The authors suggest that the plant sterol guggulsterone suppresses bacterial endotoxin-induced inflammatory signaling through the PI3K/AKT/NF-κB pathway, leading to uveitis, and that it could therefore be used as a novel therapeutic agent for the treatment of ocular inflammation, especially uveitis.
Abstract
Purpose.
To investigate the anti-inflammatory effects of guggulsterone, an antioxidant and antitumor agent, in endotoxin-induced uveitis (EIU) in rats and to elucidate the underlying molecular mechanism or mechanisms related to ocular inflammation.
Methods.
EIU was induced by subcutaneous injection of lipopolysaccharide (LPS; 150 μg) into Lewis rats treated with guggulsterone (30 mg/kg body weight, intraperitoneally) or its carrier. After 24 hours the rats were killed, eyes were enucleated, and aqueous humor (AqH) was collected. Numbers of infiltrating cells and levels of matrix metalloproteinase-2 (MMP-2), nitric oxide (NO), and prostaglandin E2 (PGE2) were determined in AqH by specific ELISAs. An antibody array was used to measure the expression of various inflammatory cytokines in AqH. The expression of MMP-2, iNOS, Cox-2, phospho-IκB, and phospho-NF-κB was determined immunohistochemically. Human primary nonpigment ciliary epithelial cells (HNPECs) were used to determine the in vitro efficacy of guggulsterone on the LPS-induced inflammatory response.
Results.
Compared with control, the EIU rat eye AqH had a significantly higher number of infiltrating cells, total protein, and inflammatory markers, such as MMP-2, NO, and PGE2, and the treatment of guggulsterone prevented EIU-induced increases. Guggulsterone also prevented the expression of MMP-2, iNOS, and Cox-2 proteins and of IκB and NF-κB in various eye tissues. Moreover, in cultured HNPECs, guggulsterone inhibited LPS-induced expression of inflammatory proteins.
Conclusions.
These results for the first time demonstrate that the plant sterol guggulsterone suppresses ocular inflammation in EIU, suggesting that the supplementation of guggulsterone could be a novel approach for the treatment of ocular inflammation.
Among all ocular inflammatory diseases, uveitis is a potent vision-threatening intraocular condition that can result in total blindness and is prevalent in many nations.1,2 So far, the etiology of the uveitis is not understood well. However, it is believed to be caused by autoimmune disorders, infections, exposure to toxins, and many other unknown factors.3 Because of uveal inflammation, the levels of cytokines and of chemokines in ocular tissues increases significantly. As a result, activation of the intracellular signaling cascades and alterations of the expression pattern of various inflammatory genes are common characteristics in ocular tissues.4–6 Activation of NF-κB is known to mediate inflammatory diseases, including uveitis.7,8 NF-κB has been shown to regulate the expression of a number of genes responsible for inflammatory markers such as iNOS, Cox-2, and various other cytokines and chemokines.9 Therefore, suppression of NF-κB activation could be a useful approach to curb uveal inflammation. Given that the activation of NF-κB and the expression of iNOS are prominent features of uveitis, therapeutic agents targeted toward the suppression of NF-κB could help in curbing ocular inflammation.
Recently, the plant sterol found in the resin of the guggul (Commiphora mukul) plant guggulsterone (4,17(20)-pregnadiene-3,16-dione) has been used as an effective herbal medicine for a plethora of disease conditions such as arthritis, cancer, cardiovascular problems, obesity, and lipid disorders.10–12 Guggulsterone was also reported to modulate cholesterol homeostasis and thereby to have an anti-obesity and anti-lipidemic effect with no significant toxicity.13–15 Most of the past studies on guggulsterone have focused on their anti-lipidemic activity. Recent studies have shown that these compounds have also shown potent anti-inflammatory effects, such as inhibiting isoproterenol-induced oxidative damage and myocardial necrosis in rats and inflammation associated with nodulocystic acne. These observations suggest that in addition to its lipid-lowering activity, guggulsterone may modulate anti-inflammatory and antioxidant responses. The efficacy of guggulsterone as an anti-inflammatory agent could be mediated through the inhibition of NF-κB and its dependent inflammatory gene expression.16,17 Therefore, we hypothesized that guggulsterone, like other NF-κB inhibitors, would also display antiocular inflammatory activities leading to the amelioration of uveitis and its complications. Recently, we have shown that the inhibition of NF-κB by the polyol pathway enzyme aldose reductase inhibitors and a fat soluble form of vitamin B1, benfotiamine, prevents inflammatory response in endotoxin-induced uveitis (EIU) in rats.7,8 Further, various studies indicate that natural products such as resveratrol,18 lutein,19 curcumin,20 and astathanthin21 have shown anti-inflammatory activity in uveitis models. Although guggulsterone has been shown as a potent anti-inflammatory agent and has also been the subject of clinical trials, the mechanism by which it prevents ocular inflammation leading to uveitis is unknown. Here we investigated the efficacy of guggulsterone in preventing EIU in rats. Our results from in vivo and in vitro studies indicate that supplementation of guggulsterone prevents ocular inflammation in rats.
Materials and Methods
Materials
LPS (from Escherichia coli) was bought from Sigma-Aldrich (St. Louis, MO). Guggulsterone-z was purchased from Steraloids Inc. (Newport, RI). Rat MMP-2 ELISA kit was purchased from BD Biosciences (San Diego, CA). Nitrite/nitrate and PGE2 assay kits were obtained from Cayman Chemical, Inc. (Ann Arbor, MI). Rat cytokine antibody array kit, IL-6 and TNF-α ELISA kits were purchased from RayBiotech Inc (Norcross, GA). Antibodies against MMP-2 were obtained from Cell Signaling (Danvers, MA), and iNOS was obtained from Cayman Chemical, Inc. Cox-2, phospho-IκB, IκB, phospho-p65, phospho-PI3K, phospho-Akt, and Actin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibodies against human recombinant aldose reductase (AR) were made for us by Alpha Diagnostic International (San Antonio, TX). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO) and were of highest purity grade.
Animals
Adult male Lewis rats (age range, 8–10 weeks; weight range, 150–200 g) were purchased and kept in 12-hour light/12-hour dark cycles for 3 days for acclimatization to the animal house facility at the University of Texas Medical Branch (Galveston, TX). Rats were randomly divided into four groups (n = 6). Animal handling, treatment, and procedures were carried out according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Uveitis was induced by LPS (150 μg/kg body weight), as described previously.8 Intraperitoneal injection of guggulsterone (30 mg/kg body weight) was given 1 hour before LPS injection to animals. Rats in the control group were injected with vehicle. The animals were euthanatized at 3 and 24 hours after LPS injection. In another set of experiments, intraperitoneal injection of guggulsterone (30 mg/kg body weight) was given 2 hours after the LPS injection. Aqueous humor (AqH) was collected from the eyes immediately by anterior chamber puncture with a 30-gauge needle under a surgical microscope. After determination of the number of infiltrating cells and the protein concentration in AqH, the samples were kept at −80°C until further use.
Determination of Infiltrating Cells and Total Proteins in AqH
The AqH samples were diluted in an equal amount of trypan-blue solution followed by infiltrating cell counting under the light microscope using a hemocytometer. Total protein concentration in the AqH samples was measured with a protein assay kit (Bio-Rad, Hercules, CA).
Measurement of MMP-2, NO, PGE2, and TNF-α
The total level of nitrate plus nitrite in the AqH was measured by using a total nitrite colorimetric assay (lactate dehydrogenase [LDH]) kit. PGE2 production and MMP-2 levels in AqH were measured by an enzyme immunoassay kit, as described by us earlier.7 The level of TNF-α in the culture media (stored at −80°C after in vitro cell culture experiment) was assessed with a commercially available ELISA kit (RayBiotech, Inc., Norcross, GA). All assays were performed according to the manufacturers' instructions.
Determination of Inflammatory Cytokines and Chemokines in AqH
Levels of inflammatory cytokines and chemokines in the AqH were determined by a commercially available rat cytokine antibody array system (AAR-CYT-1–8; Ray Biotech, Inc.) according to the manufacturer's instructions. Equal amounts of AqH obtained from pooling two rat eyes (n = 4) were used for the determination of inflammatory cytokines and chemokines in AqH. Densitometry analysis of the array was performed with an imaging system (Kodak Image Station Digital Imaging System; Rochester, NY).
Paraffin Embedding of Eyes
Enucleated eyes from the killed rats were fixed in 4% paraformaldehyde for 24 hours. After fixation, the eyes were washed in ice-cold PBS (3×) and immediately transferred in 70%, 90%, and 100% reagent alcohol for 24 hours each, followed by embedding in paraffin. Sagittal sections of 5 μm were obtained.
Histopathologic and Immunochemical Studies
Rat eye sections were stained with hematoxylin and eosin for histopathologic analysis of uveitis symptoms. For immunochemical studies, the sections were warmed at 60°C for 1 hour in the oven, deparaffinized in xylene, rehydrated by passing through 100%, 95%, 80%, and 70% reagent alcohol, and washed with deionized water. After the peroxidase reaction was blocked with 3% H2O2, the sections were rinsed in PBS twice and incubated with blocking buffer (2% BSA, 0.1% Triton X-100, and 2% normal rabbit IgG or 2% normal goat serum) overnight at 4°C. Then the sections were incubated with antibodies against MMP-2, iNOS, Cox-2, phospho-IκB, and phospho-p65 antibodies for 1 hour at room temperature, followed by staining with peroxidase (LSAB+System HRP; DakoCytomation, Carpinteria, CA). The sections were examined by bright-field or fluorescence microscopy (EPI-800 microscope; Nikon, Tokyo, Japan) and photographed with a digital camera (Olympus, Tokyo, Japan) fitted to the microscope. For fluorescence microscopy, the sections were immunostained with respective primary antibodies followed by FITC-labeled secondary antibodies and mounted with fluorescence mounting media.
In Vitro Cell Culture Study
Human primary nonpigment ciliary epithelial cells (HNPECs) and culture media were obtained from ScienCell Research Laboratories (Carlsbad, CA). HNPCECs were cultured according to the supplier's protocol using epithelial cell medium containing basal medium, antibiotics, epithelial cell growth supplement, and fetal bovine serum. The cells were grown in a humidified incubator at 37°C and 5% CO2. All incubations were performed in serum-free medium. The cells were pretreated with 40 μM guggulsterone for 30 minutes and subsequently stimulated with 1 μg/mL LPS for various time intervals, as stated in the figure legends.
Western Blot Analysis
After the treatment, HNPCECs were washed twice with ice-cold PBS and lysed in RIPA buffer containing 1 mM phenylmethylsulfonyl fluoride and 1:100 dilution of protease inhibitor cocktail (Sigma-Aldrich). Protein levels were measured from the supernatant, and aliquots were diluted with 2× SDS sample buffer and boiled for 5 minutes. Lysates were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). Membranes were then incubated in blocking solution containing 5% wt/vol dried fat-free milk and 0.1% vol/vol Tween-20 in Tris-buffered saline. Subsequently, the membranes were incubated with anti-iNOS, -Cox-2, -AR, -phospho-p65, -phospho-PI3K, -phospho-Akt, and -actin antibodies. The membranes were then probed with horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Piscataway, NJ) and visualized by chemiluminescence (Pierce Biotechnology, Rockford, IL).
Statistical Analysis
Data are expressed as mean ± SD. Wilcoxon-Mann-Whitney tests were used for the pairwise comparisons across groups. Analyses were stratified by side (left and right). All computations were performed with the SAS system (SAS/STAT: User's Guide, version 9; SAS Institute, Cary, NC). One-way ANOVA was used to compare inflammatory markers. P < 0.05 was considered statistically significant.
Results
Effect of Guggulsterone on LPS-Induced Inflammatory Cell Infiltration and Protein Concentration in Rat AqH
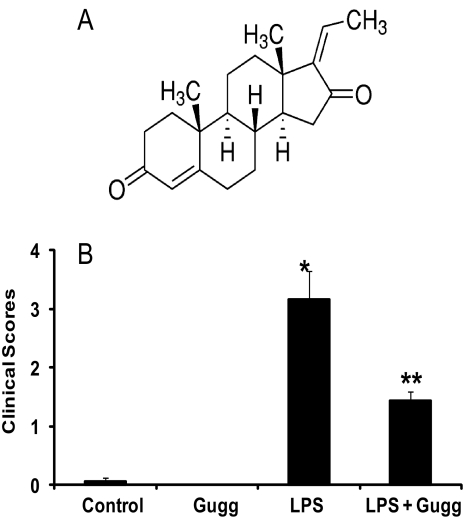
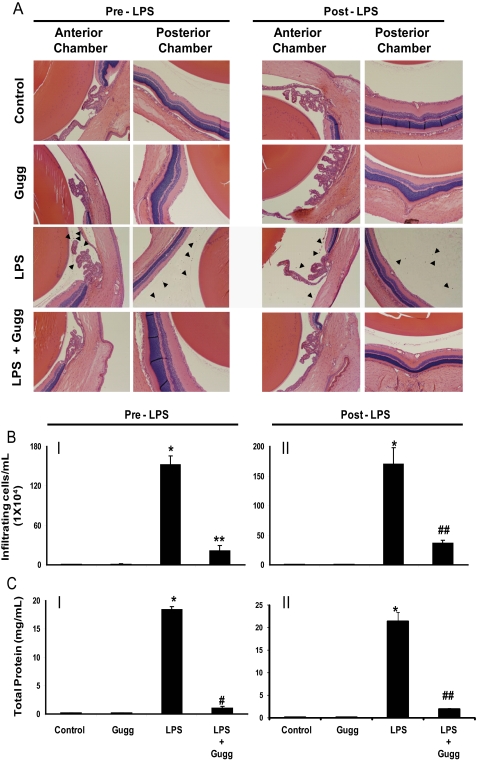
The pathologic symptoms of EIU in Lewis rat eyes injected with LPS and treated without or with guggulsterone were graded in blinded fashion with a slit lamp microscope to evaluate its efficacy. At 24 hours after LPS injection, clinical scores for the EIU rats were 3.16 ± 0.47 (P < 0.003) and were significantly reduced to 1.43 ± 0.15 (P < 0.001) with guggulsterone treatment (Fig. 1B). Examination of hematoxylin and eosin-stained sagittal sections of rat eyes revealed LPS-induced infiltration of inflammatory cells in the aqueous and in vitreous chambers. Guggulsterone significantly prevented infiltration of a large number of cells (Fig. 2A). There were no significant infiltrated cells in the control or guggulsterone-treated groups. The number of infiltrating cells in AqH was also quantified by manually counting the cells with a hemocytometer under a stereoscope microscope (Fig. 2B). Manual cell counting revealed a significant (>150-fold) LPS-induced increase in the infiltration of the inflammatory cells that was significantly (∼75%) decreased by guggulsterone (Fig. 2B). Moreover, the total protein concentration in the AqH of LPS group rat eyes was increased significantly (>18-fold) compared with the control group. However, guggulsterone significantly suppressed (∼85%) elevations in protein concentration in AqH (Fig. 2C). These results indicate the preventive role of guggulsterone in LPS-induced inflammatory cell infiltration in aqueous and vitreous chambers and in the elevation of inflammatory proteins in the AqH of rat eyes.
Figure 1.
Guggulsterone prevents LPS-induced clinical symptoms of EIU in rats. (A) Chemical structure of guggulsterone. (B) The pathologic score of EIU in Lewis rat eyes injected with LPS in the absence and presence of guggulsterone was determined at 24 hours with a slit lamp microscope. Results are given as mean ± SD (n = 6). #P < 0.003 versus control. **P < 0.001 versus EIU (Wilcoxon-Mann–Whitney test).
Figure 2.
Gugglesterone prevents LPS-induced inflammatory cell infiltration and protein concentration in AqH. (A) Histopathology results of paraffin-embedded sections showing infiltrated cells in the anterior as well as posterior chambers of EIU rat eyes without or with gugglesterone injected 1 hour before or 2 h post LPS administration. Hematoxylin and eosin–stained serial sections of rat eyes were observed under a light microscope. Original magnification, 200×. (B) The infiltrated inflammatory cells were determined by trypan blue exclusion cell counting and (C) total protein concentration in the AqH were determined by the Bradford methods. Results are expressed as the mean ± SD (n = 6). *P < 0.001 versus the control group. **P < 0.05, #P < 0.001, and ##P < 0.01 versus the 24-hour EIU group. Gugg, guggulsterone.
Effect of Guggulsterone on LPS-Induced Inflammatory Cytokines, Chemokines, and Growth Factors in Rat AqH
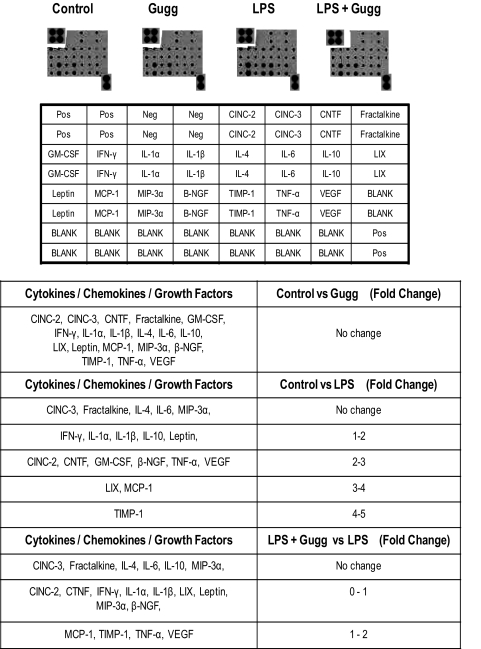
Secretion of various cytokines, chemokines, and growth factors in AqH is a prominent feature of uveitis. Hence, we determined the levels of various cytokines, chemokines, and growth factors in the rat AqH by using a specific rat inflammation array kit that can determine 19 inflammatory marker proteins from a single sample. Densitometric analysis of the respective inflammatory marker spots on the array membrane revealed a significant increase in the secretion of cytokines, chemokines, and growth factors such as IFN-γ, IL-1α, IL-1β, IL-10, and leptin (1- to 2-fold); CINC-2, CTNF, GM-CSF, β-NGF, TNF-α, and VEGF (2- to 3-fold); LIX and MCP-1 (3- to 4-fold); TIMP-1 (4- to 5-fold) in rat eye AqH (Fig. 3). However, guggulsterone suppressed the secretion of cytokines, chemokines, and growth factors significantly (P < 0.001) in the AqH of the EIU rat eye (Fig. 3).
Figure 3.
Guggulsterone inhibits LPS-induced increase in cytokines, chemokines, and growth factors in EIU eye AqH. The cytokine antibody array system was used to determine cytokines, chemokines, and growth factors from AqH. Spots on the array membrane were subjected to densitometry and are presented as fold change for individual cytokines, taking control as 0 fold value (n = 4). Densitometry results are expressed as fold change in the table. Gugg, Guggulsterone.
Effect of Guggulsterone on LPS-Induced Increase in MMP-2, NO, and PGE2 Levels in AqH and Expression of MMP-2, iNOS, and Cox-2 in Rat Ocular Tissues
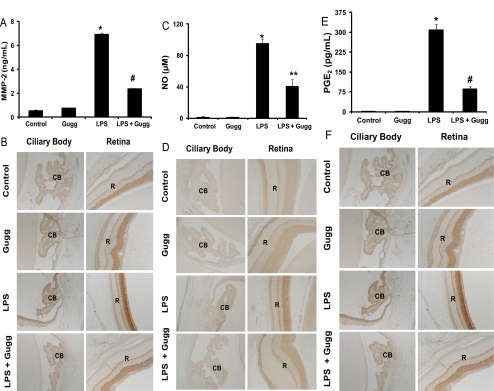
Given that guggulsterone significantly suppressed the elevation in levels of various inflammatory markers, we next determined the effect of guggulsterone on the levels of other inflammation-associated components such as MMP-2, NO, and PGE2 in the AqH and MMP-2, iNOS, and Cox-2 expression in ciliary body and retina. As shown in Figure 4, increase in the levels of MMP-2, NO, and PGE2 in the LPS-treated rat eye AqH was approximately 6.8 ng/mL (Fig. 4A), 96 μM (Fig. 4C), and 325 pg/mL (Fig. 4E), respectively. This increase was significant (P < 0.001) compared with the control group and was significantly suppressed (60%–80%) by guggulsterone (Figs. 4A, 4C, 4E). These results were further confirmed by immunohistochemistry. LPS-treated rat ocular tissues revealed increased expressions of MMP-2 (Fig. 4B), iNOS (Fig. 4D), and Cox-2 (Fig. 4F) in the ciliary body and retina of EIU rat eyes suppressed by guggulsterone. These results indicate an anti-inflammatory role of guggulsterone in the EIU model. Further, to examine whether guggulsterone prevents EIU by suppressing systemic inflammation, we measured the serum levels of cytokines such as TNF-α and IL-6 in rats. Within 3 hours of LPS treatment to rats, serum levels of TNF-α were increased from 230 ± 120 pg/mL (controls) to 1320 ± 260 pg/mL, and pretreatment of guggulsterone suppressed the LPS-induced TNF-α to 490 ± 180 pg/mL. Similarly, LPS treatment increased the serum levels of IL-6 from 140 ± 60 pg/mL (controls) to 2140 ± 190 pg/mL, and pretreatment of guggulsterone suppressed the IL-6 levels to 420 ± 130 pg/mL. The levels of these cytokines at 24 hours were marginally increased (statistically nonsignificant) in LPS-treated animals compared with controls or guggulsterone-treated rats (data not shown). These results indicate that guggulsterone could prevent EIU by suppressing the LPS-induced systemic inflammation.
Figure 4.
Gugglesterone prevents MMP-2, NO, and PGE2 secretion in the AqH of EIU rat eyes and expression of MMP-2, iNOS, and Cox-2. (A, C, E) MMP-2, NO, and PGE2 levels in the AqH (collected 24 hours) after LPS induction were determined by specific ELISAs. Data are expressed as the mean ± SD (n = 6). For MMP-2 and PGE2: *P < 0.001 versus the control group; #P < 0.001 versus the 24-hour EIU group. For NO: *P < 0.001 versus the control group; **P < 0.01 versus the 24-hour EIU group. (B, D, F) Serial sections of paraformaldehyde-fixed rat eyes were immunostained with antibodies against MMP-2 (B), iNOS (D), and Cox-2 (F) and observed under a bright-field microscope. A representative image is shown (n = 4). CB, ciliary body; R, retina; Gugg, guggulsterone. Original magnification, 400×.
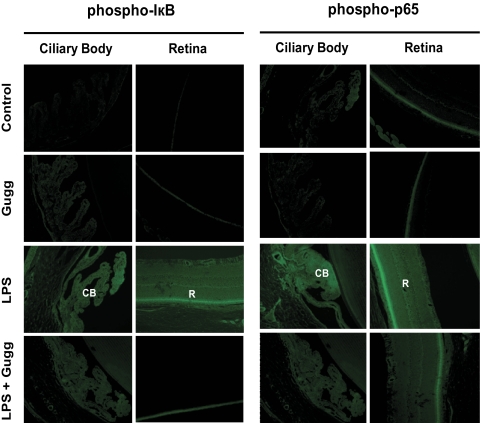
Effect of Guggulsterone on LPS-Induced IκB and NF-κB Activation in EIU Rat Eye Tissues
Given that redox-sensitive transcription factor NF-κB is well known to transcribe the genes responsible for various inflammatory markers, we next investigated the effect of guggulsterone on LPS-induced activation of IκB and of NF-κB in rat eye tissues. Paraffin sections were probed with antibodies against phospho-IκB and phospho-p65 (active subunit of NF-κB). After 3 hours of LPS induction, IκB- and NF-κB-positive cells were observed in the iris-ciliary body complex in the anterior region and the retina in the posterior region of the eye (Fig. 5). However, guggulsterone significantly suppressed the number of IκB- and NF-κB-positive cells in the anterior and posterior regions of EIU eyes.
Figure 5.
Gugglesterone suppresses the activation of IκB and NF-κB in ocular tissues of LPS-induced EIU. Serial sections of paraformaldehyde-fixed rat eyes enucleated 3 hours after EIU induction were immunostained with antibodies against phospho-IκB-α and phospho-p65. Antibody staining intensity was observed by fluorescence microscopy. Representative images are shown (n = 4). CB, ciliary body; R, retina; Gugg, guggulsterone. Original magnification, 200×.
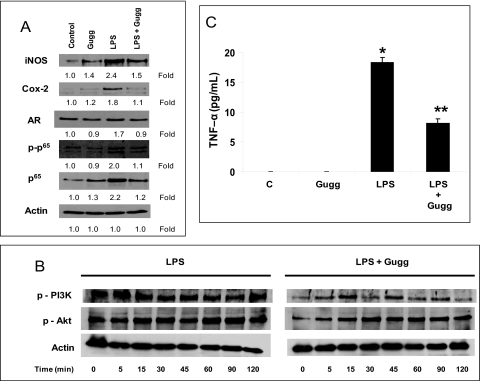
Effect of Guggulsterone on LPS-Induced Inflammatory Signals in HNPECs
Ciliary epithelial cells are major source of inflammatory markers that release into the AqH during uveitis; therefore, we used HNPECs as our in vitro model to confirm our in vivo results. On LPS stimulation, there were 1.8- and 2.4-fold increases in the expression of NF-κB–dependent inflammatory Cox-2 and iNOS proteins in HNPCECs (Fig. 6A). Consistent with these results was the activation and expression of NF-κB (2.0- and 2.2-fold, respectively). Moreover, the expression of AR, an oxidative response protein, was observed to be increased to 1.7-fold. However, guggulsterone suppressed LPS-induced expression of Cox-2, iNOS, AR, and NF-κB significantly (Fig. 6A). Similarly, phosphorylations of PI3K and Akt were inhibited by guggulsterone (Fig. 6B), suggesting that guggulsterone prevents NF-κB activation by way of PI3K/AKT. The increased inflammatory response of HNPECs was confirmed by determining the levels of inflammatory TNF-α in the culture media. There was an approximately 18-fold increase in TNF-α level on LPS-treatment in culture media compared with the control group. However, guggulsterone significantly (P < 0.001) suppressed secreted TNF-α levels in culture media (Fig. 6C), suggesting that guggulsterone prevents NF-κB–dependent expression of inflammatory markers.
Figure 6.
Gugglesterone prevents inflammatory changes in HNPCECs stimulated with LPS. (A) Growth-arrested HNPCECs pretreated with or without gugglesterone (40 μM) were incubated with 1 μg/mL LPS for 24 hours. Expression of Cox-2, iNOS, AR and phospho-p65 from cell lysates was determined by Western blot analysis using specific antibodies. (B) Growth-arrested HNPCECs pretreated with or without gugglesterone (40 μM) were incubated with 1 μg/mL LPS for 0 to 120 minutes. PI3K and Akt from total cell lysates were determined by Western blot analysis using respective phosphospecific antibodies. (C) Levels of TNF-α in the culture media were determined with ELISA kit. Data are expressed as the mean ± SD (n = 6). *P < 0.001 versus the control group. **P < 0.001 versus the LPS group. Gugg, guggulsterone.
Discussion
In this study, we have demonstrated that guggulsterone suppresses the ocular inflammatory response in the rat EIU model. Guggulsterone has been in use in Ayurvedic medicinal practice for millennia to treat a variety of pathologic conditions such as inflammation, arthritis, cardiovascular and lipid disorders.10 More recently, it has also been demonstrated to be an effective anticancer agent.22 Guggulsterone has been known to have various molecular targets, such as transcription factors, cytokines, enzymes, genes regulating apoptosis, growth factors, and growth factor receptors10 and to have cholesterol-lowering activity by acting as an farnesoid X receptor (FXR) antagonist.13,23 The inhibition of transcription factor NF-κB by guggulsterone decreased the expression of antiapoptotic gene products (IAP1, xIAP, Bfl-1/A1, Bcl-2, cFLIP, survivin).16 Further, it is also known to modulate cell proliferation through cyclin D1 and c-Myc and metastasis through MMP-9, Cox-2, and VEGF.16 Antiangiogenic activity of guggulsterone through the suppression of STAT3 has also been reported.24–26 However, the anti-inflammatory role of guggulsterone in ocular inflammation has not been investigated. In this study, for the first time, we have shown that supplementation of guggulsterone prevents LPS-induced activation of NF-κB, activation of PI3K/Akt, and expression of inflammatory cytokines and chemokines in ciliary epithelial cells. Our studies also indicate that guggulsterone prevents the ocular inflammatory response observed in EIU rats.
Our study revealed that LPS-induced infiltration of inflammatory cells, increases in protein concentration, and levels of various inflammatory marker proteins in AqH were significantly inhibited by guggulsterone in rat eyes. The mode of anti-inflammatory actions of guggulsterone has been demonstrated to occur primarily through the inhibition of iNOS and IκB degradation, which attenuate NF-κB translocation to nuclei.16,17 Consistent with findings of a previous study, our study also demonstrates that guggulsterone inhibits iNOS and the activation of IκB in EIU. To elucidate the anti-inflammatory response of guggulsterone in rat AqH, we determined the levels of various inflammatory markers such as MMP-2, NO, and PGE2 (Fig. 4) in AqH. Treatment of guggulsterone suppressed LPS-induced cytokines levels in AqH. Similarly, guggulsterone inhibited the expression of MMP-2, iNOS, and Cox-2 in ocular tissues such as ciliary bodies and retina. These results suggest that guggulsterone prevents the NF-κB–dependent expression of MMP-2, iNOS, and Cox-2 and thus the production of MMP-2, NO, and PGE2 in AqH. Our results are in agreement with those of a previous study that demonstrated the suppression of iNOS and Cox-2 expression and the subsequent NO and PGE2 production in pancreatic beta cells by guggulsterone.17,27 Increased NO levels have been detected in the AqH of patients with Behçet's disease who have uveitis.28 Moreover, the iNOS inhibitor NG-nitro-L-arginine (L-NAME) prevents the development of uveitis by inhibiting iNOS activity.29 Further, NO can activate Cox enzymes followed by a marked increase in PGE2 production.30 Because ciliary epithelial cells are well known to maintain AqH homeostasis and are considered a major source of inflammatory marker release in AqH, we also investigated the mechanism of guggulsterone in LPS-induced inflammatory response in HNPCECs in vitro. Our results suggest that guggulsterone significantly suppressed the NF-κB–dependent expression of iNOS and Cox-2 in HNPCECs. Similarly, guggulsterone also inhibited the expression of aldose reductase, an oxidative response protein. Previous investigations found that the inhibition of aldose reductase prevents EIU in rats.7 Furthermore, guggulsterone suppressed the LPS-induced levels of TNF-α, a well-known inflammatory cytokine linked with uveitis, in the culture media.31 Our previous studies and those of other investigators have shown increased levels of TNF-α in AqH and elevated expression in ocular tissues in the EIU model7,8,31,32 and in eyes of patients with Behçet's disease.33 The prominent and crucial role of TNF-α has been further substantiated by diminished inflammation in TNF receptor–deficient mice in immune complex–induced uveitis.34 In the present study, guggulsterone decreased TNF-α levels induced by LPS in vitro and in vivo.
The expression of inflammatory proteins such as iNOS, Cox-2, and TNF-α requires the activation of NF-κB,35 as have been shown in various ocular tissues of animals with uveitis.7,8 LPS and TNF-α have been demonstrated to activate NF-κB in mouse lens and human lens epithelial cells, respectively.36,37 We have observed that guggulsterone suppressed iNOS, Cox-2 and TNF-α in this study and investigated an upstream signaling event, the activation of NF-κB, in EIU rats. Our results demonstrate that guggulsterone suppresses the activation of IκB and NF-κB (Fig. 5). Similarly, guggulsterone diminished the activation of NF-κB and PI3K/Akt in HNPCECs on LPS stimulation (Fig. 6). Our results are in agreement with those of others who have shown that treatment with guggulsterone prevents the activation of IκB and NF-κB.16,27,38–41 In summary, our results indicate that supplementation with the plant sterol guggulsterone prevents ocular inflammatory responses evoked by LPS in cultured cells and in a rat model of EIU. We expect that our results will shed new light on the mechanism of regulating ocular inflammation, particularly uveitis, by using natural plant products such as guggulsterone.
Footnotes
Supported by National Institutes of Health Grants EY015891 and GM071036 (KVR).
Disclosure: N.M. Kalariya, None; M. Shoeb, None; A.B.M. Reddy, None; M. Zhang, None; F.J.G.M. van Kuijk, None; K.V. Ramana, None
References
- 1. Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Read RW. Uveitis: advances in understanding of pathogenesis. Curr Rheumatol Rep. 2006;8:260–266 [DOI] [PubMed] [Google Scholar]
- 3. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308 [DOI] [PubMed] [Google Scholar]
- 4. Sijssens KM, Rijkers GT, Rothova A, Stilma JS, de Boer JH. Distinct cytokine patterns in the aqueous humor of children, adolescents and adults with uveitis. Ocul Immunol Inflamm. 2008;16:211–216 [DOI] [PubMed] [Google Scholar]
- 5. Kim EY, Moudgil KD. Regulation of autoimmune inflammation by pro-inflammatory cytokines. Immunol Lett. 2008;30:120:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curnow SJ, Murray PI. Inflammatory mediators of uveitis: cytokines and chemokines. Curr Opin Ophthalmol. 2006;17:532–537 [DOI] [PubMed] [Google Scholar]
- 7. Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Srivastava SK, Ramana KV. Focus on molecules: nuclear factor-kappaB. Exp Eye Res. 2009;88:2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res. 2008;28:3647–3664 [PubMed] [Google Scholar]
- 11. Gujral ML, Sareen K, Tangri KK, Amma MK, Roy AK. Antiarthritic and anti-inflammatory activity of gum guggul (Balsamodendron mukul Hook). Indian J Physiol Pharmacol. 1960;4:267–273 [PubMed] [Google Scholar]
- 12. Singh BB, Mishra LC, Vinjamury SP, Aquilina N, Singh VJ, Shepard N. The effectiveness of Commiphora mukul for osteoarthritis of the knee: an outcomes study. Altern Ther Health Med. 2003;9:74–79 [PubMed] [Google Scholar]
- 13. Urizar NL, Liverman AB, Dodds DT, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706 [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–1597 [DOI] [PubMed] [Google Scholar]
- 15. Cui J, Huang L, Zhao A, et al. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003;278:10214–10220 [DOI] [PubMed] [Google Scholar]
- 16. Shishodia S, Aggarwal BB. Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J Biol Chem. 2004;279:47148–47158 [DOI] [PubMed] [Google Scholar]
- 17. Meselhy MR. Inhibition of LPS-induced NO production by the oleogum resin of Commiphora wightii and its constituents. Phytochemistry. 2003;62:213–218 [DOI] [PubMed] [Google Scholar]
- 18. Kubota S, Kurihara T, Mochimaru H, et al. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–3519 [DOI] [PubMed] [Google Scholar]
- 19. Jin XH, Ohgami K, Shiratori K, et al. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2006;47:2562–2568 [DOI] [PubMed] [Google Scholar]
- 20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318–322 [DOI] [PubMed] [Google Scholar]
- 21. Suzuki Y, Ohgami K, Shiratori K, et al. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res. 2006;82:275–281 [DOI] [PubMed] [Google Scholar]
- 22. An MJ, Cheon JH, Kim SW, Kim ES, Kim TI, Kim WH. Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 2009;279:93–100 [DOI] [PubMed] [Google Scholar]
- 23. Urizar NL, Moore DD. GUGULIPID: a natural cholesterol-lowering agent. Annu Rev Nutr. 2003;23:303–313 [DOI] [PubMed] [Google Scholar]
- 24. Ahn KS, Sethi G, Sung B, Goel A, Ralhan R, Aggarwal BB. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through activation of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–4415 [DOI] [PubMed] [Google Scholar]
- 25. Xiao D, Singh SV. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol Cancer Ther. 2008;7:171–180 [DOI] [PubMed] [Google Scholar]
- 26. Manjula N, Gayathri B, Vinaykumar KS, Shankernarayanan NP, Vishwakarma RA, Balakrishnan A. Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down-regulation of TNF-alpha, IL-1beta and IL-2. Int Immunopharmacol. 2006;6:122–132 [DOI] [PubMed] [Google Scholar]
- 27. Lv N, Song MY, Kim EK, Park JW, Kwon KB, Park BH. Guggulsterone, a plant sterol, inhibits NF-kappaB activation and protects pancreatic beta cells from cytokine toxicity. Mol Cell Endocrinol. 2008;289:49–59 [DOI] [PubMed] [Google Scholar]
- 28. Duygulu F, Evereklioglu C, Calis M, Borlu M, Cekmen M, Ascioglu O. Synovial nitric oxide concentrations are increased and correlated with serum levels in patients with active Behçet's disease: a pilot study. Clin Rheumatol. 2005;24:324–330 [DOI] [PubMed] [Google Scholar]
- 29. Mandai M, Yoshimura N, Yoshida M, Iwaki M, Honda Y. The role of nitric oxide synthase in endotoxin-induced uveitis: effects of NG-nitro L-arginine. Invest Ophthalmol Vis Sci. 1994;35:3673–3680 [PubMed] [Google Scholar]
- 30. Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santos Lacomba M, Marcos Martin C, Gallardo Galera JM, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–255 [DOI] [PubMed] [Google Scholar]
- 32. Franks WA, Limb GA, Stanford MR, et al. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11:187–191 [DOI] [PubMed] [Google Scholar]
- 33. Tugal-Tutkun I, Mudun A, Urgancioglu M, et al. Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behçet's disease: an open-label trial. Arthritis Rheum. 2005;52:2478–2484 [DOI] [PubMed] [Google Scholar]
- 34. Brito BE, O'Rourke LM, Pan Y, Anglin J, Planck SR, Rosenbaum JT. IL-1 and TNF receptor-deficient mice show decreased inflammation in an immune complex model of uveitis. Invest Ophthalmol Vis Sci. 1999;40:2583–2589 [PubMed] [Google Scholar]
- 35. Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268 [DOI] [PubMed] [Google Scholar]
- 36. Alexander G, Carlsen H, Blomhoff R. Strong in vivo activation of NF-kappaB in mouse lenses by classic stressors. Invest Ophthalmol Vis Sci. 2003;44:2683–2688 [DOI] [PubMed] [Google Scholar]
- 37. Dudek EJ, Shang F, Taylor A. H2O2-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med. 2001;31:651–658 [DOI] [PubMed] [Google Scholar]
- 38. Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin Cancer Res. 2006;12:662–668 [DOI] [PubMed] [Google Scholar]
- 39. Cheon JH, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis. 2006;12:1152–1161 [DOI] [PubMed] [Google Scholar]
- 40. Lee YR, Lee JH, Noh EM, et al. Guggulsterone blocks IL-1beta-mediated inflammatory responses by suppressing NF-kappaB activation in fibroblast-like synoviocytes. Life Sci. 2008;82:1203–1209 [DOI] [PubMed] [Google Scholar]
- 41. Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Mukhtar H. Guggulsterone modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in SENCAR mice. Carcinogenesis. 2008;29:2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]