Abstract
Amphotericin B (AMB), a potent antifungal agent, has been employed as an inhalable therapy for pulmonary fungal infections. We recently described a novel nano-sized delivery vehicle composed of phospholipid (PL) and apolipoprotein A-I, NanoDisk (ND), to which we added AMB as a payload (ND-AMB). The goal of the present study was to evaluate whether ND-AMB, compared to other formulations, preserves lung cell integrity in vitro, as AMB can be toxic to mammalian cells and reduce lung function when inhaled. Epithelial integrity was assessed by measuring K+ ion flux across a model airway epithelium, Calu-3 cells. In this assay ND-AMB was at least 8-fold less disruptive than AMB/deoxycholate (DOC). Cell viability studies confirmed this observation. Unexpectedly, the ND vehicle restored the integrity of a membrane compromised by prior exposure to AMB. An alternative formulation of ND-AMB containing a high load of AMB per ND was not protective, suggesting that ND with a low ratio of AMB to PL can sequester additional AMB from membranes. ND-AMB also protected HepG2 cells from the cytotoxicity of AMB, as determined by cellular viability and lactate dehydrogenase (LDH) levels. This study suggests that ND-AMB may be safe for administration via inhalation and reveals a unique activity whereby ND-AMB protects lung epithelial membranes from AMB toxicity.
Keywords: nanodisk, amphotericin B, Calu-3 cells, HepG2 cells, high density lipoprotein, lung epithelium, trans-epithelial K+ current, membrane protection, apolipoprotein A-I
INTRODUCTION
We have recently described NanoDisk (ND), a novel nano-sized drug delivery vehicle intended for incorporation of therapeutics with poor aqueous solubility (Oda et al., 2006). ND is a complex of apolipoprotein A-I (apoA-I) and phospholipids (1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine (DMPC) and 1,2-Dimyristoyl-sn-Glycero-3-[Phospho-rac-(1-glycerol)] (DMPG)). Structurally, ND is similar to nascent high-density lipoprotein, wherein the bilayer of phospholipid (PL) is surrounded by two molecules of apoA-I that adopt an anti-parallel extended alpha-helical “double belt” conformation around the circumference of a lipid bilayer (Martin et al., 2006). The assembled vehicle forms discoidal particles approximately 12 nm in diameter. In previous studies we demonstrated that the ND incorporates the hydrophobic antifungal drug, amphotericin B (AMB), into the complex thus forming ND-AMB (Nguyen et al., 2008; Oda et al., 2006).
Amphotericin B (AMB), is an ideal candidate for delivery via ND, as it is poorly soluble in aqueous solution but is readily incorporated into the lipid moiety of ND. The antifungal activity of AMB is related to its high affinity for ergosterol, the primary sterol in fungal membranes. AMB is known to bind sterol-containing membranes and form aqueous pores that disrupt cytosolic ion homeostasis (Bolard, 1986; Butler and Cotlove, 1971; Holz and Finkelstein, 1970; Zygmunt and Tavormina, 1966). Although AMB has a weak affinity for cholesterol-containing membranes, it can form pores in mammalian cell membranes. AMB is toxic to a number of human cells; thus, novel therapeutic formulations of AMB have focused on strategies to reduce the interaction between host cells and drug, while retaining antifungal activity (Deuticke, 1973; Guo et al., 1991; Janoff et al., 1988; Proffitt et al., 1991; Yano et al., 2009).
Currently four formulations of AMB are approved for intravenous use in man. To improve the delivery of AMB to pulmonary fungal infections, the drug has also been administered as an inhalable aerosol (Kuiper and Ruijgrok, 2009). All formulations consist of the active drug in complex with a solubilizing agent to overcome the hydrophobicity of the molecule and present the drug in a more biocompatible form. The original formulation of AMB contains the detergent deoxycholate (DOC) (AMB/DOC); however, more recently lipid-based formulations have been developed. A liposomal formulation, AmBisome (L-AMB), incorporates AMB into the bilayer of unilamellar 55–99 nm diameter liposomes. L-AMB is reported to have an LD-50 greater than 175 mg/kg in mice in contrast to 2.3 mg/kg for AMB/DOC (Proffitt et al., 1991). However, the bioactivity of L-AMB, is significantly lower than AMB/DOC (Brajtburg and Bolard, 1996; Oda et al., 2006). Abelcet (ABLC) is an AMB-lipid complex that forms a suspension of ribbon-like structures, 1.6–11 µm in diameter, composed of PL and AMB (Janoff et al., 1988). Lastly, Amphotec, AMB Colloidal Dispersion (ABCD) is a soluble wafer-like complex between cholesteryl sulfate and the drug (Guo et al., 1991). Despite the fact that lipid-based formulations are significantly less nephrotoxic and have a lower incidence of infusion-related complications, AMB/DOC remains widely prescribed due in large part to its substantially lower cost.
Immunocompromised individuals are highly susceptible to invasive pulmonary fungal infections. This population includes patients receiving immunosuppressant treatment for malignancies or organ transplants and individuals with HIV (Cornet et al., 2002; Holding et al., 2000). Although recent improvements in prophylaxis have reduced the incidence of invasive fungal infections in neutropenic individuals, mortality due to fungal infection remains high (Menzin et al., 2009; Morgan et al., 2005; Rijnders et al., 2008). To improve survival rates for pulmonary infections, nebulized AMB preparations have been administered directly to the site of infection by inhalation, yielding some success (Behre et al., 1995; Hertenstein et al., 1994; Kuiper and Ruijgrok, 2009; Mohammad and Klein, 2006). Inhaled, i.e., aerosolized, AMB/DOC does not readily enter the circulation from the lung, thus avoiding nephrotoxicity; however, irritation and decreased lung function can occur following inhalation (Gryn et al., 1993). Fewer side effects are observed with inhaled L-AMB and ABLC than AMB/DOC (Palmer et al., 2001; Rijnders et al., 2008). The safety of aerosolized ND-AMB therefore remains an important consideration.
The aim of this study was to evaluate the safety of ND-AMB for pulmonary delivery using lung epithelial cells in vitro. Specifically, the effect of ND-AMB on the integrity of the apical cell membrane of lung epithelial cells was determined by measuring changes in K+ permeability after exposure to ND-AMB and other commercially available formulations of AMB. The data show that ND-AMB induced a negligible increase in K+ current flow and unexpectedly restored the electrical resistance of lung-derived epithelia previously treated with AMB/DOC. Efficient protection depended on the ratio of AMB to PL in the formulation, suggesting that ND shielded cells from AMB toxicity by sequestering the drug from cellular membranes.
MATERIALS AND METHODS
Materials
AMB/DOC (Sigma-Aldrich, St. Louis, MO), L-AMB (Gilead, Foster City, CA), ABLC (Enzon, Bridgewater, NJ) and ABCD (Three Rivers Pharmaceuticals, Warrendale, PA) were prepared according to manufacturer’s instructions. Recombinant apoA-I was purified from Escherichia coli as described previously (Oda et al., 2006). To prepare NanoDisk, 10 mg of dehydrated DMPC:DMPG 7:3 (w/w) in glass tubes was re-suspended in phosphate buffered saline (PBS) (20 mM NaPO4, 150 mM NaCl, pH 7.4) prior to the addition of 2.5–10 mg AMB (Sigma-Aldrich, St. Louis, MO) and 4 mg apoA-I in PBS. The resulting cloudy mixture was cleared using a water bath sonicator where position of samples was kept constant. Preparations were sonicated until completely clear (1–2 hr) and then dialyzed against PBS for at least 16 hr at 4 °C. Samples were filter-sterilized (0.22 µm) before use. Amphotericin B concentration was determined by absorbance in dimethylsulfoxide at 416 nm using an extinction co-efficient of 121,400 M−1cm−1. Protein content was determined by Bicinchoninic acid method (Thermo Scientific, Waltham, MA) or Bradford method (Sigma-Aldrich, St. Louis, MO).
Cells
Calu-3 cells, a human airway epithelial cell line of adenocarcinoma origin (ATCC, Manassas, VA), were maintained in DMEM culture medium containing 4.5 g/L glucose and supplemented with MEM non-essential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin/streptomycin, 100 µg/mL GlutaMAX equivalent to 2 mM L-glutamine (all from Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (Hyclone, Logan, UT). HepG2 cells (ATCC, Manassas, VA) were maintained in DMEM containing 4.5 g/L glucose and supplemented with 100 U/mL penicillin/streptomycin, GlutaMAX equivalent to 2 mM L-glutamine (all from Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (Hyclone, Logan, UT).
Ussing chamber assay
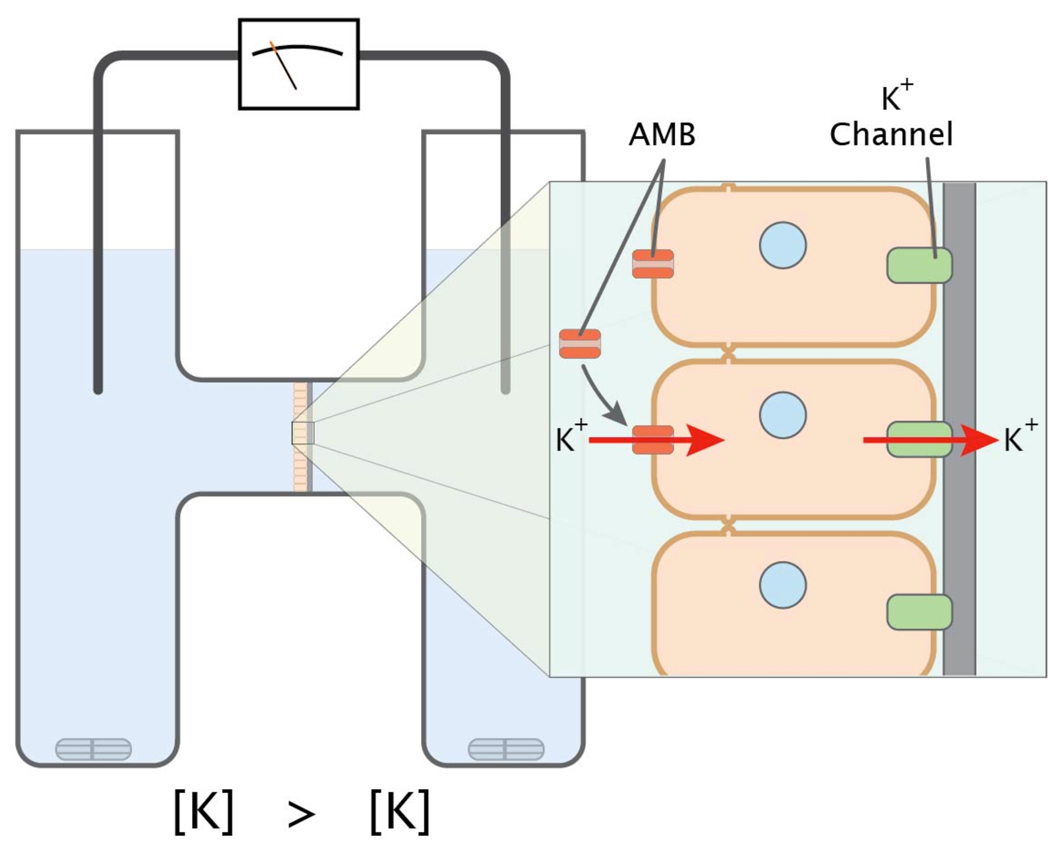
Calu-3 cells (~1×105) were seeded in clear polyester Snapwell inserts with a diameter of 12 mm (Costar, Corning, NY) and were grown to confluence. Trans-epithelial current measurements were performed with Easy Mount Ussing chambers (Physiologic Instruments, San Diego, CA) with an apical-to-basolateral directed gradient for K+ (Cowley and Linsdell, 2002; Illek et al., 1990, 1993; Illek et al., 1992) (Fig 1). High K+ buffer in the apical reservoir was (in mM): 120 KCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgSO4. The basolateral reservoir buffer was (in mM): 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgSO4. All experiments were conducted at 37 °C and solutions were continuously gassed with 95% air, 5% CO2 resulting in pH 7.4.
Figure 1.
The Ussing chamber apparatus. The apparatus was used to quantify the permeability of epithelial monolayers by measuring the flow of ions from one chamber to the other. In this experiment Calu-3 cell monolayers are positioned so that the apical face is in contact with a buffer containing a high concentration of K+ while the basolateral reservoir has low potassium. When a permeabilizing agent (AMB/DOC) is added to the apical reservoir, K+ ions are driven by a concentration gradient into the cell through the pores formed by AMB and exit the cell via K+ channels on the basolateral cell membrane. The resulting trans-epithelial K+ current is dependent on the amphotericin-induced K+ permeability of the apical membrane.
Trans-epithelial voltage was clamped to 0 mV using a standard four electrode voltage clamp (Physiologic Instruments, San Diego, CA) and gradient-driven K+ current was recorded at 5 Hz by an analog-to-digital board (DATAQ Instruments, Inc. Akron, OH) connected to a personal computer. Positive currents were defined as cation movement from apical to basal reservoir. For experiments involving pre-permeabilized Calu-3 cells, AMB/DOC was introduced to the apical reservoir of the Ussing chamber and trans-epithelial K+ was allowed to reach equilibrium (10–13 min). Once a stable K+ current was achieved, subsequent treatments were added to the apical reservoir.
Cytotoxicity assays
HepG2 or Calu-3 cells were seeded (~1×105 cells) in 24 well plates and allowed to adhere 24 hr (HepG2) or 48 hr (Calu-3). Adherent cells were washed once with Dulbecco’s PBS (Invitrogen, Carlsbad, CA) prior to incubation with treatments. Incubations were performed in growth media for the respective cell lines with fetal bovine serum content reduced to 2%. For the MTT assay, cells were incubated with 500 µg/mL 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Calbiochem, San Diego, CA) for 25 min at 37 °C before the colored formazane product was extracted with 50% isopropanol and 0.1 N HCl. Formazane was quantified by absorbance at 570 nm, subtracting background absorbance at 690 nm. To quantify the release of lactate dehydrogenase (LDH), cell culture medium was collected and centrifuged for 5 min at 2,000 RCF. Then 20 µL of medium was mixed with 100 µL LDH assay reagent (BioVision, Mountain View, CA), incubated at 37 °C for 15 min and absorbance was read at 595 nm. Assay results were confirmed by microscopic observation.
Statistical Methods
Statistical comparisons were performed using a one-way ANOVA and Tukey post test by GraphPad Prism version 5, (GraphPad Software, San Diego, CA). Statistical significance was defined as p<0.05 and reported error represents standard deviation.
RESULTS
Effect of ND-AMB and other AMB formulations on trans-epithelial K+ current
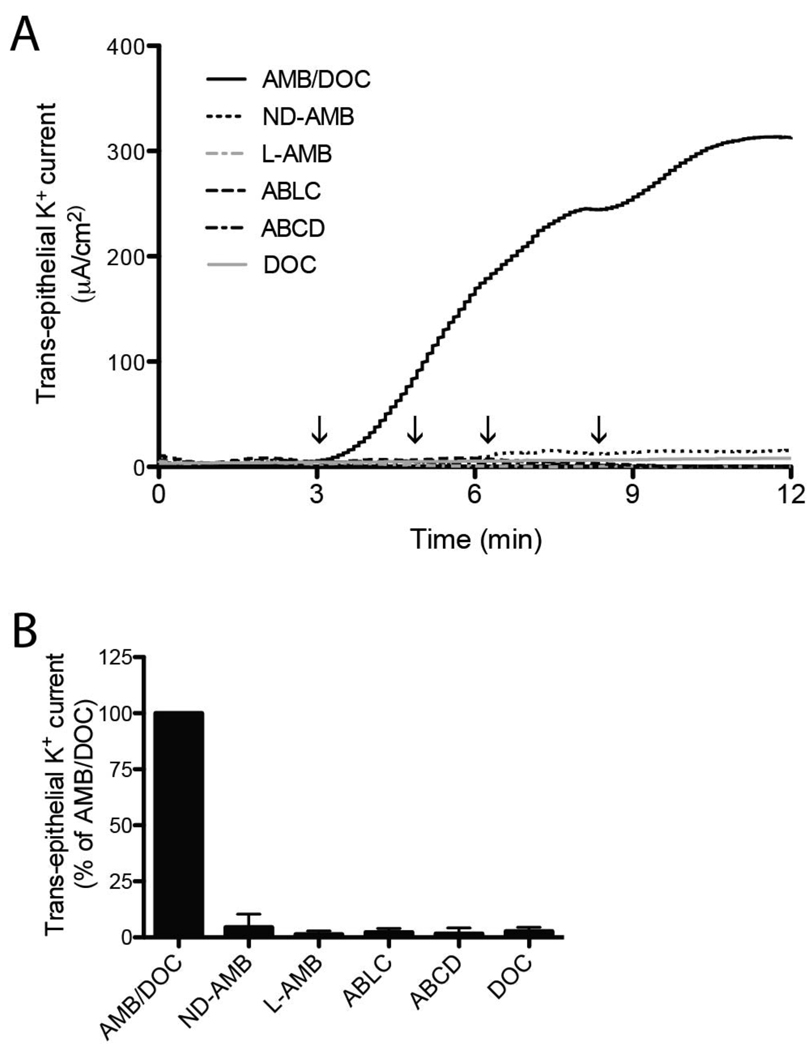
To evaluate the effect of ND-AMB on the integrity of the airway epithelium, induction of K+ current across the apical membrane of Calu-3 monolayers was measured in real-time by the Ussing chamber method. The composition of AMB formulations examined in the Ussing chamber assay is described in Table 1. The apical cell membrane of Calu-3 monolayers was exposed to ND-AMB, L-AMB, ABLC, ABCD, AMB/DOC or DOC at increasing concentrations (5–40 µg/mL AMB) over a 12 min period (Fig 2A). Extending the duration of the experiment beyond 12 min (up to 45 min) did not yield a further increase in observed current. K+ current increased rapidly within 30 sec of adding AMB/DOC at 5 µg/mL, the lowest concentration tested, indicating that the apical membrane of the monolayer had become permeable to K+ ions. Trans-epithelial K+ current continued to increase dramatically as the concentration of AMB/DOC was raised. In contrast to AMB/DOC, negligible induction of K+ current was obtained following treatment with lipid-based formulations, including ND-AMB, L-AMB, ABLC and ABCD, at a concentration of 40 µg/mL AMB. DOC alone did not elicit a change in K+ current. A comparison between the peak current elicited by each treatment highlights an acute difference between AMB/DOC and lipid-based AMB (Fig 2B).
Table I.
Amphotericin B Containing Formulations
| Formulation | Solubilizing Agent | Structure | Reference |
|---|---|---|---|
| ND-AMB | DMPC, DMPG, apoA-I | Discoidal phospholipid bilayer stabilized by apoA-I | (Oda et al., 2006) |
| AMB/DOC | DOC | Drug/detergent micelles | |
| AmBisome | Phosphatidylcholine, cholesterol distearoylphosphatidylglycerol, alpha-tocopherol | Unilamellar Liposome | (Proffitt et al., 1991) |
| Abelcet | DMPC, DMPG | Suspension of ribbon-like complexes | (Janoff et al., 1989) |
| Amphotec | Cholesteryl sulfate | Colloidal dispersion of discshaped particles | (Guo et al., 1991) |
Figure 2.
Lipid-based AMB formulations induce minimal permeability in Calu-3 monolayers compared to AMB/DOC. (A) Calu-3 monolayers were exposed to an increasing concentration AMB (5, 10, 20 and 40 µg/mL) achieved by sequential additions of either AMB/DOC, ND-AMB, L-AMB, ABLC, ABCD or an equivalent concentration of DOC. Arrows on the time axis denote an incremental increase of AMB concentration. Permeabilization of the apical cell membrane is detected as an increase in trans-epithelial K+ current. A representative current trace is shown. (B) To facilitate comparison, results were summarized relative to trans-epithelial current elicited by AMB/DOC treatment at 12 min. Data represent two independent observations.
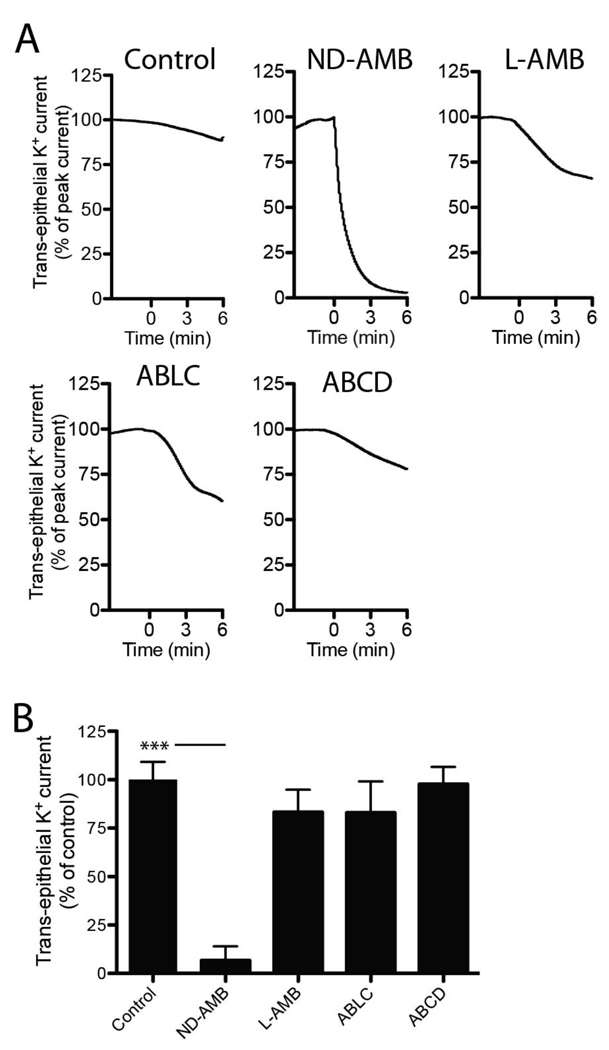
The marked absence of apical membrane permeabilization associated with lipid-based formulations of AMB could have several interpretations. The lack of K+ current induction may suggest that pore-forming interaction between AMB and Calu-3 cells is prevented when AMB is lipid-bound. Alternatively, pores formed by AMB could be blocked or disrupted by a component of the lipid-based formulations, thus restoring a previously uncompromised membrane. To test the proposed mechanisms, we established whether lipid-based formulations could restore electrical integrity of permeabilized cells. Calu-3 monolayers were first permeabilized with the addition of 5 µg/mL AMB/DOC and then treated with lipid-based AMB-formulations to test for an inhibition of current flow (Fig 3A). All treatments were normalized to an equivalent mass of AMB. Notably, ND-AMB induced a robust and rapid reduction (93.7 ± 7.3%, n=3 p<0.0001) in K+ current compared to control cells whereas L-AMB, ABLC and ABCD did not elicit a significant inhibitory response (Fig 3B). These results demonstrate that the ND-AMB formulation possesses a unique capacity to reduce cellular K+ permeability caused by AMB.
Figure 3.
ND-AMB restores the electrical resistance of Calu-3 monolayers permeabilized by AMB/DOC. (A) Monolayers were permeabilized by AMB/DOC (5 µg/mL) then exposed to an additional treatment of ND-AMB, L-AMB, ABLC or ABCD (all 0.75 µg/mL AMB) at time = 0 min or no treatment as control. Representative current traces are shown and have been normalized to peak current to account for variations in absolute values. (B) Changes in trans-epithelial current relative to control are summarized at 3 min post-treatment. Data represent three independent observations. *** p<0.0001.
Mechanism whereby ND-AMB reduces cell membrane permeability
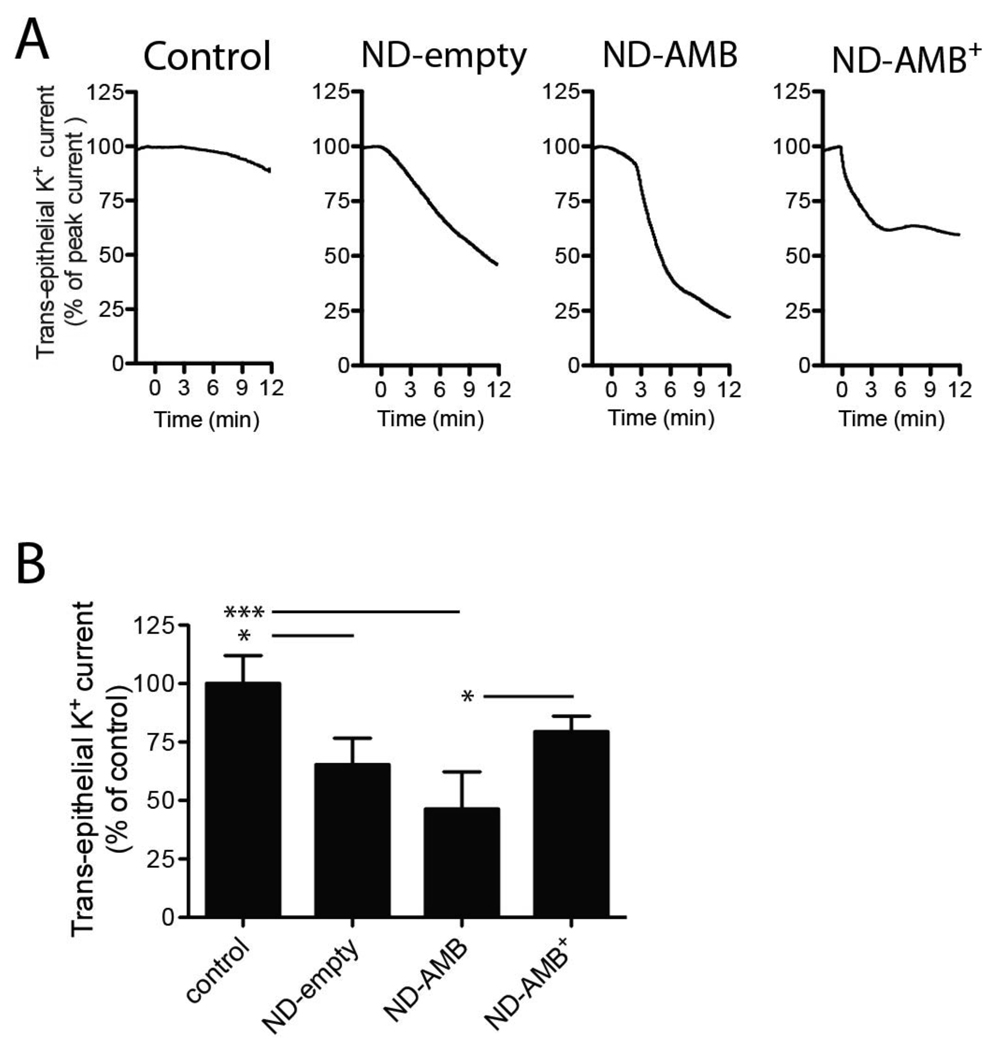
The underlying mechanism for the reduction in apical K+ permeability induced by ND-AMB was further examined. Two possibilities were considered: 1) electrically conductive pores could become blocked by the vehicle, or 2) ND-AMB could extract pore-forming AMB from membranes into the lipid bilayer of the ND, thus reducing trans-epithelial K+ current. We hypothesized that if ND-AMB extracted AMB, the process would be inversely proportional to the load of AMB on the ND. Specifically, a formulation of ND-AMB with a high load of AMB per ND would be less protective than one with a low load. Conversely, a mechanism mediated by blocking conductive pores would be unaffected by the load of AMB on the ND. To distinguish between these two proposed mechanisms, three formulations of ND, each containing a different ratio of AMB to PL, were tested for their ability to reduce K+ permeability. Monolayers that had been previously permeabilized with AMB/DOC were exposed to the following: standard ND-AMB formulation containing a AMB:PL:apoA-I weight ratio of 5:20:8, high-load ND synthesized at 20:20:8 wherein the mass of AMB was increased 4-fold (ND-AMB+) and an “empty” ND (ND-empty) lacking drug with a weight ratio of 0:20:8. Control cells received AMB/DOC and no further treatment (Fig 4 A–B). All treatments were normalized to contain an equivalent mass of apoA-I protein. In this assay ND-empty reduced trans-epithelial K+ current across a pre-permeabilized monolayer by 34.7 ± 11.4%, n=3 (p<0.05) compared to control. Similarly, standard ND-AMB reduced current by 53.7 ± 16.0%, n=4 (p<0.0001), whereas ND-AMB + did not produce a statistically significant effect, 20.6 ± 6.6%, n=4 (p>0.05). The observation, that ND-AMB with a low AMB to PL ratio reduces permeability of the epithelium, supports the hypothesis wherein ND extracts AMB from the plasma membrane, rather than simply blocking electrically conductive channels.
Figure 4.
ND-AMB is less effective at restoring cellular integrity when formulated with a high AMB load (A) Calu-3 monolayers were permeabilized by AMB/DOC (5 µg/mL). At time = 0 min, cells were exposed to ND-empty, ND-AMB or ND-AMB+ (all received 0.14 µg/mL apoA-I and 0, 0.09 or 0.35 µg/mL AMB, respectively) or no further treatment as a control. (A) Representative current traces are shown and have been normalized to peak current to account for variations in absolute values. (B) Changes in trans-epithelial current relative to control are summarized at 10 min post-treatment. Data represent four (ND-AMB, ND-AMB+) or three (control, ND-empty) independent observations. * p<0.05, *** p<0.0001.
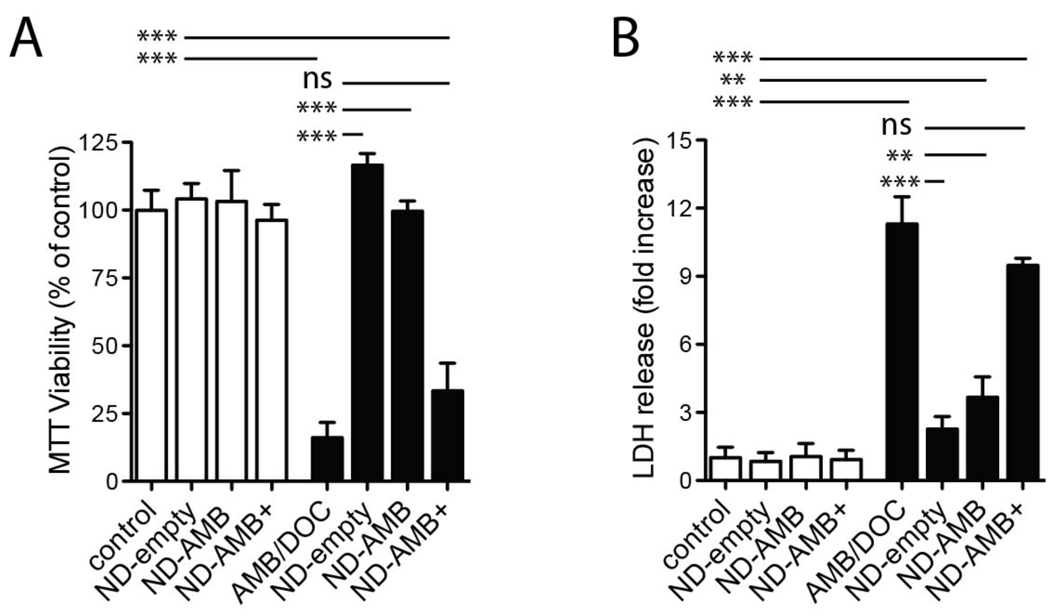
The above data suggest that ND-AMB and ND-empty, unlike ND-AMB+, can reduce toxicity and cell death caused by AMB/DOC. To evaluate this possibility, Calu-3 cells were exposed to a toxic concentration of AMB/DOC (75 µg/mL) in the presence of ND-empty, ND-AMB or ND-AMB+. Cells were incubated with each treatment for 18 hr before cellular viability and integrity were determined by MTT (Fig 5A) and LDH release (Fig 5B), respectively. Calu-3 cells incubated with AMB/DOC were significantly less viable than controls incubated with PBS alone 16.1 ± 5.6 %, n=3 (p<0.0001), assessed by MTT viability and LDH release, which was significantly increased 11.3 ± 1.2-fold, n=3 (p< 0.0001). In contrast, cells incubated in the presence of ND-empty were fully protected from the cytotoxicity of AMB/DOC and were indistinguishable from control cells by MTT and LDH release. The standard formulation of ND-AMB elicited similar protection; viability determined by MTT was not significantly different from control while LDH release from cells treated with ND-AMB was lower than AMB/DOC alone 3.7 ± 0.90-fold, n=3 (p<0.0001). Treatment with ND-AMB+ did not significantly affect MTT or LDH levels versus AMB/DOC. ND-empty, ND-AMB or ND-AMB+ in the absence of AMB/DOC did not reduce viability or promote LDH release. These data confirm that ND-AMB and ND-empty are protective against AMB/DOC exposure.
Figure 5.
ND-AMB and ND-empty protect Calu-3 cells from AMB mediated cytotoxicity. Calu-3 cells in 24 well plates were incubated in the absence (white bars) or presence (black bars) of AMB/DOC (75 µg/mL) in addition to ND-empty, ND-AMB or ND-AMB+ normalized by protein (6.3 µg/mL apoA-I and 0, 2.6 or 10.3 µg/mL AMB, respectively). After 18 hr incubation, cell viability was determined by (A) MTT assay or (B) LDH release. Measurements are representative of two independent experiments performed in triplicate. ** p<0.001, *** p<0.0001.
Effect of lipid and protein moieties on K+ current
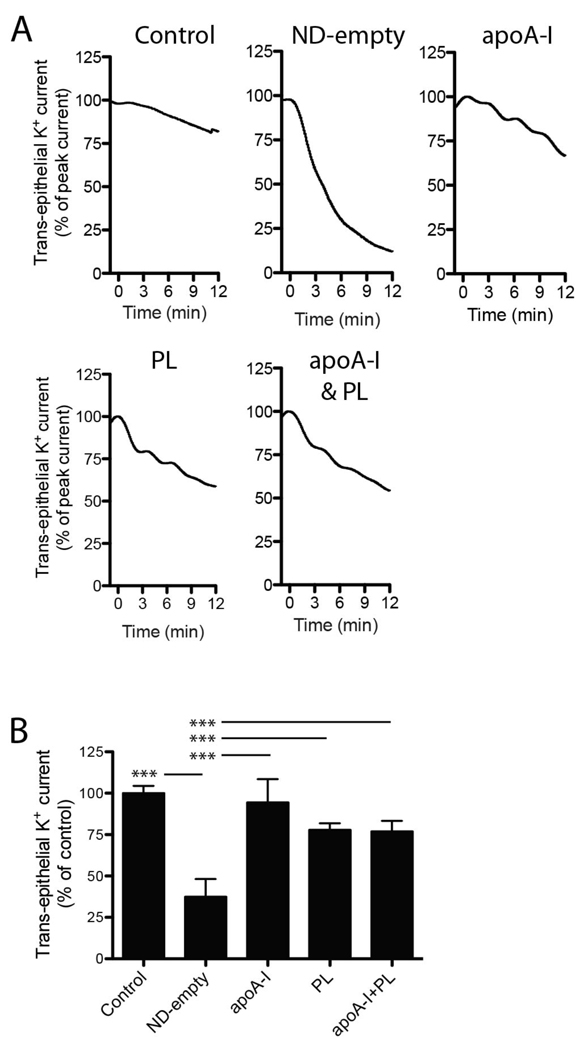
To characterize the mechanism whereby ND-AMB protects cells from AMB, the contribution of each molecular constituent of ND was assessed for the ability to restore membranes damaged by AMB/DOC in the Ussing chamber assay. A vesicle preparation of DMPC and DMPG, lipid-free apoA-I or a mixture of vesicles and lipid-free apoA-I were compared to fully assembled ND-empty (Fig 6). Compared to control, addition of ND-empty rapidly reduced trans-epithelial K+ current across Calu-3 monolayers by 62.5 ± 10.7 %, n=4 (p<0.0001). Treatment with PL vesicles, lipid-free apoA-I, or a mixture of PL vesicles and lipid-free apoA-I had no significant effect. These data demonstrate that the assembled ND elicits greater protection than its individual constituents.
Figure 6.
Efficient restoration of membrane resistance requires assembled ND. (A) Calu-3 cell monolayers were permeabilized by AMB/DOC (5 µg/mL). At time = 0 min, cells were exposed to ND-empty, lipid-free apoA-I, PL vesicles composed of DMPC:DMPG 7:3 (w/w), a mixture of PL and lipid-free apoA-I or no further treatment as control. All treatments delivered equal concentrations of apoA-I and/or PL (0.85 µg/mL apoA-I; 2.1 µg/mL PL). Representative current traces are shown and have been normalized to peak current to account for variations in absolute values. (B) Changes in trans-epithelial current relative to control are summarized at 10 min post-treatment. Data represent three (control, lipid-free apoA-I, PL, apoA-I + PL) or four (ND-empty) independent observations. ***p<0.0001.
Effect of ND-AMB on hepatic epithelial cells
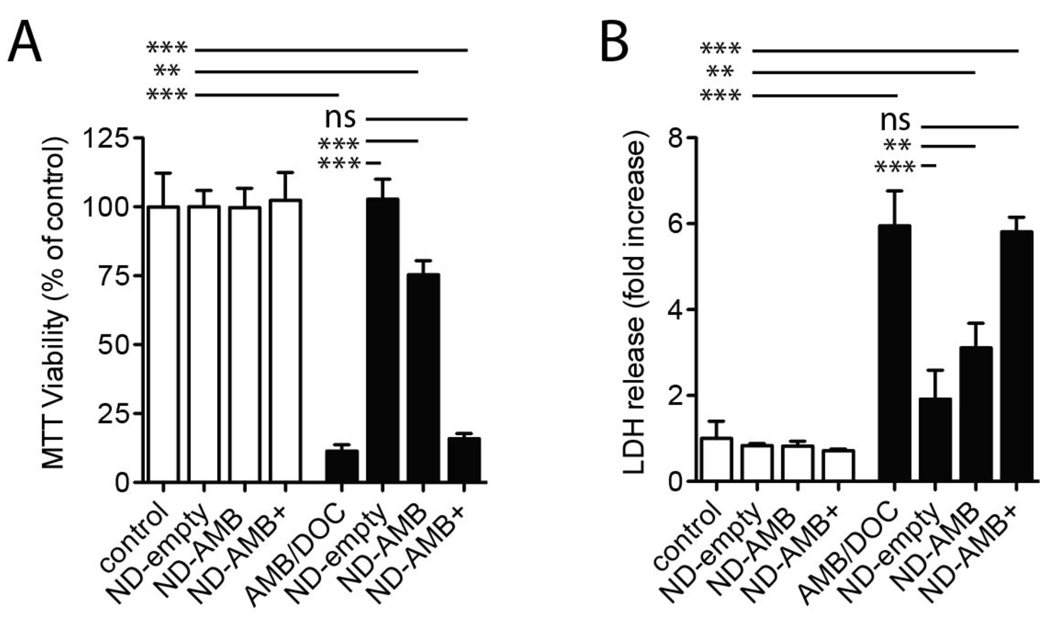
To determine whether the protective benefit of ND-AMB was limited to epithelia of the airways, we evaluated the effect of ND-AMB on a cellular model of hepatic epithelia. HepG2 cells were exposed to a toxic concentration of AMB/DOC (40 µg/mL) in the presence of ND-AMB, ND-AMB + or ND-empty. Cells were incubated with each treatment for 18 hr before cellular viability and integrity were determined by MTT assay and LDH release, respectively (Fig 7 A and B). Incubation of cells with AMB/DOC alone significantly reduced viability by 89 ± 2.3%, n=3 (p<0.0001) compared to control and increased LDH release by 5.6 ± 0.81-fold, n=3 (p<0.0001). In contrast, cells incubated in the presence of ND-empty and AMB/DOC were indistinguishable from untreated cells by MTT viability and LDH release. Similarly, treatment with ND-AMB restored viability to 75 ± 5.1%, n=3 (p<0.0001) and reduced LDH release to 3.1 ± 0.58-fold, n=3 (p<0.001) compared to AMB/DOC alone. Treatment with ND-AMB+ did not protect against AMB/DOC as cell survival remained low and LDH was elevated. These findings confirm results with airway epithelia that ND-AMB and ND-empty attenuate the cytotoxicity of AMB/DOC, whereas ND-AMB+ does not.
Figure 7.
ND protects HepG2 cells from AMB/DOC cytotoxicity. HepG2 cells in 24 well plates were incubated for 18 hr with equivalent concentrations of ND-empty, ND-AMB or ND-AMB+ normalized by protein (all 4.1 µg/mL apoA-I and 0, 2.6 or 10.3 µg/mL AMB, respectively) in the absence (white bars) or presence (black bars) of AMB/DOC (40 µg/mL). (A) Cell viability was determined using the MTT assay. (B) Cellular integrity was assessed by LDH release. Measurements are representative of two independent experiments performed in triplicate. **p<0.001, ***p<0.0001.
DISCUSSION
Pulmonary fungal infections are common and life-threatening to immunocompromised individuals. To improve the treatment of pulmonary infections, several antifungal agents including formulations of AMB, have been administered as inhaled aerosols, with some evidence of improved survival. Inhalation of AMB potentially has significant advantages over intravenous infusion including the avoidance of nephrotoxicity and infusion-related reactions. Aerosolized formulations of AMB may improve the treatment and prophylaxis of pulmonary fungal infections; however, safety remains an important consideration. The goal of the present study was to determine in vitro whether a novel nano-particle delivery platform for AMB, ND-AMB, was less disruptive to lung epithelium than existing formulations of aerosolized AMB. We hypothesized that AMB would cause minimal damage to the lung when formulated within the ND. The data describe the protective benefit of ND-AMB whereby the ND with or without drug not only minimizes AMB-induced cell membrane damage to lung epithelium in vitro but also reverses damage caused by prior exposure to lipid-free AMB.
We observed that lipid-based formulations of AMB, including ND-AMB, caused negligible permeabilization to our model airway system under the experimental conditions tested. Notably, lipid-based formulations were more than 8-fold less potent at inducing permeability than AMB/DOC. This observation is consistent with clinical results for lipid-based L-AMB, ABLC and ABCD that find these formulations to be significantly less toxic than detergent-based AMB/DOC therapy in both intravenous and inhaled applications.
A key finding of the present study was the ability of ND-AMB to restore membrane resistance that had been previously compromised by exposure to AMB/DOC. In the Ussing chamber assay, addition of ND-AMB to AMB-permeabilized monolayers initiated the complete and rapid restoration of baseline trans-epithelial K+ current. This protective activity was not observed for L-AMB, ABLC or ABCD. The difference between our formulation and that of the latter may, in part, be explained by the presence of apoA-I that could facilitate a beneficial interaction between the ND vehicle and cellular membranes by binding to cell surface receptors and/or membrane lipid.
In addition to the presence of apoA-I in the ND formulation, the ratio of AMB to PL was identified as an important modulator of protective activity. Notably, ND-AMB and ND-empty were protective in both Ussing chamber and cytotoxicity assays in contrast to ND-AMB+, synthesized with a higher ratio of AMB to PL, that did not confer significant protection. This behavior could be explained by a prior biophysical study of a related ND-AMB formulation (Nguyen et al., 2008). These authors observed that, when a higher mass of AMB was incorporated into a complex of apoA-I and DMPC, thinning of the PL bilayer occurred. The authors concluded that bilayer thinning resulted from interdigitation of the acyl chains from opposing PL bilayer leaflets, leading to a denser complex. Although speculative, these changes could preclude an interaction between ND-AMB+ and cell membranes thus preventing sequestration of AMB.
Cell viability results of Calu-3 and HepG2 monolayers exposed to AMB/DOC demonstrated that ND-AMB and ND-empty, but not ND-AMB+, are able to protect against cell death. These findings confirm that ND can protect cellular membranes from the cytotoxic effects of AMB. Furthermore, we demonstrated that the protective activity of ND was largely dependent on the assembled ND complex rather than on its constituent parts, i.e., lipid-free apoA-I or phospholipid vesicles. Taken together these findings suggest a mechanism whereby ND-AMB with a low AMB to PL ratio can alleviate or reverse the cytotoxic consequences of AMB exposure by removing toxic AMB from the cellular milieu.
The fact that standard ND-AMB can protect epithelial membranes from AMB cytotoxicity has significant implications for its efficacy in pulmonary fungal treatment. Firstly, it is possible that a higher dose of inhalable ND-AMB could be safely administered to infected airways compared to the other formulations examined, due to the anti-permeability and anticytoxicity activities described. Secondly, the safety of inhaled ND-AMB may be maximized by synthesizing it with a low ratio of AMB to PL. The protective activity of ND also has ramifications for existing treatment regimens involving aerosolized AMB, wherein inclusion of ND-empty into commercially available AMB aerosols may reduce side effects of AMB inhalation.
In conclusion, this study finds that in a lung epithelial model, ND-AMB provides greater protection from AMB toxicity than current clinically approved lipid-based formulations of AMB. Future studies will determine whether inhaled ND-AMB is superior in vivo for the treatment of fungal infections in the lung.
ACKNOWLEDGEMENTS
This work supported by R01 award number HL77268 from the National Heart, Lung, Blood Institute and the NIH Small Business Initiative Research award number 5R44A166444 from the National Institute of Allergy and Infection Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, Blood Institute, National Institute of Allergy and Infections Diseases, or the National Institutes of Health. We thank Dr. Robert Ryan for many helpful discussions during the course of these studies. BI is supported by the Cystic Fibrosis Foundation (Illek08G0). GC is supported by a new investigator award of the Tobacco Related Disease Program of California (18KT-0021). MNO, TMF and BLB are supported by Lypro Biosciences, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Behre GF, Schwartz S, Lenz K, Ludwig WD, Wandt H, Schilling E, Heinemann V, Link H, Trittin A, Boenisch O, et al. Aerosol amphotericin B inhalations for prevention of invasive pulmonary aspergillosis in neutropenic cancer patients. Ann Hematol. 1995;71:287–291. doi: 10.1007/BF01697981. [DOI] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WT, Cotlove E. Increased Permeability of Human Erythrocytes Induced by Amphotericin B. J Infect Dis. 1971;123:341–350. doi: 10.1093/infdis/123.4.341. [DOI] [PubMed] [Google Scholar]
- Cornet M, Fleury L, Maslo C, Bernard JF, Brucker G. Epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the Greater Paris area. J Hosp Infect. 2002;51:288–296. doi: 10.1053/jhin.2002.1258. [DOI] [PubMed] [Google Scholar]
- Cowley EA, Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol. 2002;538:747–757. doi: 10.1113/jphysiol.2001.013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B, Kim M, Zollner C. The Influence of Amphotericin B On The Permeability of Mammalian Erythrocytes To Nonelectrolytes, Anions and Cations. Biochimica et Biophysica Acta. 1973;318:345. [Google Scholar]
- Gryn J, Goldberg J, Johnson E, Siegel J, Inzerillo J. The toxicity of daily inhaled amphotericin B. Am J Clin Oncol. 1993;16:43–46. doi: 10.1097/00000421-199302000-00011. [DOI] [PubMed] [Google Scholar]
- Guo L, Fielding R, Lasic D, Hamilton R, Mufson D. Novel antifungal drug delivery: stable amphotericin B-cholestryl sulfate discs. Int J Pharma. 1991;75:45–54. [Google Scholar]
- Hertenstein B, Kern WV, Schmeiser T, Stefanic M, Bunjes D, Wiesneth M, Novotny J, Heimpel H, Arnold R. Low incidence of invasive fungal infections after bone marrow transplantation in patients receiving amphotericin B inhalations during neutropenia. Ann Hematol. 1994;68:21–26. doi: 10.1007/BF01695915. [DOI] [PubMed] [Google Scholar]
- Holding KJ, Dworkin MS, Wan PC, Hanson DL, Klevens RM, Jones JL, Sullivan PS. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Adult and Adolescent Spectrum of HIV Disease Project. Clin Infect Dis. 2000;31:1253–1257. doi: 10.1086/317452. [DOI] [PubMed] [Google Scholar]
- Holz R, Finkelstein A. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970;56:125–145. doi: 10.1085/jgp.56.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illek B, Fischer H, Clauss W. Aldosterone regulation of basolateral potassium channels in alveolar epithelium. Am J Physiol. 1990;259:L230–L237. doi: 10.1152/ajplung.1990.259.4.L230. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Clauss W. Quinidine-sensitive K+ channels in the basolateral membrane of embryonic coprodeum epithelium: regulation by aldosterone and thyroxine. J Comp Physiol [B] 1993;163:556–562. doi: 10.1007/BF00302114. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Kreusel KM, Hegel U, Clauss W. Volume-sensitive basolateral K+ channels in HT-29/B6 cells: block by lidocaine, quinidine, NPPB, and Ba2+ Am J Physiol. 1992;263:C674–C683. doi: 10.1152/ajpcell.1992.263.3.C674. [DOI] [PubMed] [Google Scholar]
- Janoff AS, Boni LT, Popescu MC, Minchey SR, Cullis PR, Madden TD, Taraschi T, Gruner SM, Shyamsunder E, Tate MW, et al. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci U S A. 1988;85:6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper L, Ruijgrok E. A review on the clinical use of inhaled amphotericin B. J Aerosol Med Pulm Drug Deliv. 2009;22:213–227. doi: 10.1089/jamp.2008.0715. [DOI] [PubMed] [Google Scholar]
- Martin DD, Budamagunta MS, Ryan RO, Voss JC, Oda MN. Apolipoprotein A-I assumes a "looped belt" conformation on reconstituted high density lipoprotein. J Biol Chem. 2006;281:20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- Menzin J, Meyers JL, Friedman M, Perfect JR, Langston AA, Danna RP, Papadopoulos G. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm. 2009;66:1711–1717. doi: 10.2146/ajhp080325. [DOI] [PubMed] [Google Scholar]
- Mohammad RA, Klein KC. Inhaled amphotericin B for prophylaxis against invasive Aspergillus infections. Ann Pharmacother. 2006;40:2148–2154. doi: 10.1345/aph.1G477. [DOI] [PubMed] [Google Scholar]
- Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, Walsh TJ, Fridkin SK, Pappas PG, Warnock DW. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43 Suppl 1:S49–S58. doi: 10.1080/13693780400020113. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Weers P, Raussens V, Wang Z, Ren G, Sulchek T, Hoeprichjr P, Ryan R. Amphotericin B induces interdigitation of apolipoprotein stabilized nanodisk bilayers. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2008;1778:303–312. doi: 10.1016/j.bbamem.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda MN, Hargreaves PL, Beckstead JA, Redmond KA, van Antwerpen R, Ryan RO. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. J Lipid Res. 2006;47:260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- Palmer SM, Drew RH, Whitehouse JD, Tapson VF. Safety of aerosolized amphotericin B lipid complex in lung transplant recipients. Transplantation. 2001;72:545–548. doi: 10.1097/00007890-200108150-00036. [DOI] [PubMed] [Google Scholar]
- Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991;28 Suppl B:49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- Rijnders B, Cornelissen J, Slobbe L, Becker M, Doorduijn J, Hop W, Ruijgrok E, Löwenberg B, Vulto A, Lugtenburg P, De Marie S. Aerosolized Liposomal Amphotericin B for the Prevention of Invasive Pulmonary Aspergillosis during Prolonged Neutropenia: A Randomized, Placebo- Controlled Trial. Clin Infect Dis. 2008;46:1401–1408. doi: 10.1086/586739. [DOI] [PubMed] [Google Scholar]
- Yano T, Itoh Y, Kawamura E, Maeda A, Egashira N, Nishida M, Kurose H, Oishi R. Amphotericin B-induced renal tubular cell injury is mediated by Na+ Influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob Agents Chemother. 2009;53:1420–1426. doi: 10.1128/AAC.01137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt WA, Tavormina PA. Steroid Interference with Antifungal Activity of Polyene Antibiotics. Appl Microbiol. 1966;14:865–869. doi: 10.1128/am.14.6.865-869.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]