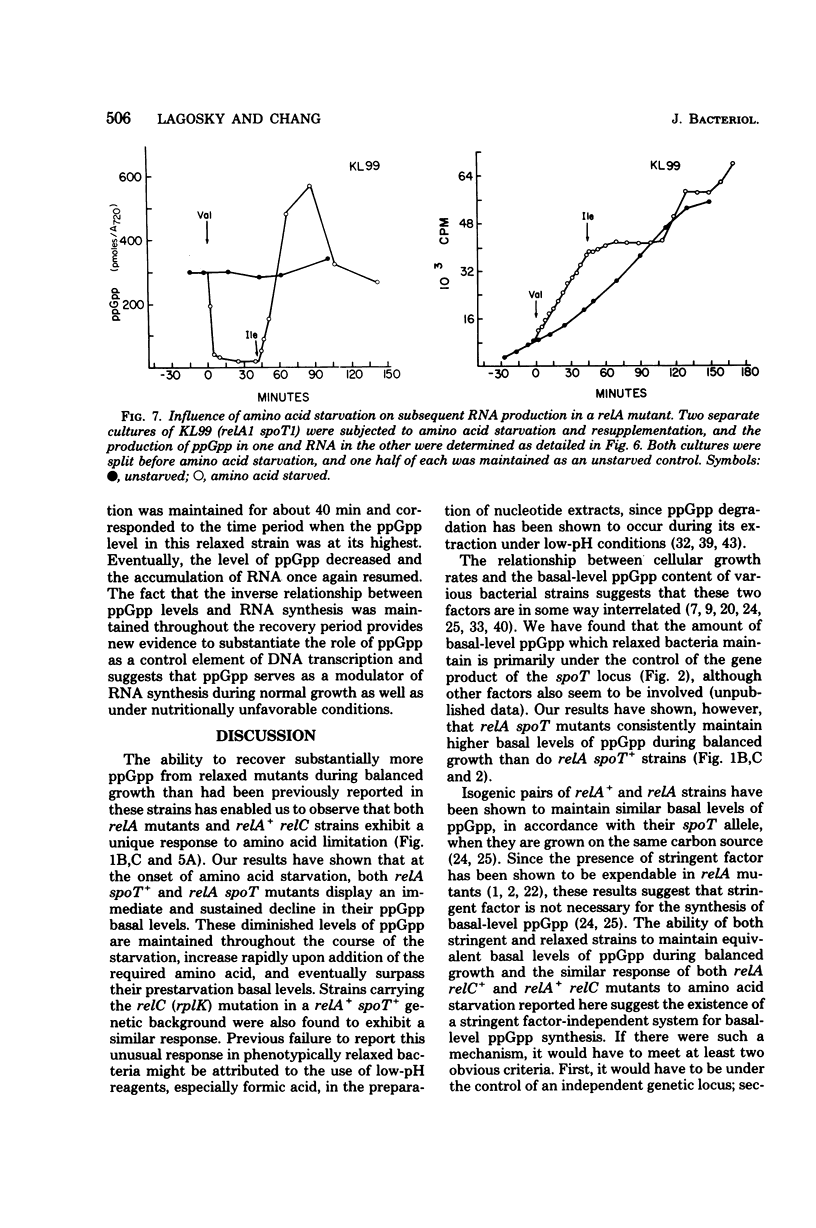
We observed that the synthesis of basal-level guanosine 5'-diphosphate 3'-diphosphate (ppGpp) in both relA mutants and relA+ relC strains of Escherichia coli decreased in response to amino acid limitation and that this was accompanied by an increase in ribonucleic acid (RNA) synthesis. Addition of the required amino acid to starved cultures of relaxed bacteria resulted in the resumption of ppGpp synthesis and a concomitant decrease in RNA production. Our results indicate that relA mutants retain a stringent factor-independent ribosomal mechanism for basal-level ppGpp synthesis. They also suggest that in relA+ bacteria, stringent factor-mediated ppGpp synthesis and the production of basal-level ppGpp are mutually exclusive. These findings substantiate the hypothesis that there are two functionally discrete mechanisms for ppGpp synthesis in E. coli. Through these studies we have also obtained new evidence which indicates that ppGpp serves as a modulator of RNA synthesis during balanced growth as well as under conditions of nutritional downshift and starvation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G. Escherichia coli mutant containing a large deletion from relA to argA. J Bacteriol. 1979 May;138(2):530–534. doi: 10.1128/jb.138.2.530-534.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOREK E., ROCKENBACH J., RYAN A. Studies on a mutant of Escherichia coli with unbalanced ribonucleic acid synthesis. J Bacteriol. 1956 Mar;71(3):318–323. doi: 10.1128/jb.71.3.318-323.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOREK E., RYAN A., ROCKENBACH J. Nucleic acid metabolism in relation to the lysogenic phenomenon. J Bacteriol. 1955 Apr;69(4):460–467. doi: 10.1128/jb.69.4.460-467.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block R., Haseltine W. A. Thermolability of the stringent factor in rel mutants of Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):625–629. doi: 10.1016/0022-2836(73)90228-3. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Chaloner-Larsson G., Yamazaki H. Effects of the spoT and relA mutation on the synthesis and accumulation of ppGpp and RNA during glucose starvation. Can J Biochem. 1978 Apr;56(4):264–272. doi: 10.1139/o78-041. [DOI] [PubMed] [Google Scholar]
- Chaloner-Larsson G., Yamazaki H. Relationship between ppGpp levels and rates of protein and RNA synthesis in Escherichia coli. Can J Biochem. 1976 Mar;54(3):291–295. doi: 10.1139/o76-043. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Chang C. N., Paik W. K. Methylation of ribosomal proteins in Escherichia coli. J Bacteriol. 1974 Nov;120(2):651–656. doi: 10.1128/jb.120.2.651-656.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran J. W., Byrne R. W. Isolation and properties of a ribosome-bound factor required for ppGpp and ppGpp synthesis in Escherichia coli. J Biol Chem. 1974 Jan 25;249(2):353–360. [PubMed] [Google Scholar]
- De Boer H. A., Bakker A. J., Weyer W. J., Gruber M. The role of energy-generating processes in the degradation of guanosine tetrophosphate, ppGpp, in Escherichia coli. Biochim Biophys Acta. 1976 May 19;432(3):361–368. doi: 10.1016/0005-2787(76)90146-5. [DOI] [PubMed] [Google Scholar]
- Diderichsen B., Fiil N. P., Lavallé R. Genetics of the relB locus in Escherichia coli. J Bacteriol. 1977 Jul;131(1):30–33. doi: 10.1128/jb.131.1.30-33.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N. P., Willumsen B. M., Friesen J. D., von Meyenburg K. Interaction of alleles of the relA, relC and spoT genes in Escherichia coli: analysis of the interconversion of GTP, ppGpp and pppGpp. Mol Gen Genet. 1977 Jan 7;150(1):87–101. doi: 10.1007/BF02425329. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., von Meyenburg K., Friesen J. D. Accumulation and turnover of guanosine tetraphosphate in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):769–783. doi: 10.1016/s0022-2836(72)80037-8. [DOI] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill N. A functional analysis of the rel gene in Escherichia coli. J Mol Biol. 1969 Oct 28;45(2):195–203. doi: 10.1016/0022-2836(69)90099-0. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., An G., Fiil N. P. Nonsense and insertion mutants in the relA gene of E. coli: cloning relA. Cell. 1978 Dec;15(4):1187–1197. doi: 10.1016/0092-8674(78)90045-4. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., von Meyenburg K. Synthesis and turnover of basal level guanosine tetraphosphate in Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):304–309. [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Gallant J., Shell L., Bittner R. A novel nucleotide implicated in the response of E. coli to energy source downshift. Cell. 1976 Jan;7(1):75–84. doi: 10.1016/0092-8674(76)90257-9. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. R., Yamazaki H. Correlation between guanosine tetraphosphate accumulation and degree of amino acid control of ribonucleic acid accumulation during nutritionally slowed growth in Escherichia coli. Biochemistry. 1974 Jun 18;13(13):2785–2788. doi: 10.1021/bi00710a019. [DOI] [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. The extraction of guanosine 5'-diphosphate, 3'-diphosphate (ppGpp) from Escherichia coli using low pH reagents: a reevaluation. Biochem Biophys Res Commun. 1978 Oct 30;84(4):1016–1024. doi: 10.1016/0006-291x(78)91685-6. [DOI] [PubMed] [Google Scholar]
- Lavallé R. Nouveaux mutants de régulation de la synthèse de l'ARN. Bull Soc Chim Biol (Paris) 1965;47(8):1567–1570. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Molin S., Von Meyenburg K., Maaloe O., Hansen M. T., Pato M. L. Control of ribosome synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):7–17. doi: 10.1128/jb.131.1.7-17.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A gene involved in the metabolic control of ppGpp synthesis. Mol Gen Genet. 1978 Jan 17;158(3):271–277. doi: 10.1007/BF00267198. [DOI] [PubMed] [Google Scholar]
- Parker J., Watson R. J., Friesen J. D. A relaxed mutant with an altered ribosomal protein L11. Mol Gen Genet. 1976 Feb 27;144(1):111–114. doi: 10.1007/BF00277313. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Sokawa J., Kaziro Y. Regulation of stable RNA synthesis and ppGpp levels in growing cells of Escherichia coli. Cell. 1975 May;5(1):69–74. doi: 10.1016/0092-8674(75)90093-8. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Tetu C., Dassa E., Boquet P. L. Unusual pattern of nucleoside polyphosphate hydrolysis by the acid phosphatase (optimum pH = 2.5) of Escherichia coli. Biochem Biophys Res Commun. 1979 Mar 15;87(1):314–322. doi: 10.1016/0006-291x(79)91681-4. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Parker J., Fiil N. P., Flaks J. G., Friesen J. D. New chromosomal location for structural genes of ribosomal proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2765–2769. doi: 10.1073/pnas.72.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer W. J., de Boer H. A., de Boer J. G., Gruber M. The sequence of ppGpp and pppGpp in the reaction scheme for magic spot synthesis. Biochim Biophys Acta. 1976 Aug 2;442(1):123–127. doi: 10.1016/0005-2787(76)90183-0. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Raué H. A., Ab G., Gruber M. Role of the ribosome in stringent control of bacterial RNA synthesis. Biochim Biophys Acta. 1971 Aug 12;246(1):157–160. doi: 10.1016/0005-2787(71)90081-5. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Weyer W. J., de Boer J. G., van der Heide S., Gruber M. Synthesis of guanosine 5'-diphosphate, 3'-diphosphate in spot mutants of Escherichia coli. Biochim Biophys Acta. 1977 Jan 20;474(2):165–172. doi: 10.1016/0005-2787(77)90190-3. [DOI] [PubMed] [Google Scholar]



