Although keratinocytes are the primary constituents of the hair follicle and generate the hair shaft, mesenchymal cells also play important roles. These include the follicular dermal papilla (DP) and the connective tissue sheath or dermal sheath (CTS). The DP is embedded in the hair bulb during the anagen phase and forms a compact ball during the telogen phase, while CTS cells line the outside of the epithelial follicle from the bulge to its base. The central role of the dermal papilla in regulating the activity of keratinocytes during hair follicle regeneration and hair shaft morphogenesis has been established by extirpation and grafting studies (Ibrahim and Wright, 1977; Jahoda et al., 1984; Jahoda et al., 1993; McElwee et al., 2003), and more recently by direct manipulation of gene expression in the DP in vivo (Enshell-Seijffers et al., 2010). A strong correlation between DP size, hair bulb diameter and hair caliber has been noted (Elliott et al., 1999; Ibrahim and Wright, 1982; Van Scott and Ekel, 1958). The CTS is less accessible to experimental manipulation and its role in the intact follicle is more poorly defined. However, the proximal CTS shares properties with the DP that include the capacity to reform the dermal papilla in grafting studies (McElwee et al., 2003). DP cells undergo comparatively few divisions and the constituents of a DP are a largely static population when compared with the dynamic changes in the keratinocyte populations that abut them. Modest expansion and contraction of cell numbers in the DP occurs over the course of the hair cycle. Tobin and colleagues quantified these changes for the mouse hair cycle, reporting an increase in DP cell numbers during Anagen I–V and a decrease in Anagen VI- telogen (Tobin et al., 2003b). They noted that the increase in DP cell number observed in early anagen precedes detectable proliferation in the DP, and suggested that it results from migration of connective tissue sheath cells into the DP.
We have generated a mouse line, Cor-cre, that expresses cre recombinase in the DP (Enshell-Seijffers et al., 2010). When coupled with a cre-dependent reporter gene, this provides a method to trace the fate of DP cells. The reporter gene contains the sequences encoding Yellow Fluorescent Protein (YFP) in the ubiquitously expressed Rosa26 locus that are separated from a promoter by a transcriptional termination cassette flanked by LoxP sites (Srinivas et al., 2001). When this stop cassette is excised in cells expressing cre-recombinase, YFP is expressed in the cell and its progeny regardless of their position in the tissue or changes in expression of the cell type-specific cre recombinase allele. In Cor-cre/+; rYFP/+ mice, YFP is not detected until p3 and effective deletion across the DP population is not complete until p7 (Enshell-Seijffers et al., 2010). By the end of the anagen phase, virtually all DP cells express YFP, while the proximal CTS is variably labeled. Corin is not expressed in catagen or telogen, and although Corin expression returns during early anagen, cre recombinase is not reliably detected until anagen V (data not shown) (Enshell-Seijffers et al., 2008).
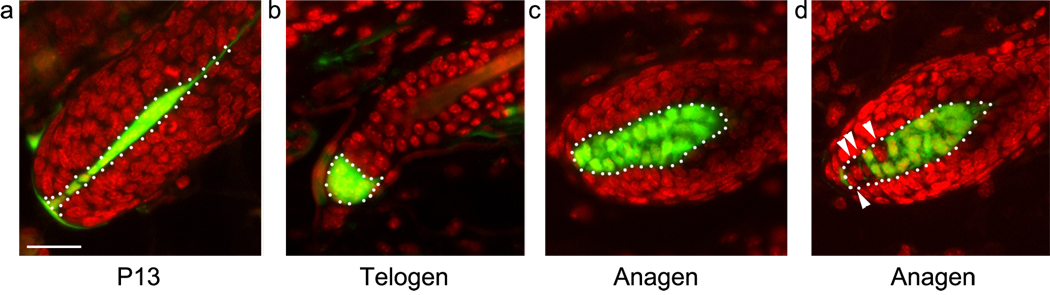
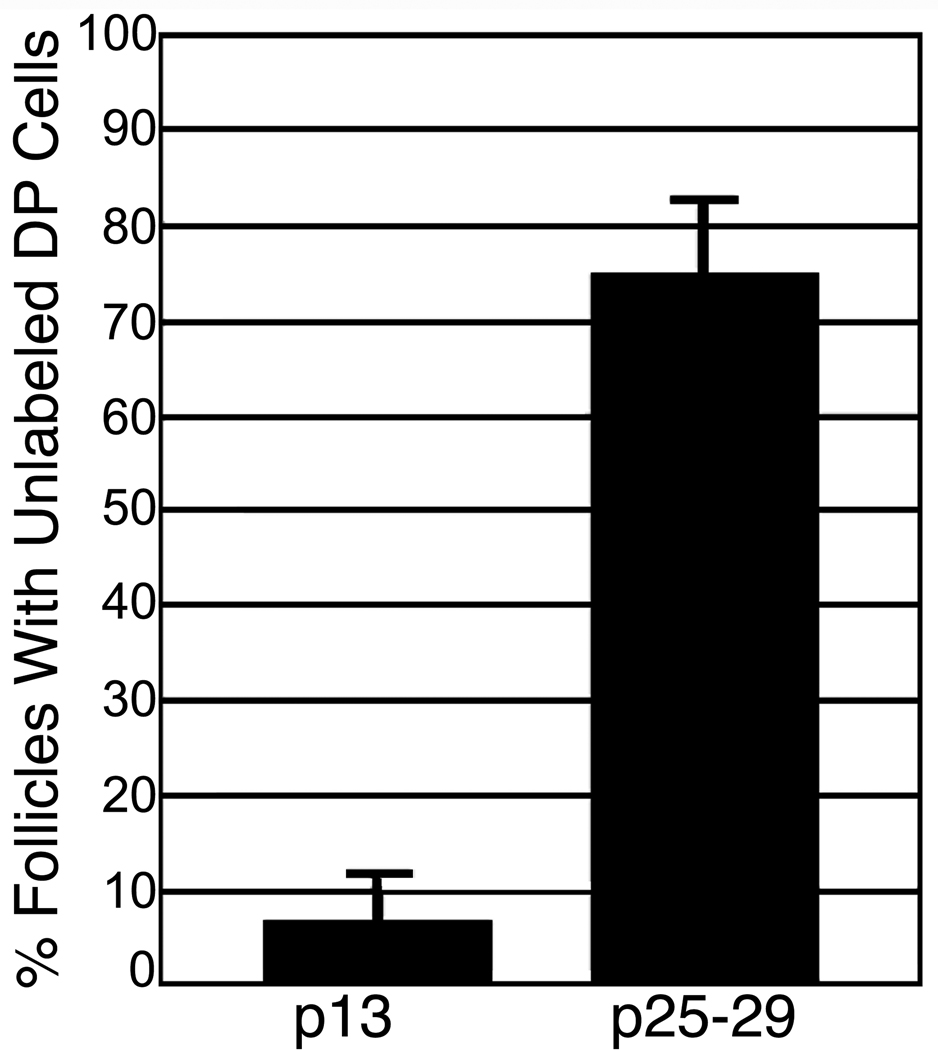
If all DP cells in a follicle are labeled with YFP at the end of one growth phase, the appearance of unlabelled cells in the subsequent anagen phase would provide evidence of recruitment of new cells to the DP. Mice of the genotype Cor-cre/+; rYFP/+ were sacrificed at p13 and the extent of labeling in the DP was determined in tissue sections (Fig. 1a). In a survey of follicles from 5 mice, no unlabelled DP cells were observed in 94 ±5% of the follicles scored (n=129) (Fig. 2). While most DP cells were labeled in the remaining follicles, one or two cells were unlabelled. During the catagen phase, a compact ball of YFP+ cells is associated with the regressing epithelial strand. In telogen, the descendents of the anagen DP form a compact ball of contiguous labeled cells, surrounded by more elongated cells that are variably labeled (Fig. 1b). The variable labeling of these peripheral cells is consistent with the assumption they derive from the CTS and confirms that most are not descendents of the dermal papilla. However, definitive distinction between DP and CTS derivatives at this stage is not possible.
Figure 1.
Recruitment of cells to the DP during the early anagen phase of the hair cycle. Representative hair bulbs from (a) post-natal day 13, (b) first telogen, and (c, d) first post-natal anagen are shown. While some DP are completely labeled in early anagen (c) unlabeled, newly recruited DP cells (arrowheads) are observed in most follicles (d). Nuclei are shown in red, YFP is shown in green, white dotted lines demarcate the DP. Scale bar = 25 microns.
Figure 2.
The frequency of DP with unlabelled cells at p13 and during first anagen (p25–29). Error bars represent S.D., n=5 mice per sample.
In contrast to p13, many unlabeled DP cells are observed during the early anagen phase of the first post-natal hair cycle (Fig. 1 c, d). Skins in the early anagen phase were harvested (n=5) and follicles in anagen stage III–IV were analyzed (Muller-Rover et al., 2001). Prior to stage III, distinction between DP and adjacent CTS is open to debate. At stage III, the DP is engulfed by the hair bulb thereby allowing unambiguous definition of the boundary between DP and CTS with a line between the keratinocytes at the base of the follicle. 75 % ± 8 of the follicles showed one or more DP cells that did not express YFP (n=256) (Fig. 2). To confirm that this was not a failure of the rYFP reporter transgene, mice in which rYFP had been activated in the germ line were examined. No unlabelled DP cells were observed (n= 68 follicles).
This data demonstrates either that new cells are recruited to the DP during early anagen, or that a rare unlabelled population of DP cells in each follicle escaped detection in the random section analysis at P13. To rule out this latter possibility, 10 follicles were optically sectioned at 1 micron intervals through the entire hair bulb. All of the DP cells were labeled in each of these follicles. Given the preponderance of follicles containing unlabelled DP cells in the first true anagen this result clearly demonstrates that these unlabelled cells do not arise from unlabelled DP cells persisting from the prior hair cycle (p<.0001). We therefore conclude that most follicles incorporate new cells into the DP during the early anagen phase.
Previous work had demonstrated that engrafted DP/CTS cells could be incorporated into the DP of existing follicles (Biernaskie et al., 2009; McElwee et al., 2003). Induction of anagen enhanced the efficiency with which engrafted SKPs, a multi-potent population that can be derived from DP in culture, are incorporated into existing hair follicles (Biernaskie et al., 2009). This suggests that the early anagen environment includes conditions conducive to recruitment of new cells to the DP. The results reported here demonstrate that new cells are incorporated into the DP during the early anagen phase of a natural hair cycle.
It has been suggested that emigration from the DP to the CTS and or dermis also occurs to achieve the reduction of DP cell numbers observed during anagen VI-telogen (Tobin et al., 2003b). Both Corin and cre recombinase are detected in the proximal CTS and sporadic labeling of the proximal CTS is observed during morphogenesis of the follicle as early as p4 (Enshell-Seijffers et al., 2010), well prior to the mature anagen stages when export of cells from the DP is postulated. As a result we cannot address the possibility of export to the CTS. If YFP expressing CTS cells are incorporated into the DP during early anagen, this would lead to an underestimate of the magnitude of cellular recruitment to the DP.
When the DP was viewed as a static lineage compartment, depletion of that restricted population could be invoked as the cause of reduction in follicle size. The observation of de novo generation of DP cells has important implications for the way we view the consistency of hair growth across hair cycles and its decay during aging or pathological hair loss (Tobin et al., 2003a). It emphasizes that regulation of DP size is an active process, and that conservation of hair size is not the passive result of a fixed DP compartment, nor is decay in follicle size an inevitable consequence of damage within this compartment. While it is reasonable to infer, based on close proximity and shared properties that the CTS is a likely source for new DP cells, identifying the pool of potential DP progenitors and the mechanisms that regulate DP cell number remain important steps towards improved management of hair loss.
Acknowledgements
The authors are grateful for expert technical assistance from E. Wu and B. Czyzewski. This work was supported by grants 5R01AR055256 and 5R01AR052339 from NIAMS to BAM. W.C was supported in part by training grant 5T32AR007098-35 from NIAMS.
Abbreviations used
- DP
Follicular dermal papilla
- CTS
Connective tissue sheath or dermal sheath
- YFP
Yellow Fluorescent Protein
- p
post-natal day
References
- Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J Invest Dermatol. 1999;113:873–877. doi: 10.1046/j.1523-1747.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Kashiwagi M, Lindon C, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Developmental Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Wright EA. Inductive capacity of irradiated dermal papillae. Nature. 1977;265:733–734. doi: 10.1038/265733a0. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Wright EA. A quantitative study of hair growth using mouse and rat vibrissal follicles. I. Dermal papilla volume determines hair volume. J Embryol Exp Morphol. 1982;72:209–224. [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101:584–590. doi: 10.1111/1523-1747.ep12366039. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003a;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Gunin A, Magerl M, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme during the hair growth cycle: implications for growth control and hair follicle transformations. J Investig Dermatol Symp Proc. 2003b;8:80–86. doi: 10.1046/j.1523-1747.2003.12177.x. [DOI] [PubMed] [Google Scholar]
- Van Scott EJ, Ekel TM. Geometric relationships between the matrix of the hair bulb and its dermal papilla in normal and alopecic scalp. J Invest Dermatol. 1958;31:281–287. doi: 10.1038/jid.1958.121. [DOI] [PubMed] [Google Scholar]