Abstract
Chronic allergic asthma is the result of a Th2- biased immune status. Current asthma therapies control symptoms in some patients, but a long lasting therapy has not been established. ASHMI™, a Chinese herbal formula improved symptoms and lung function, and reduced Th2 responses in a controlled trial of patients with persistent moderate to severe asthma.
We evaluated the persistence of ASHMI™ beneficial effects following therapy in a murine model of persistent asthma and the immunological mechanisms underlying such effects. BALB/c mice sensitized intraperitoneally with ovalbumin (OVA) received 3 weekly intratracheal OVA challenges to induce airway hyperreactivity (AHR) and inflammation (OVA mice). Additional OVA mice were treated with ASHMI™ (OVA/ASHMI™) or water (OVA/Sham) for 4 weeks, and then challenged immediately and eight weeks post-therapy. In other experiments OVA mice received ASHMI™ treatment with concomitant neutralization of IFN-γ or TGF-β. Effects on airway responses, cytokine and OVA-specific IgE levels were determined 8 weeks post-therapy.
Prior to treatment, OVA mice exhibited AHR and pulmonary eosinophilic inflammation following OVA challenge, which was almost completely resolved immediately after completing treatment with ASHMI™ and did not re-occur following OVA re-challenge up to 8 wks post-therapy. Reduced allergen-specific IgE and Th2 cytokine levels, and increased IFN-γ levels also persisted at least 8 wks post-therapy. ASHMI™ effects were eliminated by neutralization of IFN-γ, but not TGF-β, during therapy.
Conclusion
ASHMI™ induced long-lasting post-therapy tolerance to antigen-induced inflammation and AHR. IFN-γ is a critical factor in ASHMI™ effects.
Keywords: Allergic asthma, Mice, Traditional Chinese Medicine, Th-2 cytokines, Interferon-γ, IgE
INTRODUCTION
Allergic asthma is an increasingly common disease in western societies.[1] Standard therapy for asthma employing a combination of steroids and β2-adrenergic agonists control symptoms in most but not all asthmatics. [2;3] Relapse after therapy withdrawal is common, [4] and adverse effects of steroids on growth in children, steroid-induced immunosuppression and HPA-axis disturbances are of some concern. [5–7] Anti-IgE therapy is beneficial for some asthmatics, however its effects are transient and cysteinyl leukotriene inhibitors have shown only limited efficacy. [8;9] Recently, safety concerns regarding both of these therapies has been raised. [10;11] Thus, a safe, effective and long-lasting therapy remains to be established.
In recent years, a small, but increasing number, of well-controlled clinical trials provide evidence that some traditional Chinese medicines may have potential for development of asthma medications.[12] In preclinical studies we showed that the Anti-Asthma Simplified Herbal Medicine Intervention (ASHMI™), an extract of three Chinese medicinal herbs, exerted broad-based protective effects on asthma pathology and skewed Th2-dominant responses to Th1 in mice. We also found that Glycerrhiza uralensis, a key herbal component of ASHMI ™ reduced eotaxin production by human pulmonary fibroblasts. [13;14] In humans, ASHMI™ was effective in a controlled trial of subjects with moderate to severe asthma.[15] ASHMI™ suppressed Th2 cytokines while increasing IFN-γ. This beneficial immunomodulatory effect was also reported in children receiving ASHMI™ treatment as complementary therapy to inhaled steroid.[12] ASHMI™ is currently in clinical trials in the US and early reports indicate it is well-tolerated.[16] However, whether this treatment has a long lasting effect, and whether INF-γ or perhaps other cytokines such as IL-10 or TGF-β, are essential in ASHMI™-mediated clinical effect and Th2 suppression is unknown.
Using a well-established murine model of asthma, our current study seeks to test the persistence of ASHMI™- mediated protection from allergic asthma. Furthermore, we sought to determine the functional contribution of ASHMI™-induced elevation of IFN-γ to the persistent benefits of ASHMI™ therapy. We found that 4 week ASHMI™ treatment completely reversed asthmatic characteristics. ASHMI™-treated mice were tolerant to OVA challenges for at least 8 weeks post-therapy. Lung Th2 cytokine and OVA-specific IgE levels were also significantly lower, but IFN-γ levels was higher 8 weeks post-therapy. In additional experiments, mice were treated with concomitant neutralization of IFN-γ or TGF-β. ASHMI™-induced therapeutic effects were virtually eliminated by neutralization of IFN-γ, but not TGF-β during therapy, indicating that increased IFN-γ is essential to persistent benefits of ASHMI™ treatment in mice.
METHODS
ASHMI™ preparation and quality control
ASHMI™ is an extract of 3 TCM herbs-Ganoderma lucidum (Ling-Zhi), Sophora flavescens Ait (Ku-Shen), Glycyrrhiza uralensis Fischer (Gan-Gao). ASHMI™ was prepared by the Sino-Lion Pharmaceutical Company ( a GMP certified facility) Weifang, China) as described previously[16] .Composition, quality control and chemistry analysis have been described in extensive detail previously[16;17]. Endotoxin levels in ASHMI™ were tested using the Pyrogent Plus assay kit (Lonza, MA). No endotoxin was detected in ASHMI™ [<0.03 EU/ml, the limit of sensitivity for this kit].
Protocol for induction of asthma, ASHMI™ treatment and post therapy evaluation
Female 6-week- old BALB/c (Jackson Laboratory, ME) mice sensitized twice with weekly intraperitoneal (i.p.) injections of 200µg, ovalbumin (OVA, Type V; Sigma-Aldrich, , MO) and 2 mg of alum (Thermo Scientific, IL) in 0.4 ml of phosphate buffered saline (PBS), were challenged intratracheally (i.t.) with 100 µg OVA in 50 µl PBS weekly for 3 weeks (Fig 1A). Forty-eight hours after the third challenge, airway pressure time index (APTI) measurements and bronchoalveolar lavage were performed in a group of OVA mice compared to naïve mice (n=5 per group) Additional groups of OVA mice were given 9 mg ASHMI™ in 0.5 ml of drinking water twice daily (OVA/ASHMI™) or water (OVA/Sham) by oral gavage for 4 weeks. The ASHMI™ dose was determined by a conversion table of equivalent human to animal dose ratios based on body surface area.[18] Naïve mice served as normal controls. Post-therapy evaluations for AHR and airway inflammation were performed at 1 day, 4 weeks and 8 weeks post therapy on separate groups of mice (Fig 1A).
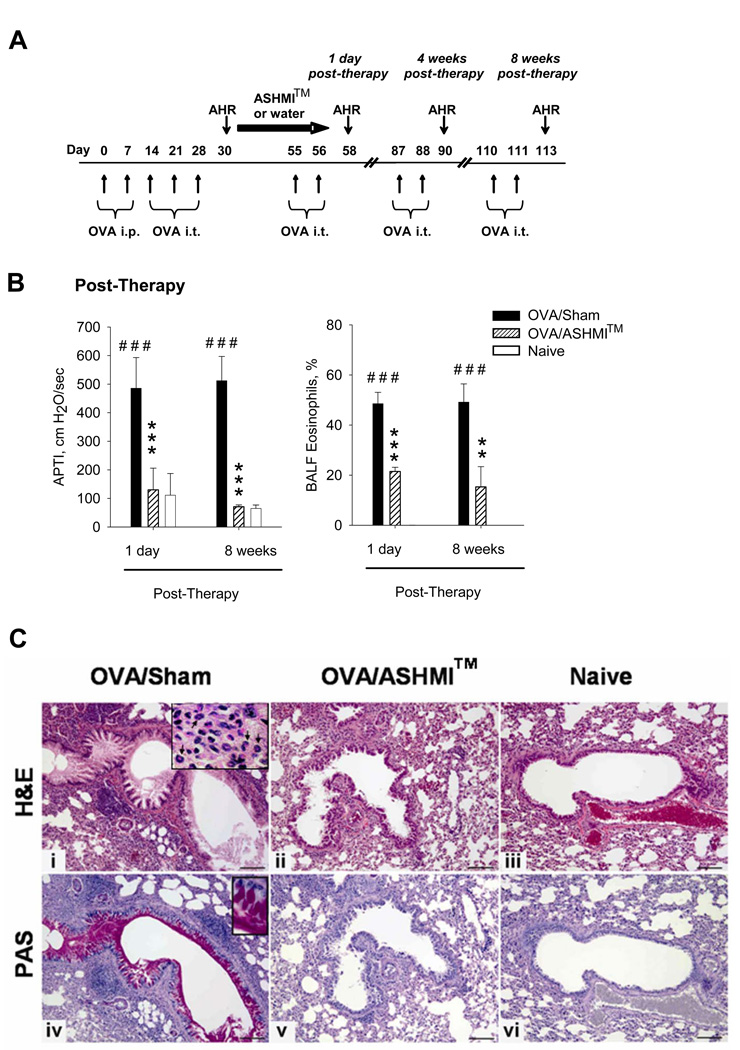
Figure 1.
A-Protocol: Mice were sensitized and challenged with OVA at times indicated. Daily oral ASHMI™ treatment lasted for 4 weeks. Mice were subsequently challenged 1 day-, 4- and 8 weeks post therapy.. B-AHR (left panel) and % of BAL eosinophils (right panel) were measured 48 hrs after 5th OVA challenge (day 58) and the 9th OVA challenge (day 113). Data shown as Mean ± SD in individual samples (N=4–5 mice/group). *, p<0.05;**, p<0.01; *** p<0.001 vs. sham; #, p<0.05; # # #, p<0.001 vs naïve. C, panels i-iii- H&E staining of lung sections from formalin fixed unlavaged left lung. Inset in panel ii shows eosinophils indicated by arrows. C, Panels iv-vi- and inset shows lung sections stained with PAS which stains mucin with a magenta color. Bar indicates 100µM.
Protocol for examining the role of IFN-γ or TGF-β in ASHMI™ long-lasting post-therapy effect
ASHMI™ was administered to OVA mice as above for 6 weeks in additional experiments (Fig 4A). Anti-IFN-γ ( clone H22, 100µg/week, i.p.) or Anti-TGF-β (clone ID11, 100µg/week, i.p.) antibodies (Abs), both purchased from R&D Systems, MN, or appropriate isotype control Abs (Hamster IgG1 for Anti-IFN-γ and Mouse IgG1 for Anti-TGF-β, 100 µg/week, BD Biosciences, CA) were administered i.p. to ASHMI™ or sham-treated mice, one day prior to treatment and then weekly for 6 weeks. All mice (except naïve mice) were re-challenged as above up to 8 weeks post-therapy. Forty-eight hours after the final OVA challenge, airway responses and cytokine profiles were determined in all mice.
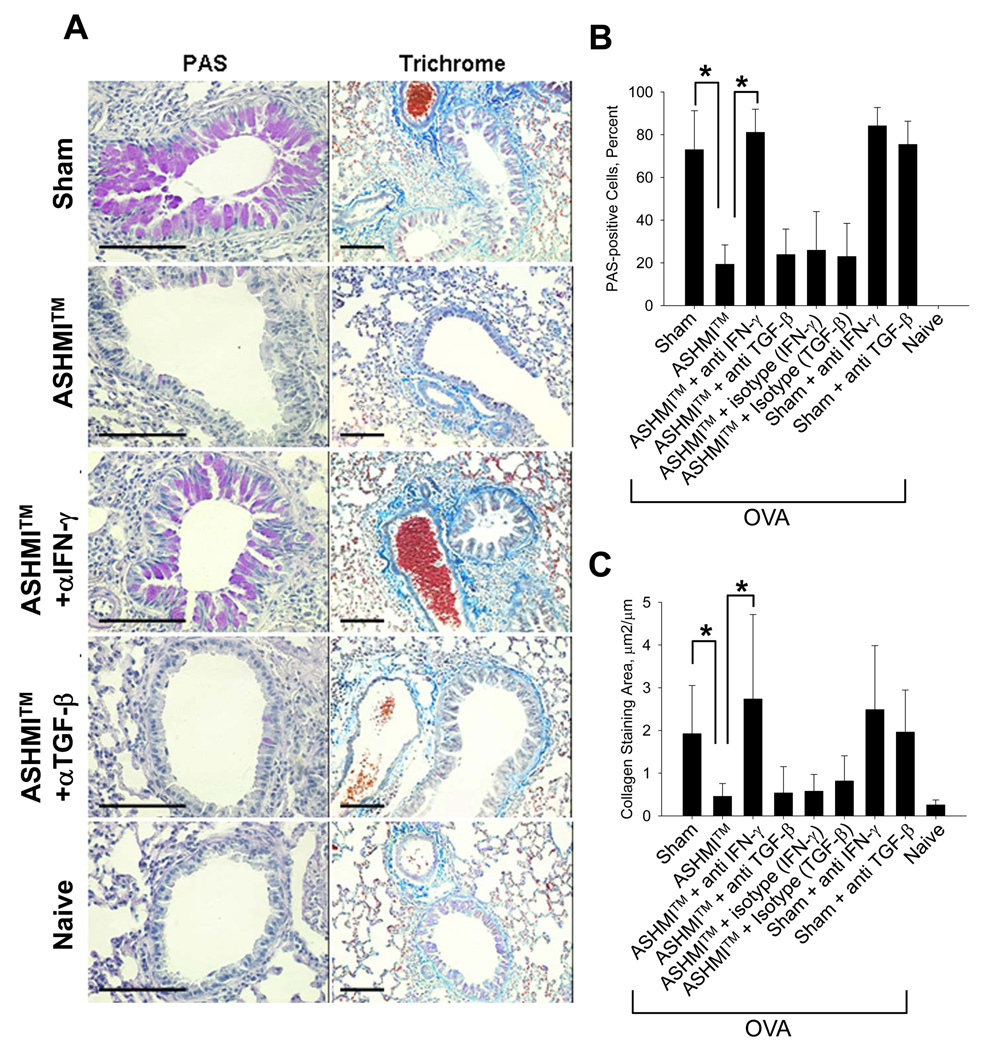
Figure 4.
A (Left panel)- PAS staining of sections of unlavaged lung. Mucin-positive cells stain magenta. 4B shows percent of PAS-positive cells/airway. 4A (Right Panel)- Trichrome staining of lung sections. Collagen stains turquoise blue. 4C shows area of peribronchial collagen staining. All data shown as Mean ± SD of 3–5 airways per animal (N=4/group) Bars indicate 100µM. *, P<0.05.
AHR measurement and bronchoalveolar lavage fluid cell differential counts
AHR to acetylcholine (ACh) provocation were measured following challenge at time-points indicated in Figure 1A and Figure 4A by measuring airway pressure time index (APTI), a classical invasive measure of AHR.[19;20] Mice were sacrificed immediately following APTI measurements and bronchoalveolar lavage fluid (BALF) and lung tissues were collected. Cytospin slides of BALF cells were stained with HEMA 3 stain (Fisher Scientific, NJ) for differential cell counting. [21]
Lung histology
Formalin-fixed lung samples were processed by the Mount Sinai Histology Shared Facilities. Sections were stained with hematoxylin and eosin (H&E) for evaluation of inflammation. Periodic acid Schiff (PAS) stain was used for assessment of mucus secretion and percentage of PAS-positive cells/bronchiole were calculated. 3–5 bronchioles were counted per slide (12–20/group). Collagen deposition was stained using trichrome staining, and peribronchial collagen deposition was analyzed by morphometric methods used previously. [22] The area of peribronchial trichrome staining was outlined and quantified on high-resolution digital images captured by an Olympus BX60 microscope and analyzed using Image-Pro Plus (Media Cybernetics, MD). Results are expressed as the area of trichrome staining per micron length of basement membrane of bronchioles. Three bronchioles per slide were analyzed (12/group).
Splenocyte culture and measurement of splenocyte and BALF cytokines
Following APTI measurements, splenocytes from each animal were prepared and BALF was collected. Splenocytes were cultured in the presence or absence of 50µg/ml OVA or 2.5 µg/ml Concanavalin A (Con A) (Data not shown) for 72 hrs.. Cytokine levels (IL-4, IL-5, IL-10, IL-13, IFN-γ and TGF-β) in Splenocyte or BALF supernatants were assayed by commercial ELISA kits according to the manufacturer’s instructions (R&D Systems, MN for IL-13 and TGF-β and BD Biosciences, San Diego, CA for all others) as previously described. [23]
Measurements of Ag-specific IgE levels
Serum was harvested from blood collected immediately after APTI measurements. OVA-specific IgE was measured as described previously.[20] Serum samples were evaluated using OVA coated-plates and detected using sheep anti-mouse IgE(The Binding Site, CA) followed by biotinylated donkey anti-sheep IgG (Accurate Chemical and Scientific Co., NY). Avidin-horseradish peroxidase and ABTS substrate were used for color development.
Statistical methods
Data were analyzed by SigmaStat 2.03 software (SPSS Inc., IL) using Two-way ANOVA for time course IgE data and One-way ANOVA for all other data , followed by pair-wise testing using Bonferroni’s adjustment. For data that failed tests for normality and equal variance, differences between groups were analyzed by Kruskal-Wallis One Way Analysis of Variance on Ranks followed by all pair-wise comparisons. p values ≤0.05 were considered significant.
RESULTS
ASHMI™ produced long-term post therapy suppression of allergic airway responses
ASHMI™ treatment commenced after the 3rd OVA challenge (Fig 1A). At this time asthmatic responses were fully established (Supplementary Figure 2). Immediately after completing treatment, OVA/sham mice showed sustained AHR following OVA re-challenge, evidenced by significantly higher APTI levels than naïve mice (p<0.001, Fig 1 B, left panel). In contrast, OVA/ASHMI™ mice showed complete resolution of AHR evidenced by normal APTI levels. Importantly, OVA/ASHMI™ mice showed no AHR in response to subsequent OVA re-challenges up to 8 wks post-therapy, whereas OVA/Sham mice showed sustained AHR. Consistently, immediately after completing treatment, eosinophil numbers were significantly reduced in OVA/ASHMI™ mice as compared to the OVA/Sham mice (P<0.01–0.001, Fig 1B, right panel), and similar results were observed 8 weeks post-therapy 48 hours following the final antigen challenge (P<0.01–0.001 vs sham).
Histological analysis also showed near normal features in OVA/ASHMI™ mice, similar to naïve mice immediately (data not shown) and 8 weeks post-therapy (Fig 1C). Consistent with the BAL data, OVA/Sham mice exhibited pronounced peribronchial inflammation containing numerous eosinophils (Fig 1 C, panel i and indicated by arrows in inset). As illustrated in Fig 1 C panel ii, inflammation was dramatically reduced in the lungs from OVA/ASHMI™ mice. Numerous goblet cells were present in airways of OVA/sham mice as shown by PAS-positive staining (Fig 1C, panel iv and inset). Goblet cells were almost absent in airways of OVA/ASHMI™ mice (Fig 1C, panel v), which were similar in appearance to those of naïve mice (Fig 1 C, panel vi). Taken together these findings show that ASHMI™ was effective in reversing established allergic airway changes in an established asthma model, and that this effect lasted at least 8 weeks post-therapy during which time 6 OVA re-challenges were administered.
ASHMI™ induced persistent suppression of Th-2 immune responses
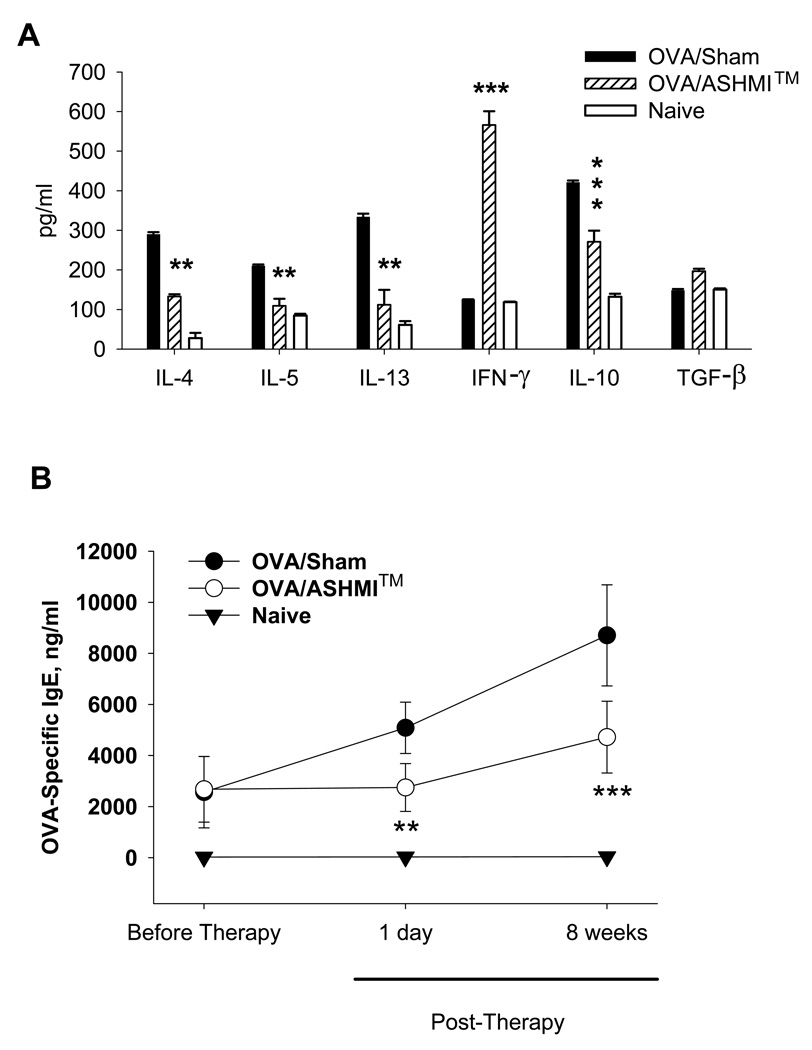
Th-2 cytokines, IL-4, IL-5 and IL-13 were significantly lower in BALF from OVA/ASHMI™ mice, compared to BAL from OVA/Sham mice at 8 weeks post-therapy (P<0.01 to P<0.001, Fig 2 A). However, the Th-1 cytokine IFN-γ was markedly elevated in BALF from OVA/ASHMI™ mice (P<0.001 vs Sham). In addition, IL-10 production was significantly reduced in BALF (P<0.001 vs sham). TGF-β levels were slightly but not significant increased in BALF from OVA/ASHMI™ mice compared to BAL from OVA/Sham mice. These results were similar to those observed immediately following challenge (data not shown), and demonstrate that the therapeutic effect of ASHMI™ was associated with persistent, specific suppression of Th-2 responses and increased IFN-γ levels.
Figure 2.
A-Cytokines in BALF harvested 48 hours after the final set of OVA challenges were determined by ELISA. B- OVA-specific IgE in serum that was collected from mice one day prior to ASHMI™ therapy, 4-weeks and 8-weeks after stopping ASHMI™ therapy was measured by ELISA. Results are expressed as Mean ± SD of pooled samples in triplicate for cytokines and individual samples for IgE. (n=4–5/group). Results were replicated at least once in a separate experiment. **, p<0.01 and ***p<0.001 vs. sham.
In addition, elevated OVA-specific IgE levels of OVA mice were significantly lower in OVA/ASHMI™ mice than OVA/sham mice immediately after completing the treatment (p<0.001). Following the stoppage of treatment, a trend towards increased IgE was observed in both Sham and ASHMI™-treated mice likely due to additional challenges. However at all post-therapy time-points, OVA-specific IgE levels were in significantly reduced in OVA/ASHMI™-treated, compared to OVA/Sham mice (P<0.001, Fig 2 B).
ASHMI™ protection against AHR and inflammation was eliminated by IFN-γ, but not TGF-β neutralization
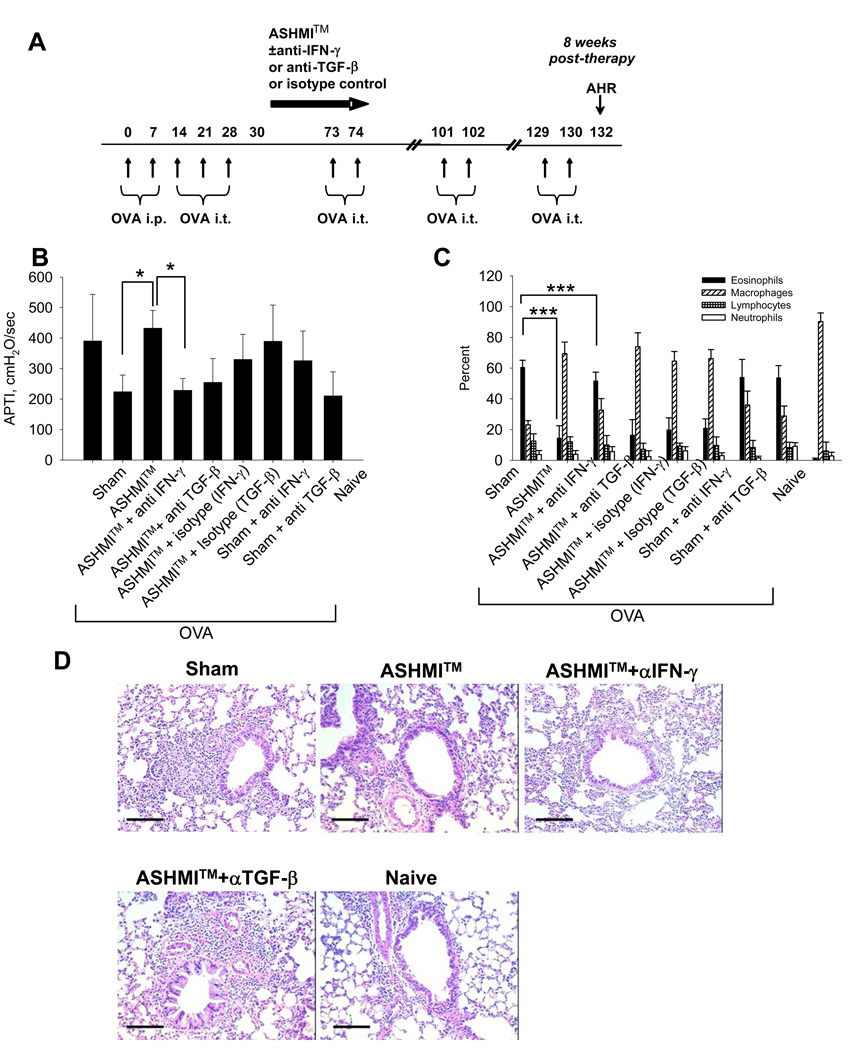
Using an antibody neutralization protocol described in Fig 3A, ASHMI™ suppression of AHR was abolished with co-administration of anti-IFN-γ but not isotope control Abs during treatment (Fig 3 B ASHMI™ vs ASHMI™ +anti-IFN-γ, p<0.05) when evaluated 8 weeks post-therapy. ASHMI™ suppression of AHR was not affected by anti-TGF-β or isotype control Abs. Administration of anti-IFN-γ or anti-TGF-β Abs during treatment had no effect on APTI of OVA-sham mice. Similarly, ASHMI™ suppression of eosinophilic inflammation was eliminated by co-administration of anti IFN-γ Abs (Fig 3 C, ASHMI™ vs ASHMI™ +anti-IFN-γ, p<0.001), but not isotype control Abs. ASHMI™ suppression of eosinophils was not affected by anti-TGF-β or isotype control Abs. Administration of anti-IFN-γ or anti-TGF-β Abs during treatment had no effect on the percentage of eosinophils in the BAL of OVA/sham mice. Histological analysis also showed that ASHMI™ + anti IFN-γ Abs, but not ASHMI™ + anti-TGF-β Abs restored peribronchial inflammatory infiltration, similar to the OVA/sham (Fig 3 D).
Figure 3.
A- Mice were sensitized and challenged at times indicated. 6 weeks of daily oral ASHMI™ was given with or without neutralizing IFN-γ, TGF-β or isotype control antibodies. Neutralizing antibodies were also given to groups of sham treated mice. Responses were assessed 8 weeks post-therapy. B- AHR measured 48 hours after last OVA challenge. Data shown as Mean ± SD of individual APTI values (n=4). C-Percentages of inflammatory cells in BALF counted after differential staining. Data shown as Mean ± SD of percentages in individual samples. D-H&E staining of sections of unlavaged lung (n=4). Bars indicate 100µM. #, p<0.05; ###, p<0.001 vs Naïve. *, p<0.05; ***p<0.001 vs. Sham. +, p<0.05;+++, P<0.001 vs Naïve.
IFN-γ neutralization abrogates ASHMI™-induced reduction of mucus cell generation and collagen deposition
Mucus-positive goblet cells (stained by PAS) were abundant in airways of sham treated OVA mice but dramatically reduced in those of ASHMI™-treated OVA mice (Fig 4 A Left panel and Fig 4 B p<0.05 vs Sham). The number of goblet cells in ASHMI™-treated mice given IFN-γ neutralizing antibodies was similar to that of sham-treated animals and significantly higher than ASHMI™ only-treated (Fig 4 A Left panel and Fig 4 B, p<0.05 vs ASHMI™ alone). Also, we observed strong positive peribronchial and perivascular staining for collagen in lung sections of sham-treated mice (Fig 4, Right panel). This was greatly reduced in ASHMI™-treated mice (P<0.05 vs Sham, Fig 4 C) but not in ASHMI™-treated, IFN-γ neutralized animals (P<0.05 vs ASHMI™ alone). TGF-β neutralization or isotype control antibodies did not alter ASHMI™ effect on mucus hyperplasia or collagen deposition.
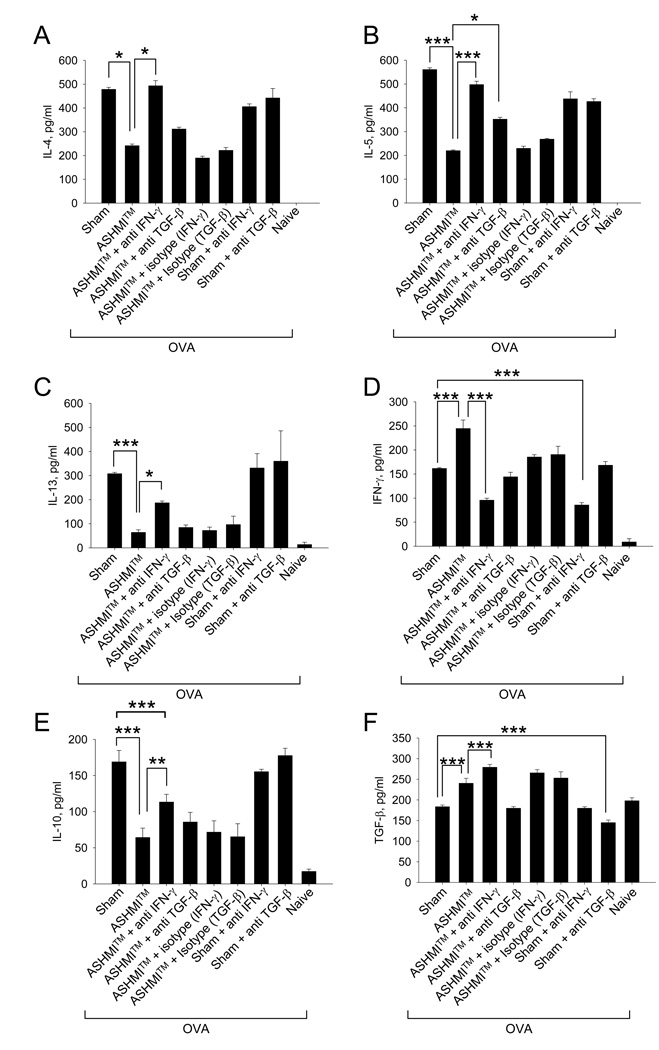
ASHMI™ suppression of Th2 cytokines is largely IFN-γ dependent
BALF IL-4, IL-5, IL-13 and IL-10 levels from ASHMI™ treated OVA mice (second bars) were significantly lower than those from sham-treated mice (first bars) (P<0.05- P<0.001, Fig 5 A, B, C, and E). IFN-γ and TGF-β levels were higher (p<0.001 and 0.01 respectively, Fig 5 D and F). Neutralization of IFN-γ (third bars) completely attenuated ASHMI™ reduction of IL-4, IL-5 (p<0.05-–P<0.001vs ASHMI™ alone) where levels of these cytokines were similar to those observed in the Sham group. ASHMI suppression of IL-10 and IL-13 was dramatically but not completely reversed by IFN-γ neutralization since IL-10 and IL-13 levels in this group were still lower than Sham (P<0.001 for IL-10 and P=0.055 for IL-13, ASHMI+Anti-IFN-γ vs Sham). As expected ASHMI+ anti-IFN-γ treatment reduced IFN-γ (p<0.001), but it had no effect on TGF-β, implying that ASHMI™ –mediated reduction in Th2 cytokines is largely IFN-γ -dependent. TGF-β neutralization had minimal effect on ASHMI™ reduction of IL-4 and IL-13, but significantly increased IL-5 levels (P<0.05 ASHMI™ + anti TGF-β Ab vs ASHMI™). Anti-IFN-γ or TGF-β isotype control Abs did not affect ASHMI™ effects. In addition, anti-IFN-γ and TGF-β Abs had no effects on cytokine production in the sham-treated OVA mice. This might be attributable to anti-IFN-γ Abs being administered when Th2-mediated allergic airway responses were already established, and IFN-γ levels were negligible. These results were similar to those observed in splenocyte culture supernatants to recall OVA-stimulation 8 weeks post-therapy (Supplementary figure 3).
Figure 5.
BALF supernatants were pooled for each group of mice and cytokine levels at eight weeks post-ASHMI™ therapy were determined by ELISA (A)-IL-4, (B)-IL-5, (C)-IL-13, (D)-IFN-γ, (E)-IL-10, (F)-TGF-β. Results are expressed as means ± SD of pooled samples measured in triplicate. (N=4 mice/group). *, P<0.05 ; **, P<0.01; ***p<0.001
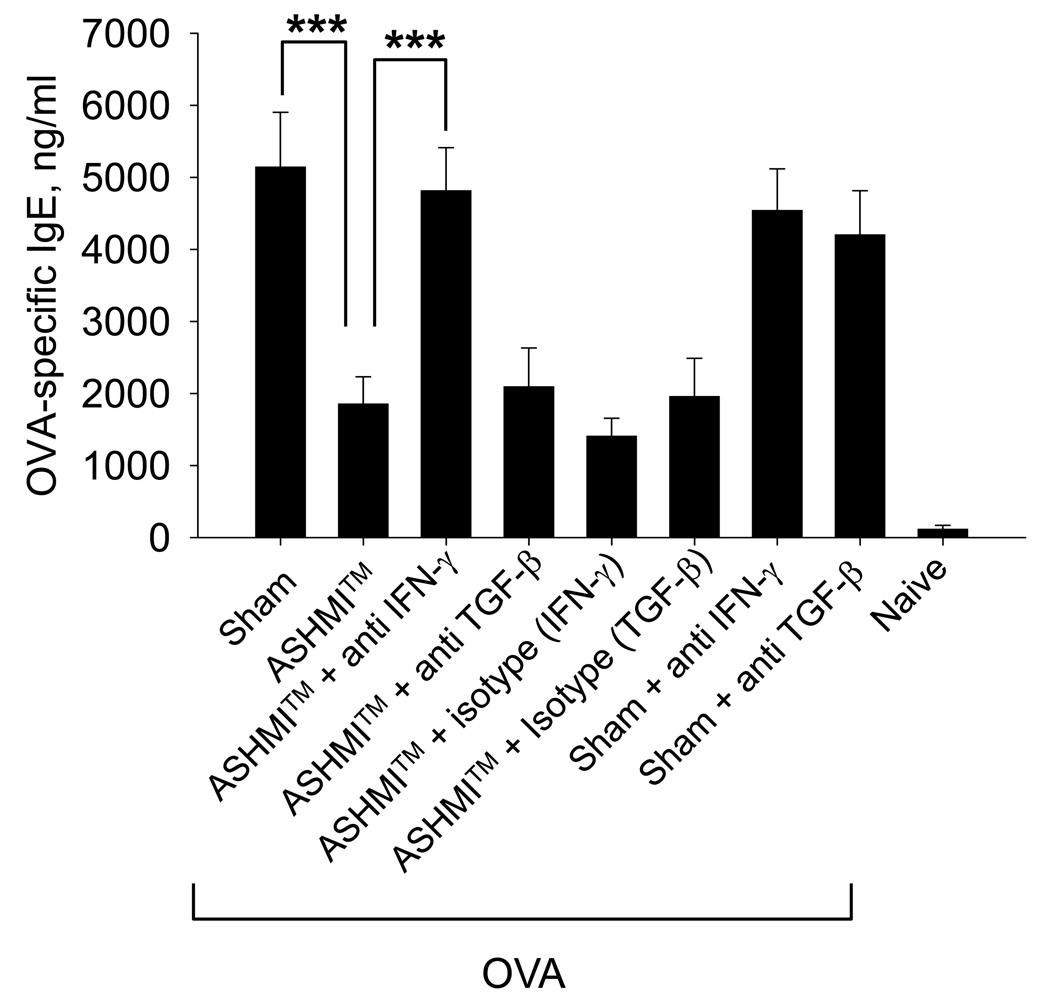
ASHMI™ persistently suppressed OVA-specific IgE production is in an IFN-γ-dependent manner
OVA-specific IgE levels in ASHMI™-treated mice were greatly reduced 8 weeks post-therapy, compared to sham treated OVA mice (P<0.001 vs Sham, Fig 6). Neutralization of IFN-γ abrogated ASHMI™-mediated IgE suppression (P<0.001 vs ASHMI™ alone, Fig 6). Neutralization of TGF-β or administration of isotype control antibodies had no effect. IFN-γ or TGF-β neutralization did not affect IgE levels in OVA/sham mice.
Figure 6.
Serum harvested from mice at the time of AHR evaluation was assayed for OVA-specific IgE by ELISA. Data are shown as Mean ± SD of individual samples measured in duplicate. N=4 mice/group. ***, P<0.001
DISCUSSION
Growing concern over the increased incidence and prevalence of asthma in westernized countries has intensified attempts to find new drug therapies. We report here that, in a murine model of chronic allergic asthma, treatment with the herbal preparation, ASHMI™, eliminated AHR, and reduced pulmonary inflammation and airway remodeling in animals with established asthmatic responses. Therapeutic effects were associated with suppression of specific-IgE and Th2 cytokines well known to play key roles in the pathology of allergic asthma. Importantly, ASHMI™ benefits persisted for at least 8 weeks after treatment was discontinued. Such persistent effects are in marked contrast to steroid withdrawal which is often accompanied by asthmatic exacerbation and amplified airway inflammation less than 4–6 weeks after discontinuing treatment.[24;25] Sustained suppression of IgE levels post-ASHMI™ therapy, despite additional OVA challenges, suggests that this therapy may be superior to anti-IgE therapy, in which free-IgE increased as early as 2 weeks after discontinuation of treatment [8] It is a common practice in preclinical drug development to treat animals before disease establishment which is of limited use regarding treatment of human asthma. In this study, allergic airway responses were established before initiating treatment, thereby mimicking human asthma therapy. Furthermore, as in other previously described chronic models of asthma, [26;27] OVA mice received repetitive antigen challenges, and by week 8 post-therapy, mice had received 6 sets of challenges over ∼100 days. If airway tolerance is defined by the loss of induction of AHR to antigen exposure, all ASHMI™-treated mice, but no sham-treated mice, developed tolerance, which persisted up to 8 weeks post-therapy. Although, animal models of asthma are not identical to human asthma, this study suggests that clinical investigation of potential long term ASHMI™ effects in asthma patients is warranted.
The findings of the present study that ASHMI™ significantly up-regulated IFN-γ production, slightly reduced IL-10 production, and only modestly increased TGF-β production suggest that ASHMI™ efficacy is attributable to shifting a Th-2-mediated allergic immune response towards a Th-1 response, thereby distinguishing it from steroid therapy where in similar murine models IFN-γ was reduced in dexamethasone –treated mice. [28;29] To confirm that IFN-γ functionally contributes to ASHMI™ effects we gave ASHMI™ concomitantly with IFN-γ neutralizing antibodies. This study also used TGF-β neutralizing antibodies to rule out a cell-cell contact mechanism of TGF-β function, determinable by measuring TGF-β levels. Anti IFN-γ Ab administered during treatment, completely abrogated ASHMI™- reductions of systemic Th2 cytokines (supplementary figure 3), AHR, OVA-specific IgE, pulmonary inflammation and airway remodeling. Neutralizing INF-γ dramatically but not completely reversed ASHMI-induced reductions in BALF IL-13 and IL-10, implying that yet undetermined IFN-γ independent mechanisms acting locally may be contributing to ASHMI effects and warrant further investigation. We have yet to identify the cellular source of ASHMI™-induced increased IFN-γ secretion in our study, however CD8+ T cells and iNKT cells are attractive targets for investigation based on work by us and others.[30–33]. We previously demonstrated that ASHMI™ and its parent formula MSSM-002 inhibited GATA-3 expression and activity in T cells.[17;34] This may work to decrease the GATA-3/T-bet ratio in these cells leading to upregulated IFN-γ production as shown in previous reports.[35;36] A role for regulation of T-cell /iNKT cell IFN-γ output by macrophages and dendritic cells also warrants investigation since Ganoderma lucidum, a major component of ASHMI™ has been shown to be a potent modulator of macrophage and dendritic cell responses.[37–39]
Interestingly neutralization of TGF-β during treatment although moderately decreasing ASHMI™-suppression of IL-5, did not decrease therapeutic effects. The role of IL-10/TGF-β in chronic asthma remains controversial, [40;41]. A recent study by Goplen et al in a murine model of asthma induced by combined sensitization of mice dust mite, ragweed and Aspergillus, closely mimicking chronic asthma features in humans, showed increased IL-10 and TGF-β in chronic asthmatic mice. [42] Our findings showing suppression of IL-10 by ASHMI™ and lack of effect of TGF-β neutralization on ASHMI™ effects suggest that long-term therapeutic effects of ASHMI™ were not due to Treg activity. That IFN-γ elevation underlies ASHMI™ effects is not surprising. Similar immune deviation has been implicated in previous work in which established asthmatic responses were improved by strategies such as CD137 ligation and allergen vaccination with bacterial adjuvants.[43–46] The protective role of IFN-γ in the allergic setting is well known. Mice with defective IFN-γ production show elevated AHR and specific-IgE.[32] In humans, low IFN-γ levels in early life was found to be a predictor of asthma.[47;48] It must be noted however, that clinical trials of asthma therapies that increased IFN-γ levels have yielded mixed results. Nebulized IFN-γ was shown not to benefit asthmatics and 1018 ISS, a CpG-ODN therapy despite elevating IFN-γ responses failed to show symptomatic improvement, though concerns regarding effective dose were cited.[49;50] In contrast , BCG vaccinations, associated with elevated IFN-γ were shown to improve lung function and inhibit Th2 responses in moderate-to-severe asthmatics . [51] While we have demonstrated that ASHMI™ therapy in humans is beneficial and elevates IFN-γ, the precise contribution of this elevation in humans is unknown as depletion of the cytokine during human ASHMI™ therapy cannot be undertaken. Other mechanisms such as direct suppression of Th2 cells, [52] chemokine production[53] and modulation of airway smooth muscle contractility[54] may also contribute to clinical benefit of ASHMI™ and require further investigation.
In conclusion, the herbal formula ASHMI™ protected allergic mice against AHR, airway inflammation and remodeling for 8 weeks post-therapy. Since AHR remained in complete remission following OVA challenges for 8 weeks post-therapy, research is underway to determine how long the effect persists to subsequent allergy challenges, and identify the active components in ASHMI™ responsible for its benefits on asthma. ASHMI™ may be a promising option for human asthma therapy as it provides persistent protection without global immune suppression.
Supplementary Material
Acknowledgments
Funding source: This work was supported by the National Institutes of Health grants # PO1 AT002647 to XM Li
Abbreviations
- AHR
airway hyperreactivity
- APTI
airway pressure time index
- ASHMI™
anti-asthma herbal medicine intervention
- BALF
bronchoalveolar lavage fluid
- CA
conalbumin
- CS
Corticosteroid
- i.p.
intraperitoneally
- i.t.
intratracheally
- OVA
ovalbumin
- OVA mice
OVA sensitized and challenged mice
- PBS
phosphate buffered saline
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130 doi: 10.1378/chest.130.1_suppl.4S. 4S–12S. [DOI] [PubMed] [Google Scholar]
- 2.Lemanske RFJ. A review of the current guidelines for allergic rhinitis and asthma. J Allergy Clin Immunol. 1998;101:S392–S396. doi: 10.1016/s0091-6749(98)70228-3. [DOI] [PubMed] [Google Scholar]
- 3.Hall IP. The beta-agonist controversy revisited. Lancet. 2004;363:183–184. doi: 10.1016/S0140-6736(03)15369-X. [DOI] [PubMed] [Google Scholar]
- 4.Sheth A, Reddymasu S, Jackson R. Worsening of Asthma with Systemic Corticosteroids. J Gen Intern Med. 2005 doi: 10.1111/j.1525-1497.2005.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF, Jr, Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 6.Aun MV, Ribeiro MR, Costa Garcia CL, Agondi RC, Kalil J, Giavina-Bianchi P. Esophageal candidiasis--an adverse effect of inhaled corticosteroids therapy. J Asthma. 2009;46:399–401. doi: 10.1080/02770900902777783. [DOI] [PubMed] [Google Scholar]
- 7.Zora JA, Zimmerman D, Carey TL, O'Connell EJ, Yunginger JW. Hypothalamic-pituitary-adrenal axis suppression after short-term, high-dose glucocorticoid therapy in children with asthma. J Allergy Clin Immunol. 1986;77:9–13. doi: 10.1016/0091-6749(86)90315-5. [DOI] [PubMed] [Google Scholar]
- 8.Saini SS, MacGlashan DW, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, Bochner BS. Down-regulation of human basophil IgE and FC epsilon RI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 9.Papadopoulos NG, Philip G, Giezek H, Watkins M, Smugar SS, Polos PG. The efficacy of montelukast during the allergy season in pediatric patients with persistent asthma and seasonal aeroallergen sensitivity. J Asthma. 2009;46:413–420. doi: 10.1080/02770900902847727. [DOI] [PubMed] [Google Scholar]
- 10.FDA.Consum. 9 A.D. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm172218.htm. Ref Type: Magazine Article.
- 11.Holbrook JT, Harik-Khan R. Montelukast and emotional well-being as a marker for depression: results from 3 randomized, double-masked clinical trials. J Allergy Clin Immunol. 2008;122:828–829. doi: 10.1016/j.jaci.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XM. Complementary and alternative medicine in pediatric allergic disorders. Curr Opin Allergy Clin Immunol. 2009 doi: 10.1097/ACI.0b013e328329226f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang T, Srivastava K, Wen MC, Yang N, Cao J, Busse P, Birmingham N, Goldfarb J, Li XM. Pharmacology and immunological actions of a herbal medicine ASHMI(TM) on allergic asthma. Phytother Res. 2009 doi: 10.1002/ptr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57:820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, Mu DZ, Du JB, Li GH, Wallenstein S, Sampson H, Kattan M, Li XM. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116:517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Wisnivesky J, Li X-M, Kattan M. Safety and tolerability of an antiasthma herbal formula (ASHMI™) in adult asthmatics: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. Journal of Alternative and complementary Medicine. 2009;15:735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Srivastava K, Wen M-C, Yang N, Busse P, Birmingham N, Goldfarb J, Li X-M. Pharmacology and immunological actions of an herbal medicine, ASHMI™ for allergicasthma. Phytotherapy Research. 2009 doi: 10.1002/ptr.3077. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiu SY. The Experimental Method of Pharmacology. Beijing: The People's Public Health Publisher. 1986 [Google Scholar]
- 19.Li XM, Huang CK, Zhang TF, Teper AA, Srivastava K, Schofield BH, Sampson HA. The chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol. 2000;106:660–668. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- 20.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37:1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XM, Schofield BH, Wang QF, Kim KH, Huang SK. Induction of pulmonary allergic responses by antigen-specific Th2 cells. J Immunol. 1998;160:1378–1384. [PubMed] [Google Scholar]
- 22.Lim DH, Cho JY, Song DJ, Lee SY, Miller M, Broide DH. PI3K gamma-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling1. Am J Physiol Lung Cell Mol Physiol. 2009;296:L210–L219. doi: 10.1152/ajplung.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava K, Teper AA, Zhang TF, Li S, Walsh MJ, Huang CK, Kattan M, Schofield BH, Sampson HA, Li XM. Immunomodulatory effect of the anti-asthma Chinese herbal formula, MSSM-002 on Th2 cells. J Allergy Clin Immunol. 2004;113:268–276. doi: 10.1016/j.jaci.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 24.Castro M, Bloch SR, Jenkerson MV, DeMartino S, Hamilos DL, Cochran RB, Zhang XE, Wang H, Bradley JP, Schechtman KB, Holtzman MJ. Asthma exacerbations after glucocorticoid withdrawal reflects T cell recruitment to the airway. Am J Respir Crit Care Med. 2004;169:842–849. doi: 10.1164/rccm.200208-960OC. [DOI] [PubMed] [Google Scholar]
- 25.Wiley RE, Cwiartka M, Alvarez D, Mackenzie DC, Johnson JR, Goncharova S, Lundblad L, Jordana M. Transient corticosteroid treatment permanently amplifies the Th2 response in a murine model of asthma. J Immunol. 2004;172:4995–5005. doi: 10.4049/jimmunol.172.8.4995. [DOI] [PubMed] [Google Scholar]
- 26.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epstein MM. Do mouse models of allergic asthma mimic clinical disease? Int Arch Allergy Immunol. 2004;133:84–100. doi: 10.1159/000076131. [DOI] [PubMed] [Google Scholar]
- 28.Mathur M, Herrmann K, Li X, Qin Y, Weinstock J, Elliott D, Monahan J, Padrid P. TRFK-5 reverses established airway eosinophilia but not established hyperresponsiveness in a murine model of chronic asthma. Am J Respir Crit Care Med. 1999;159:580–587. doi: 10.1164/ajrccm.159.2.9712018. [DOI] [PubMed] [Google Scholar]
- 29.Rogerio AP, Kanashiro A, Fontanari C, da Silva EV, Lucisano-Valim YM, Soares EG, Faccioli LH. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma1. Inflamm Res. 2007;56:402–408. doi: 10.1007/s00011-007-7005-6. [DOI] [PubMed] [Google Scholar]
- 30.Huang TJ, MacAry PA, Kemeny DM, Chung KF. Effect of CD8+ T-cell depletion on bronchial hyper-responsiveness and inflammation in sensitized and allergen-exposed Brown-Norway rats. Immunology. 1999;96:416–423. doi: 10.1046/j.1365-2567.1999.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123:443–451. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 32.Karwot R, Maxeiner JH, Schmitt S, Scholtes P, Hausding M, Lehr HA, Glimcher LH, Finotto S. Protective role of nuclear factor of activated T cells 2 in CD8+ long-lived memory T cells in an allergy model. J Allergy Clin Immunol. 2008;121:992–999. doi: 10.1016/j.jaci.2007.12.1172. [DOI] [PubMed] [Google Scholar]
- 33.Hachem P, Lisbonne M, Michel ML, Diem S, Roongapinun S, Lefort J, Marchal G, Herbelin A, Askenase PW, Dy M, Leite-de-Moraes MC. Alpha-galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-gamma. Eur J Immunol. 2005;35:2793–2802. doi: 10.1002/eji.200535268. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava Kamal, Teper Ariel A, Zhang Teng-Fei, Li Side, Walsh Martin J, Huang Chih-Kang, Kattan Meyer, Schofield Brian H, Sampson Hugh A, Li Xiu-Min. Immunomodulatory effect of the anti-asthma Chinese herbal formula, MSSM-002 on Th2 cells. J Allergy Clin Immunol. 2003 doi: 10.1016/j.jaci.2003.10.062. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- 35.Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. J Immunol Methods. 2003;278:157–169. doi: 10.1016/s0022-1759(03)00200-x. [DOI] [PubMed] [Google Scholar]
- 36.ayre-Orthez C, Becker J, Auwerx J, Frossard N, Pons F. Suppression of allergen-induced airway inflammation and immune response by the peroxisome proliferator-activated receptor-alpha agonist fenofibrate. Eur J Pharmacol. 2008;581:177–184. doi: 10.1016/j.ejphar.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 37.Chang YH, Yang JS, Yang JL, Wu CL, Chang SJ, Lu KW, Kuo CL, Hsia TC, Chung JG. Gandoderma lucidum extract promotes immune responses in normal BALB/c mice In vivo. In Vivo. 2009;23:755–759. [PubMed] [Google Scholar]
- 38.Lin YL, Liang YC, Lee SS, Chiang BL. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J Leukoc Biol. 2005;78:533–543. doi: 10.1189/jlb.0804481. [DOI] [PubMed] [Google Scholar]
- 39.Lin ZB. Cellular and molecular mechanisms of immuno-modulation by Ganoderma lucidum. J Pharmacol Sci. 2005;99:144–153. doi: 10.1254/jphs.crj05008x. [DOI] [PubMed] [Google Scholar]
- 40.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–624. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salib RJ, Howarth PH. Transforming growth factor-beta in allergic inflammatory disease of the upper airways: friend or foe? Clin Exp Allergy. 2009;39:1128–1135. doi: 10.1111/j.1365-2222.2009.03239.x. [DOI] [PubMed] [Google Scholar]
- 42.Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J Allergy Clin Immunol. 2009;123:925–932. doi: 10.1016/j.jaci.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polte T, Jagemann A, Foell J, Mittler RS, Hansen G. CD137 ligand prevents the development of T-helper type 2 cell-mediated allergic asthma by interferon-gamma-producing CD8+ T cells. Clin Exp Allergy. 2007;37:1374–1385. doi: 10.1111/j.1365-2222.2007.02785.x. [DOI] [PubMed] [Google Scholar]
- 44.Polte T, Foell J, Werner C, Hoymann HG, Braun A, Burdach S, Mittler RS, Hansen G. CD137-mediated immunotherapy for allergic asthma. J Clin Invest. 2006;116:1025–1036. doi: 10.1172/JCI23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniel C, Repa A, Wild C, Pollak A, Pot B, Breiteneder H, Wiedermann U, Mercenier A. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy. 2006;61:812–819. doi: 10.1111/j.1398-9995.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 46.Korf JE, Pynaert G, Tournoy K, Boonefaes T, Van OA, Ginneberge D, Haegeman A, Verschoor JA, De BP, Grooten J. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am J Respir Crit Care Med. 2006;174:152–160. doi: 10.1164/rccm.200507-1175OC. [DOI] [PubMed] [Google Scholar]
- 47.Guerra S, Lohman IC, Halonen M, Martinez FD, Wright AL. Reduced interferon gamma production and soluble CD14 levels in early life predict recurrent wheezing by 1 year of age. Am J Respir Crit Care Med. 2004;169:70–76. doi: 10.1164/rccm.200304-499OC. [DOI] [PubMed] [Google Scholar]
- 48.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–841. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Boguniewicz M, Martin RJ, Martin D, Gibson U, Celniker A, Williams M, Leung DY. The effects of nebulized recombinant interferon-gamma in asthmatic airways1. J Allergy Clin Immunol. 1995;95:133–135. doi: 10.1016/s0091-6749(95)70162-1. [DOI] [PubMed] [Google Scholar]
- 50.Gauvreau GM, Hessel EM, Boulet LP, Coffman RL, O'Byrne PM. Immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses1. Am J Respir Crit Care Med. 2006;174:15–20. doi: 10.1164/rccm.200601-057OC. [DOI] [PubMed] [Google Scholar]
- 51.Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial8. Ann Allergy Asthma Immunol. 2002;88:584–591. doi: 10.1016/S1081-1206(10)61890-X. [DOI] [PubMed] [Google Scholar]
- 52.Patil SP, Ji WJ, Singha A, Hendricks DE, Busse PJ, Sampson HA, Wisnivesky JP, Li X. In Vitro Immunomodulatory Effect Of ASHMI (Anti-asthma Herbal Medicine Intervention) On PBMCs From Asthmatics. Journal of Allergy and Clinical Immunology. 2010;125:AB198. [Google Scholar]
- 53.Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57:820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang T, Srivastava K, Wen M-C, Yang N, Busse P, Birmingham N, Goldfarb J, Li X-M. Pharmacology and immunological actions of an herbal medicine, ASHMI™ for allergic asthma. Phytotherapy Res. 2009 Dec 8; doi: 10.1002/ptr.3077. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.