Abstract
BACKGROUND
Modification of Notch receptors by O-linked fucose and its further elongation by the Fringe family of glycosyltransferase has been shown to be important for Notch signaling activation. Our recent studies disclose a myeloproliferative phenotype, hematopoietic stem cell (HSC) dysfunction, and abnormal Notch signaling in mice deficient in FX, which is required for fucosylation of a number of proteins including Notch. The purpose of this study is to assess the self-renewal and stem cell niche features of fucose-deficient HSCs.
STUDY DESIGN AND METHODS
Homeostasis and maintenance of HSCs derived from FX-/- mice were studied by serial bone marrow transplantation, homing assay, and cell cycle analysis. Two-photon intravital microscopy was performed to visualize and compare the in vivo marrow niche occupancy by fucose-deficient and wild type (WT) HSCs.
RESULTS
Marrow progenitors from FX-/- mice had mild homing defects that could be partially prevented by exogenous fucose supplementation. Fucose-deficient HSCs from FX-/- mice displayed decreased self-renewal capability compared with the WT controls. This is accompanied with their increased cell cycling activity and suppressed Notch ligand binding. When tracked in vivo by 2-photon intravital imaging, the fucose-deficient HSCs were found localized further from the endosteum of the calvarium marrow than the WT HSCs.
CONCLUSION
The current reported aberrant niche occupancy by HSCs from FX-/- mice, in the context of a faulty blood lineage homeostasis and HSC dysfunction in mice expressing Notch receptors deficient in O-fucosylation, suggests that fucosylation modified Notch receptor may represent a novel extrinsic regulator for HSC engraftment and HSC niche maintenance.
Introduction
The selective adhesion of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) to the extracellular matrix and the stromal cells plays an important role in the regulation of stem cell self-renewal, lineage commitment, and differentiation.1 HSCs reside in the endosteal niche along the bone surface where the osteoblasts adhesively interact with HSCs.2,3 A variety of intrinsic factors, including c-Myc, p21, p27, and p53 are involved in the regulation of HSC self-renewal.4-7 In addition, extrinsic cues, including Notch ligand, cdc42, Ang-1/Tie2, retinoid acid, BMP, and CXCR4/CXCL12 may coordinate to regulate the ability of HSCs to lodge and engraft at the endosteal niche.2,3,8-10
The Notch gene family is important for cell fate determination in many processes including mammalian hematopoiesis.11 Notch receptors are decorated by various post-translation modifications including O-fucosylation, with fucose moieties attached to the conserved serine or threonine residues of EGF-like repeats present on the Notch receptor extracellular domain. The O-fucose residues can then be further modified by the Fringe family of glycosyltransferases. These fucose-dependent modifications modulate Notch signaling through ligand-receptor interactions.12,13 Studies have shown that Notch activation promotes HSC expansion in vitro and favors lymphoid over myeloid lineage outcome in vivo.14-17 In addition, Notch activation by osteoblasts expressing the Notch ligand, Jagged1, in the bone marrow niche promotes self-renewal of primitive hematopoietic cells.3 However, loss-of-function studies using the conditional knockout of Notch1, Notch1 combined with Notch ligand Jagged1, or expression of a dominant negative regulator of canonical Notch transcriptional activation, did not reveal an essential role of Notch1 signaling on HSC self-renewal.18-20 It remains possible that other Notch/Notch ligand pairs may be important for the maintenance of the HSC pool. Also, it is unclear whether aspects of Notch pathways upstream of Notch activation, eg, its association with ligand-expressing osteoblasts which dictates the physical association of HSC with marrow micro-environment, may be critical for HSC biology.
We previously reported a chronic myeloproliferative phenotype and abnormal Notch signaling in FX-/- mice.13 Deletion of the FX locus eliminates constitutive GDP-fucose synthesis, but leaves intact a salvage pathway for GDP-fucose synthesis that uses exogenous fucose.21 Therefore fucosylation of glycans and proteins are conditionally dependent on exogenous fucose via the salvage pathway in FX-/- mice. We disclosed that chronic myeloproliferation found in FX-/- mice is a result of loss of controlled suppression of myelopoiesis exerted by Notch. In the context of a wild-type fucosylation phenotype, O-fucosylation of Notch, expressed on HSCs and hematopoietic progenitor cells (HPCs), is required for effective interaction of these cells with Notch ligand and the transduction of Notch signal activation. This effective interaction is required for Notch exerted suppression of overt myeloid development. By contrast, fucosylation-deficient myeloid progenitors have lost the wild-type Notch ligand-binding phenotype, do not transcribe Notch target genes, and display uncontrolled myeloid differentiation. More recently, we found that HSCs deficient in cellular fucosylation have decreased lymphoid but increased myeloid developmental potentials. These features were accompanied with their suppressed in vitro binding ability with recombinant Notch ligand. Further, these cells displayed a 13.7-fold reduction of HSC frequency by limiting dilution transplant analysis (Yan et al, manuscript in press).22 In this study, we studied the mechanism underlying the dysfunction of HSCs expressing fucosylation-deficient Notch by examining their in vivo self-renewal capability. In order to explore whether an altered self-renewal capability of fucose-deficient HSC is associated with their aberrant stem cell niche features, we performed two-photon intravital microscopy to visualize and compare the in vivo marrow niche occupancy by fucose-deficient and wild type HSCs.
Materials and Methods
Mice
The animal research described in this manuscript was approved by Case Western Reserve University Institutional Animal Care and Use Committee. Mice used include 8~24-wkold WT and FX-/- mice maintained and prepared as described.21 The Notch reporter transgenic (NTg) mouse was purchased from the Jackson Laboratory (stock # 005854). The NTg mouse carries a hemizygous allele of Notch-signaling CBF-1 response element and a minimal SV40 promoter driven enhanced green fluorescent protein (eGFP). This mouse has been used to define active Notch signaling in HSCs in vivo.23 FX-/--NTg mouse was generated by crossing the NTg with the FX-/- mouse, and maintained as described.13
Flow cytometry analysis and cell sorting
Flow cytometric analyses and cell sorting were performed as described.13 Briefly, marrow mononuclear cells were incubated with biotin conjugated rat anti-mouse antibody cocktails (CD3, CD4, CD8, Gr-1, CD11b, B220, Ter119), followed by lineage depletion with goat anti-rat IgG magnetic beads (Miltenyi Biotec, Auburn, CA). Lineage-depleted cells were further stained with streptavidin-APC-Cy7, fluorescein isothiocyante (FITC)-anti-Sca-1, and allophycocyanin (APC)-anti-c-kit, and sorted using FACSAria (BD Biosciences, San Jose, CA). For cell cycle G1/G0 analysis in the stem cell compartment, bone marrow nucleated cells were incubated with DNA dye Ho (4 μg/liter) and RNA dye PY (1 μg/ml) at 37°C for 45 min, respectively (17), then labeled with FITC-anti-lineage antibodies, APC-anti-c-kit and PE-Cy7-anti-Sca-1. For S-G2/M cell cycle ananlysis, mice were injected intraperitoneally with one dose of Brdu and fed with water containing Brdu for 3 days. The percent of LSKs in S-G2/M phase of the cell cycle was analyzed by anti-Brdu and 7-amino-actinomycin D (7-AAD). Flow cytometry was performed on FACSAria and BD LSR II Instruments (BD Biosciences).
Recombinant mouse Notch ligand (Dll4) binding assay
Recombinant Notch ligand comprised of the extracellular domains of Dll4 fused with human IgG Fc were constructed as described.24 Recombinant Dll4 were prepared from HEK 293 T cells transfected with hIgG-Dll4 or the vector, and quantified by ELISA. Binding assay was performed by incubating LSK cells with recombinant Dll4 in Hanks balanced salt solutions supplemented with Ca2+, and analyzed by flow using PE-anti-human IgG Fc.
Bone marrow transplantation and homing assay
Primary and secondary bone marrow transplantation was performed as described.13 Briefly, 2×106 donor marrow cells were injected into lethally irradiated (950 Rad) female recipient mice. Recipient mice were monitored daily for survival for more than 30 days. The mice were sacrificed at 2 to 4 months, and bone marrow cells were prepared from those mice and injected into new female recipients. To perform homing assays, bone marrow cells from donor animals (WT or FX-/- mice) (Ly5.2) were prepared as 2×106 cells/200 μl volume in PBS, and were injected into lethally irradiated WT recipient mice. An aliquot is also plated in methylcellulose cultures (M3434, Stem Cell Technologies, Vancouver, BC, Canada) to quantify committed progenitor cells of various lineages. Sixteen hours later, single-cell suspensions were prepared from the bone marrow of the recipients, and cultured in duplicate to assess donor colony forming unit (CFU) recovery in the recipient animals. The number of homed CFU per femur was corrected to represent the whole BM (multiplied by 16.9).25 The number of donor CFUs recovered after 16 h in BM was expressed as a percentage of total CFUs infused.
Multi-photon intravital imaging studies
Intravital imaging of adoptively transplanted hematopoietic progenitors was performed as described.26 Briefly, isolated Lineage-c-kit+ (Lin-c-Kit+) cells (5-50×105) were injected into the tail vein of lethally-irradiated recipient mouse. At indicated times after i.v. transfer, mice were anaesthetized and a small incision was made in the scalp so as to expose the underlying dorsal skull surface. Donor cell homing to the skull marrow was imaged using a SP5/AOBS/2-photon microscope tuned to 860 nm (Leica Microsystems & Coherent Inc., Lawernceville, GA) while mice are under inhaled anesthesia (1-2% isoflurane) on a warmed microscope stage (37°C). To highlight the bone marrow vasculature, TRITC-Dextran (Sigma, St. Louis, MO) was injected into recipient mice 5 min prior to imaging experiments. Simultaneous visualization of bone endosteum, vasculature and HSC was achieved by second harmonic generation (SHG) microscopy, Dextran dye, and cells with GFP signals, respectively. Fluorescent images from optical sections of individual xy-planes were collected through pre-determined, fixed optical z-slices. This data set was then analyzed using imaging software (Imaris; BitPlane, Inc., Saint Paul, MN), which allows simultaneous tracking of object positions in 3 dimensions over time with statistical calculations.
Statistical analysis
Data are presented as means plus or minus SD, unless otherwise stated. Statistical significance was assessed by Student t test.
Results
Mild marrow homing defects of FX-/- cells can be partially prevented by exogenous fucose
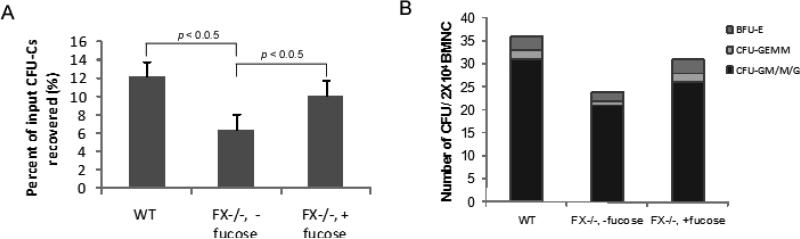
The deletion of the FX locus abolishes the expression of all fucosylated glycans, including the counter receptors for the selectins, which are involved in the rolling and homing of hematopoietic stem cells to the marrow.27 To assess the contribution of defective homing to the stem cell defects we observed in the FX-/- mice, we compared the CFUs of marrow cells recovered from recipient mice 16 h after transplantation of donor marrow cells derived from WT or FX-/- mice. As shown in Figure 1A, the percentage of recovered HPCs that homed to the BM was 12.2 ± 0.9% in the WT control group. As expected, the fucose-deficient FX-/- HPCs had decreased homing by 52% when compared to the control group. However, the homing ability of fucose-deficient FX-/- HPCs could be partially prevented with HPCs that were derived from FX-/- mice that had been reared on fucose-supplemented chow. Although the progenitors of myeloid lineage were relatively increased in FX-/- mice maintained on standard chow, the proportion of different lineages of progenitors recovered was not changed compared to the cells infused (Fig 1B). Therefore to ensure equal homing of donor cells to the recipient marrow, the transplantation experiments described thereafter were performed using cells from WT or FX-/- mice maintained on fucose-supplemented chow, and the recipients were provided with fucose-supplemented drinking water for 9-12 days after receiving intravenous injection of donor cells, and then maintained on standard chow.13
Figure 1. Mild homing defects associated with FX-/- marrow progenitors are corrected by exogenous fucose.
Lethally irradiated WT recipients were injected with bone marrow cells derived from WT mice, mice raised on standard chow (FX-/-, - fucose), or mice raised on fucose-supplemented chow (FX-/-, + fucose). (A) CFUs were determined from the recipient bone marrow 16 h after injection, and expressed as percentage recovery of infused CFU-Cs (n=5 per group). (B) Proportions of different lineages of CFUs were determined from the recovered marrow progenitors, including Burst-forming unit-erythroid (BFU-E), myeloid lineage colonies of granulocyte/macrophage/granulocyte macrophage (CFU-G/M/GM), and multi-potential progenitors CFU-GEMM (CFU-granulocyte, erythroid, macrophage, megakaryocyte).
FX-/- HSCs have decreased self-renewal, are less quiescent, and have increased cell cycling activity
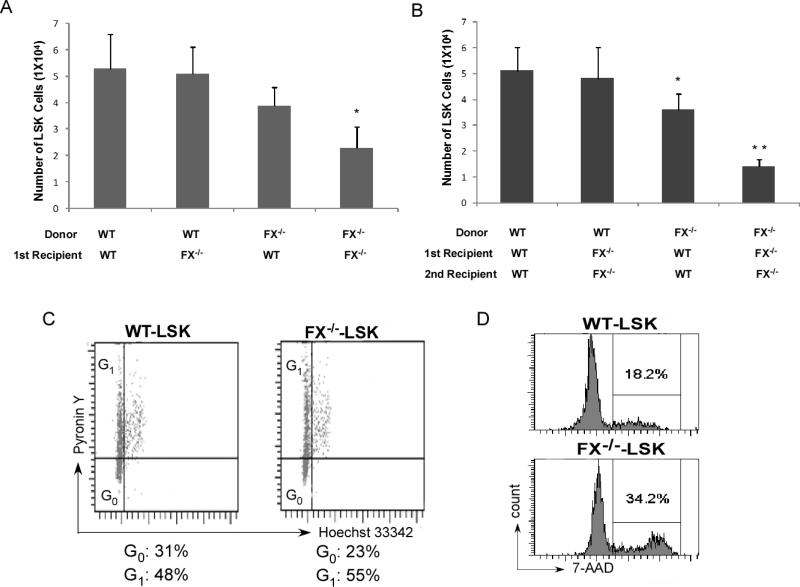
The frequency of LSK cells (lineage-Sca-1+c-kit+) under steady state is mildly decreased in FX-/- mice compared to WT controls, or FX-/- mice with fucose-supplementation (0.13% in WT, 0.10% and 0.12% in FX-/- mice without or with fucose-supplementation, respectively). However, a reduction of HSC frequency in FX-/- mice by competitive transplant after myeloablation suggests that fucose-deficient HSCs may have decreased engraftment or decreased self-renewal.22 Serial bone marrow transplantation was performed to test this hypothesis. We found that in the primary transplant setting, the pool of donor-derived bone marrow LSK cells was modestly decreased in the WT recipient when the marrow cells are derived from FX-/- mice. LSKs were further decreased in the secondary recipients receiving marrow cells derived from the FX-/- donor. Interestingly, although the LSK frequency was not changed in the FX-/- recipients receiving WT donor cells in both primary and secondary transplant settings, LSK pools were severely diminished when both marrow cells and the stroma are of the fucose-deficient genotype, indicating a marrow stromal dependent mechanism that maintains HSC pool by interacting with HSCs in these settings. Because stem cell quiescence is critical for protection from myelotoxic injury and preservation of the stem cell pool, we decided to examine the cell cycling status of the HSCs in FX-/- mice. Using the RNA dye Pyronin Y as a measure of quiescence among the LSK and Hoechst 33342 low-staining bone marrow cells,28 we found that FX-/- marrow progenitors were less quiescent by displaying less cells in G0 phase (Fig 2C). Further, we found that more FX-/- marrow LSKs were engaged in active cell division by using bromodeoxyuridine (BrdU) incorporation and 7-amino-actinomycin D staining (7-AAD) (Fig 2D). These results indicate that decreased self-renewal of HSCs expressing Notch receptors deficient in O-fucosylation could be accounted for by enhanced HSC cycling and depletion of the long-term HSC pool.
Figure 2. FX-/- HSCs have decreased self-renewal in serial trasnplantation, are less quiescent, and have increased cell cycling activity.
Flow cytometry analysis of LSK cells in the primary recipients 4 months after transplantation (A) and secondary recipients 3 months after transplantation (B). N=5 for each group, *p < 0.05, ** p< 0.01 compared with primary (A) or secondary (B) transplant WT recipients receiving WT donor cells. (C) Shown is a representative distribution of G0 versus G1 in the LSKs defining an increased cycling fraction in FX-/- mice. Mouse bone marrow cells were stained with lineage antibodies, APC-anti-ckit, PE-anti-Sca-1, Pyronin Y (RNA dye), and Hoechst 33342 (DNA dye). LSK cells were gated by means of a stringent parameter. Cells residing in G0 appear at the bottom of the G0/G1 peak, and G1 cells are the upper part as indicated. (D) Mice were injected intraperitoneally with one dose of Brdu and fed with water containing Brdu for 3 days. The percent of LSKs in S-G2/M phase of the cell cycle was analyzed by anti-Brdu and 7-amino-actinomycin D (7-AAD). Data shown in (C) and (D) is one representative of 3 similar experiments.
FX-/- HSCs display decreased Notch ligand binding and aberrant endosteal niche occupancy
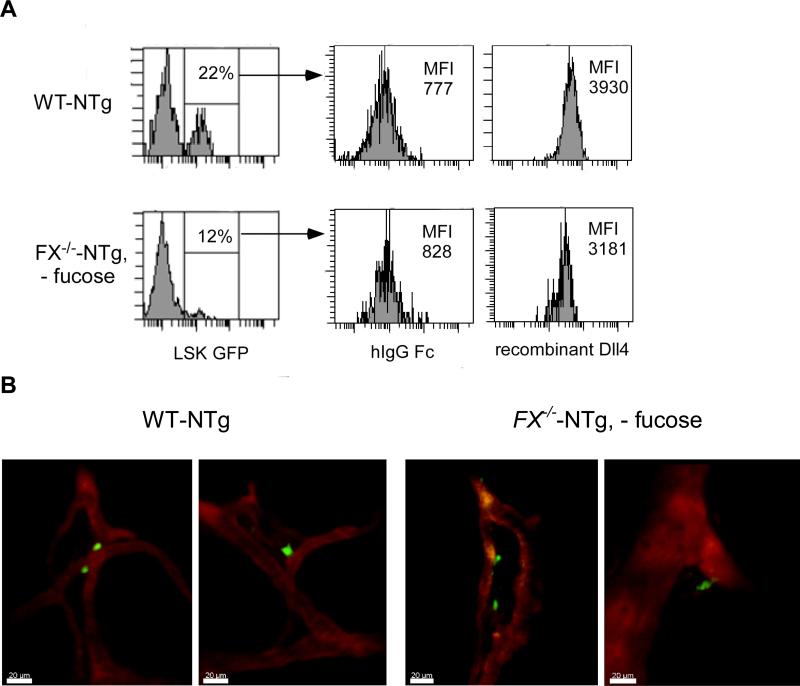
Proper endosteal niche occupancy has been shown to be essential for HSC self-renewal and marrow engraftment. In addition, there is evidence that perivascular space may delineate another important niche for HSC engraftment or expansion, as the major cell type, osteoblasts, in the endosteum, were also found in the perivascular regions.26 In order to understand the cellular mechanism by which the differential cellular positioning of HSCs may be regulated by Notch and its modification by O-fucose, we first examined the binding of Notch-expressing HSCs with Notch ligand.3 We then performed transplant experiments followed by 2-photon microscopy to visualize the niche positioning of transplanted HSC/HPC that are either fucose-replete or fucose-depleted in the calvarium marrow.29 The calvarium marrow has been shown to have HSC frequencies comparable to long bones,26 and is easily accessible for intravital imaging by 2-photon microscopy. Marrow cells were isolated from either FX-/--NTg mice that had been fed standard chow (FX-/--NTg, - fucose) or WT-NTg mice. The NTg mouse carries a hemizygous allele of Notch-signaling CBF-1 response element and a minimal SV40 promoter driven enhanced green fluorescent protein (eGFP). GFP expression indicates Notch activation, which is a hallmark of primitive HSC, whereas HSCs undergoing proliferation and differentiation display down-regulated Notch activation.23 By flow cytometric analysis, we found that GFP expression was decreased by ~50% in fucose-depleted LSK cells when compared to WT LSK cells. In addition, these GFP+ cells showed decreased binding ability to recombinant Notch ligands in vitro, such as Dll4 (Fig 3A) and Jagged1 (data not shown).
Figure 3. FX-/- HSCs have decreased Notch ligand binding but similar physical relationship to the vasculature compared to WT HSCs after homing in mouse calvarium bone marrow.
(A) Marrow LSK cells from WT-NTg or FX-/--NTg mice without fucose supplementation were examined for GFP expression. GFP+ cells were gated and analyzed for their binding with vector control or recombinant Notch ligand Dll4 using PE-anti-hIgG Fc by flow cytometric analysis. Data shown is one representative of five similar experiments as indicated by the mean fluorescent intensity (MFI) after binding of LSKs with vector or Dll4. (B) Shown are 2 representative images of each taken by 2-photon intravital microscopy of engrafted GFP+ marrow progenitors isolated from either WT-NTg (left 2 panels) or FX-/--NTg (- fucose) (right 2 panels) mouse donors, respectively. Calvarium blood vessels were highlighted by TRITC-Dextran.
Five million marrow Lin-c-kit+ cells from either WT-NTg mice or FX-/--NTg mice maintained on standard chow (no fucose supplementation), were transplanted into WT recipient mice. High-resolution 2-photon microscopy images were obtained 16 h later. Consistent with our previous finding that fucose-deficient progenitors have decreased homing capability (Fig 1), we observed less engrafted cells that are derived from FX-/--NTg donors compared to that from the WT-NTg donors in the recipient calvarium. About 35% of GFP+ cells (green) from WT-NTg mice were found lining along the blood vessels. In comparison, only ~20% of GFP+ cells from FX-/--NTg donor (no fucose) were bound to the vascular segments (Fig 3B), while more cells were found scattered in the central marrow cavity (data not shown). No statistical difference was identified when the average distance of GFP+ cells from WT-NTg mice to the vessels was compared to that from GFP+ cells from FX-/--NTg mice. Whether FX-/- and WT HSCs show any difference in their physical relationship to the osteoblasts that are embedded in the vasculature remains to be determined.26
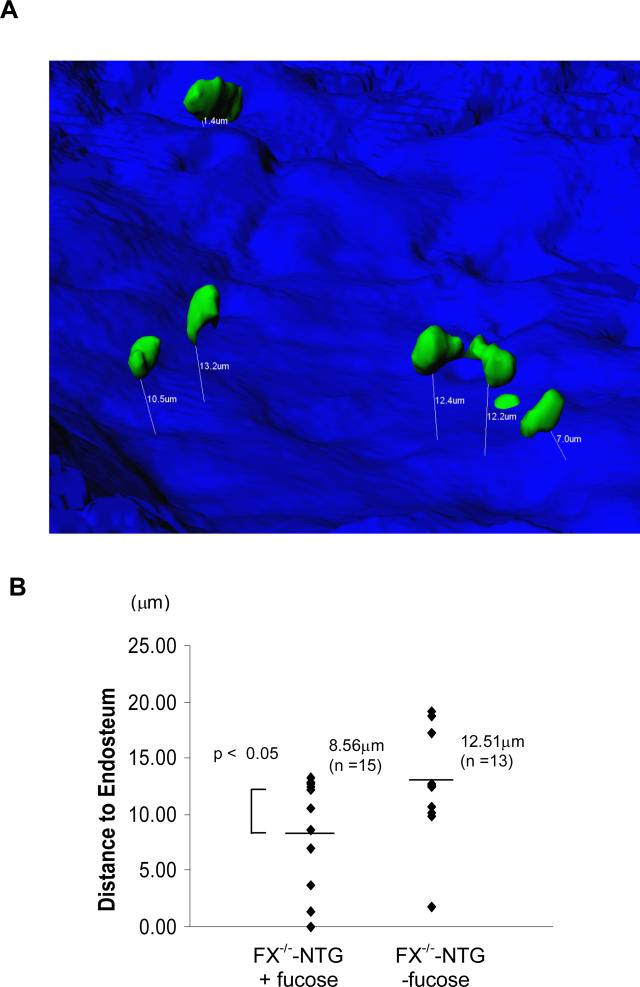
We then compared the endosteal niche localization of HSCs that are either fucose-replete or fucose-depleted. Visualization of bone endosteum and HSC was achieved by second harmonic generation (SHG; blue) microscopy and GFP signals (green) from HSCs, respectively.26 XYZ stack Images were collected and processed using Imaris Software (Fig 4A). The shortest three-dimensional distance between GFP+ cells and the endosteal surface was determined (Fig 4B). The average distance of the fucose-depleted cells to the endosteum was 12.51μm, which is greater than the 8.56μm observed in fucose-replete cells. These findings suggest that the niche positioning of marrow progenitors from FX-/- mice is affected by the absence of fucose on these cells, which correlates with their decreased Notch ligand binding and Notch activation.
Figure 4. Marrow HSCs without fucose are localized more distant to the endosteum in the calvarium.
(A) A representative image of 3 similar experiments showing HSCs (GFP+, green) localized in the marrow after transplantation. The shortest three-dimensional distance (μm) between HSC and the endosteum (blue) was determined and marked with white lines and numbers. (B) GFP+ cells from fucose-replete mice are closer to the endosteum than GFP+ cells from fucose-depleted mice.
Discussion
HSCs ensure sustained production of all blood cells through complex processes regulated by intricate signaling pathways and interactions with the marrow micro-environment/ stem cell niche. HSCs expressing members of the Notch family of receptors are located in bone marrow where they interact with surrounding stromal cells expressing Notch ligands. 3,30-32 Such direct cell-cell interaction via Notch and Notch ligand coupling may provide an important means for HSCs to position themselves in the stem cell niche where they are exposed to cytokines and signals released from the niche supporting cells. This hypothetical role of Notch has been suggested by recent studies done by Celso et al using a combination of confocal microscopy and 2-photon imaging system, which showed that quiescent HSCs are significantly closer to osteoblasts lining the endosteal surface than cells undergoing rapid proliferation. Further, HSCs are found localized closer to the endosteal surface in a transgenic mouse model in which increased numbers of osteoblasts have been shown to drive stem cell expansion.3,26 Significantly, Calvi et al showed osteoblasts that exert this regulatory role express increased level of the Notch ligand, Jagged1.3 These findings suggest that HSC endosteal niche localization may be facilitated by Notch and Notch ligand coupling.
This notion is now supported by our observation that HSCs with Notch activation are found localized closer to the endosteal surface. Since the Notch/Notch ligand coupling can be modulated by Notch receptor O-fucose modification,13,33 and the endosteal niche is essential for HSC quiescence maintenance, we speculate that HSC niche occupancy can be influenced by the status of Notch receptor glycosylation that may dictate the strength of interaction between Notch expressed on HSC and Notch ligand expressed on osteoblasts. If this is the case, then HSCs expressing non-fucosylated Notch molecules may exhibit aberrant niche occupancy resulting in more active cell division and therefore are prone to differentiation along the myeloid lineag.13 Indeed, we found that fucose-deficient HSCs are positioned further from the endosteal surface than fucose-replete HSCs. The altered physical relationship of fucose-deficient HSCs with the marrow endosteum correlates with their decreased quiescence, increased cell cycling, suppressed ligand binding ability and suppressed Notch activation. Our observations are consistent with the finding by Duncan et al who found that HSCs undergoing rapid proliferation and differentiation display down-regulated Notch activation.23 Because the observed phenotype in the FX-/- mouse model may not be specific for Notch, future studies will be targeted to confirm these results by using genetically-engineered animal models targeting Notch-specific fucose modification, or by agents that directly target the Notch /Notch ligand binding.
Notch signaling is essential for T cell fate specification and differentiation in other hematopoietic lineages. Whether canonical Notch intracellular signaling activation is required for HSC self-renewal is controversial.34 The negative finding of an essential role of Notch canonical signaling in HSC self-renewal suggests that aspects of Notch pathways that are independent of the transcriptional control of Notch signaling mediated by Mastermind-like 1 may be critical for HSC functional control.20 To assess the contribution of Notch intracellular signaling to HSC self-renewal that is independent of glycosylation-modified Notch and ligand coupling, we examined the consequence of restoring Notch1 intracellular activation on cell fate of marrow progenitors that are fucose-deficient. Interestingly, forced expression of Notch1 appears to be able to reverse the aberrant lineage commitment in the marrow progenitors associated with fucose deficiency, but it also decreases the LSK frequency.22 This latter finding could be caused by the secondary leukemogenic effect of non-physiological does of Notch activation in promoting aberrant T cell development in the marrow HSC population.35 It remains to be determined whether signaling from other Notch family members alone, or in concert with Notch1, may be required for HSC maintenance in the HSC compartment.
In summary, our observations indicate that Notch and Notch ligand interaction may represent an important mechanism being part of a complex network mediated by a group of adhesion and signaling molecules in a specialized micro-environment that supports the stem cell niche.1 Therefore, similar to what has been described in Rb-/- mice,36 we believe that the defective HSC phenotype and the myeloproliferation are inter-related in FX-/- mice, and is likely reflective of the role of fucosylation-modified Notch as a novel extrinsic regulator of HSC self-renewal and fate determination. Although evidences presented in this study as well as our previous reports strongly support that abnormal Notch ligand binding and Notch signaling could account for the observed aberrant hematopoietic phenotypes and HSC defects in FX-/- mice, it remains possible and unknown yet whether fucose deficiency could impact hematopoiesis mediated by other fucosylated proteins. O-fucose is present on EGF repeats of uninary plasminogen activator and some of the clotting factors, as well as the thrombospondin type 1 repeats (TSRs) in many secreted and transmembrane proteins.37-40 Recently, it was shown that O-fucosylation sites on TSR of ADAMTS like-1/punctin-1 and ADAMTS13 are functionally significant for secretion of these proteins. 41,42 In addition, other forms of fucosylated cell surface glycans and matrix proteins may be implicated in a variety of biological processes including cell growth and cell-cell adhesion. 43 It remains possible that lack of fucose on fucosylated glycans other than Notch may be directly or indirectly cause HSC defects mediated through altered intercellular signaling. Therefore a clear link between Notch fucosylation and hematopoietic and stem cell defect awaits further analysis of fucose deficiency specific for Notch pathway. Nevertheless, we hope the findings from this study may provide a rationale for future research in understanding the importance of Notch fucosylation, and fucosylation of other relevant biological molecules, for HSC niche competency and HSC functional control, and may also advance efforts in the future to use such knowledge in stem cell therapy.
Acknowledgment
We thank Dr. John Lowe for providing FX-/- mouse line. This study was supported in part by grants from the National Blood Foundation Research Grant and NIH to Lan Zhou, by grants from the St. Baldrick's Foundation, the Dana Foundation, and the Gabrielle's Angel's Foundation to Jay Myers and Alex Huang. Alex Huang is a designated St. Baldrick's Scholar.
Footnotes
The authors certify that they have no conflict of interest of financial involvement with this manuscript.
References
- 1.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 3.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A, Murphy MJ, Oskarsson T, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18(22):2747–63. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Elf SE, Miyata Y, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4(1):37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walkley CR, Fero ML, Chien WM, et al. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2005;7(2):172–8. doi: 10.1038/ncb1214. [DOI] [PubMed] [Google Scholar]
- 7.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–8. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 8.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Purton LE, Bernstein ID, Collins SJ. All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood. 2000;95(2):470–7. [PubMed] [Google Scholar]
- 10.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29(9):1041–52. doi: 10.1016/s0301-472x(01)00676-2. [DOI] [PubMed] [Google Scholar]
- 12.Haltiwanger RS. Regulation of signal transduction pathways in development by glycosylation. Curr Opin Struct Biol. 2002;12(5):593–8. doi: 10.1016/s0959-440x(02)00371-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, Li LW, Yan Q, et al. Notch-dependent control of myelopoiesis is regulated by fucosylation. Blood. 2008;112(2):308–19. doi: 10.1182/blood-2007-11-115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6(11):1278–81. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 15.Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93(3):838–48. [PubMed] [Google Scholar]
- 16.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J Clin Invest. 2002;110(8):1165–74. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stier S, Cheng T, Dombkowski D, et al. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99(7):2369–78. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 18.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10(5):547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 19.Mancini SJ, Mantei N, Dumortier A, et al. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105(6):2340–2. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 20.Maillard I, Koch U, Dumortier A, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2(4):356–66. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PL, Myers JT, Rogers CE, et al. Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J Cell Biol. 2002;158(4):801–15. doi: 10.1083/jcb.200203125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Q, Yao D, Wei L, et al. O-fucose Modulates Notch-Controlled Lineage Commitment. American Journal of Pathology. 2010 doi: 10.2353/ajpath.2010.090702. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan AW, Rattis FM, DiMascio LN, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6(3):314–22. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 24.Heinzel K, Benz C, Martins VC, et al. Bone marrow-derived hemopoietic precursors commit to the T cell lineage only after arrival in the thymic microenvironment. J Immunol. 2007;178(2):858–68. doi: 10.4049/jimmunol.178.2.858. [DOI] [PubMed] [Google Scholar]
- 25.Chervenick PA, Boggs DR, Marsh JC, et al. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968;215(2):353–60. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- 26.Celso CL, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2008 doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katayama Y, Hidalgo A, Furie BC, et al. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102(6):2060–7. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 28.Gothot A, Pyatt R, McMahel J, et al. Functional heterogeneity of human CD34(+) cells isolated in subcompartments of the G0 /G1 phase of the cell cycle. Blood. 1997;90(11):4384–93. [PubMed] [Google Scholar]
- 29.Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435(7044):969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varnum-Finney B, Purton LE, Yu M, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91(11):4084–91. [PubMed] [Google Scholar]
- 31.Schroeder T, Kohlhof H, Rieber N, Just U. Notch signaling induces multilineage myeloid differentiation and up-regulates PU.1 expression. J Immunol. 2003;170(11):5538–48. doi: 10.4049/jimmunol.170.11.5538. [DOI] [PubMed] [Google Scholar]
- 32.Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different Notch ligands. Blood. 2007;109(2):507–15. doi: 10.1182/blood-2006-05-025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okajima T, Xu A, Irvine KD. Modulation of notch-ligand binding by protein O-fucosyltransferase 1 and fringe. J Biol Chem. 2003;278(43):42340–5. doi: 10.1074/jbc.M308687200. [DOI] [PubMed] [Google Scholar]
- 34.Sandy AR, Maillard I. Notch signaling in the hematopoietic system. Expert Opin Biol Ther. 2009;9(11):1383–98. doi: 10.1517/14712590903260777. [DOI] [PubMed] [Google Scholar]
- 35.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11(3):299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 36.Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129(6):1081–95. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez de Peredo A, Klein D, Macek B, et al. C-mannosylation and o-fucosylation of thrombospondin type 1 repeats. Mol Cell Proteomics. 2002;1(1):11–8. doi: 10.1074/mcp.m100011-mcp200. [DOI] [PubMed] [Google Scholar]
- 39.Hofsteenge J, Huwiler KG, Macek B, et al. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem. 2001;276(9):6485–98. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- 40.Buko AM, Kentzer EJ, Petros A, et al. Characterization of a posttranslational fucosylation in the growth factor domain of urinary plasminogen activator. Proc Natl Acad Sci U S A. 1991;88(9):3992–6. doi: 10.1073/pnas.88.9.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LW, Dlugosz M, Somerville RP, et al. O-fucosylation of thrombospondin type 1 repeats in ADAMTS-like-1/punctin-1 regulates secretion: implications for the ADAMTS superfamily. J Biol Chem. 2007;282(23):17024–31. doi: 10.1074/jbc.M701065200. [DOI] [PubMed] [Google Scholar]
- 42.Ricketts LM, Dlugosz M, Luther KB, et al. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem. 2007;282(23):17014–23. doi: 10.1074/jbc.M700317200. [DOI] [PubMed] [Google Scholar]
- 43.Zhao YY, Takahashi M, Gu JG, et al. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008;99(7):1304–10. doi: 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]