Abstract
The prevalence of obesity and diabetes is rising to epidemic proportions worldwide. Insulin resistance is central to the pathogenesis of type 2 diabetes. The role of insulin sensitive tissues and organs, such as adipose, liver, muscle and hypothalamus, in regulating insulin sensitivity has been extensively studied. However, the existence and nature of inter-tissue communication in the regulation of insulin action is unresolved. A major factor in the development of insulin resistance is obesity, especially abdominal obesity. Chronically positive energy balance leads to a process gradually progressing to insulin resistance in obese states. Increased adipocyte size is positively correlated with the frequency of adipocyte death in obesity. The death of the hypertrophic adipocytes facilitates the infiltration of macrophages, which further perpetuates adipose inflammation and insulin resistance, characterized by low liposynthetic capacity and high lipolytic capacity, causing increased release of free fatty acids (FFA). The inflamed adipose tissue releases FFA, inflammatory cytokines, and hormones that act as circulatory factors to regulate insulin sensitivity in distant organs. In addition, perivascular adipose tissue participates in regulating insulin sensitivity, vascular inflammation and function in an endocrine/paracrine manner through adipose-derived relaxing and contracting factors, cytokines and infiltration of inflammatory cells. Thus, adipose tissue releases cytokines, hormones and lipids that signal distant organs to regulate systemic metabolic homeostasis, as well as signal local blood vessels to modulate vascular inflammation and function. These findings may lead to uncovering new therapeutic targets to fight obesity and type 2 diabetes by restructuring the adipokine profile and lipid metabolism.
Keywords: obesity, inflammation, adipokines, toll-like receptor 4
Introduction
In the United States, the incidence of obesity in adults over 15 years of age is predicted to increase from 32% to 44.2% in males and from 37.8% to 48.3% in females between 2002 and 2010 (1). Obesity plays a principal and causative role in the development of insulin resistance and type 2 diabetes (2). However, mechanism(s) that describe how excess fat causes impairment of insulin sensitivity and vascular dysfunction have not yet been elucidated. Recent advances in obesity research strongly indicate that adipose tissue is an active endocrine organ that secretes multiple bioactive factors categorized as adipokines and lipokines (3–4). These adipose derived factors may provide a link between adipose tissue lipid accumulation and the metabolic function of other insulin sensitive organs such as liver, muscle and hypothalamus, as well as the crosstalk between perivascular adipose and blood vessels in the regulation of vascular function.
1. Chronically Positive Energy Balance (Obesity) Gradually Progresses to Insulin Resistance
Chronic nutrient overload leads to an increase in adipose depots that, if adipose tissue expandability is low, are characterized by an increased presence of hypertrophic adipocytes (5). This adipocyte hypertrophy causes endoplasmic reticulum (ER) stress (6). ER stress plays a critical role in insulin resistance found in diabetes by modifying the expression of oxygen-regulated protein 150 (ORP150), a molecular chaperone that protects cells from ER stress by affecting the phosphorylation state of insulin receptor substrate-1 (IRS-1) and protein kinase B (Akt) (6). Meanwhile, increased adipocyte size in obese mice and humans is positively correlated with the frequency of adipocyte death. Adipocyte death in diabetic (db/db) mice is 30 fold of that in control mice and “appears to involve an alternative death pathway exhibiting morphological features of necrosis and the leukocyte-eliciting profile of apoptosis” (7). More than 90% of all macrophages in white adipose tissue (WAT) of obese mice and humans are localized to dead adipocytes, where they “fuse to form syncytia that sequester and scavenge the residual free adipocyte lipid droplet and eventually form multinucleate giant cells”, which is a hallmark of chronic inflammation (7–8). In vitro studies revealed that co-culture of macrophages and adipocytes with toll-like receptor 4 (TLR4) ligand, lipopolysaccharide (LPS), markedly up-regulated interleukin-6 (IL-6) production (nearly 100-fold higher than that of adipocyte culture alone) (9), suggesting that dead adipocytes preceded by macrophage infiltration may synergistically orchestrate the inflammatory response in adipose tissue. This provides a novel framework whereby the death of the hypertrophic adipocyte facilitates the infiltration of macrophages, which in turn release inflammatory proteins causing further recruitment of macrophages and the release of inflammatory cytokines (7). Moreover, T-lymphocyte infiltration in visceral adipose acts as a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance (10). In a mouse model of obesity-mediated insulin resistance, a 5 week high fat diet (HFD)-induced insulin resistance was associated with a marked T-lymphocyte infiltration in visceral adipose tissue. In contrast, recruitment of macrophages was delayed with an increase of MAC-3 positive staining and F4/80 mRNA expression after 10 weeks of HFD. In patients with type 2 diabetes, most macrophages were HLA-DR-positive, reflecting activation through interferon-gamma (IFNγ), a cytokine released from CD4-positive lymphocytes (10). Obese IFNγ-deficient animals had significantly reduced adipose expression of mRNA encoding inflammatory genes such as tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein-1 (MCP-1), decreased adipose inflammatory cell accumulation, and better glucose tolerance than control animals fed the same diet (11). These suggest that adipose T-lymphocytes serve as early contributors to obesity-mediated insulin resistance and adipose tissue macrophage invasion.
Furthermore, insulin-resistant adipocytes, characterized by low liposynthetic capacity and high lipolytic capacity, lead to increased release of free fatty acids (FFA), which activate TLR4- induced IkappaB kinase (IKKβ)/c-jun N-terminal kinase (JNK) signaling, and then further stimulate chemokines and cytokines release, amplifies insulin resistance, lipolysis and inflammation in adipose tissue (12–13),
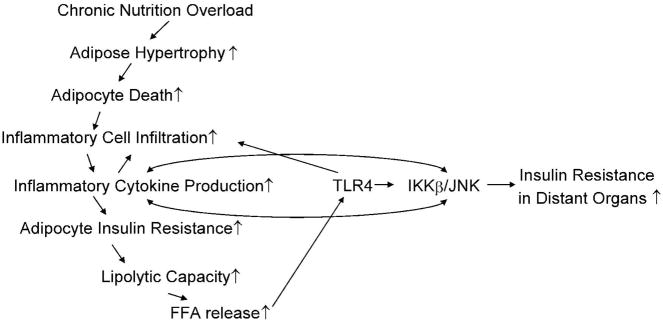
Thus, there is a process of gradual progression to insulin resistance in obese states (5). Increased circulating FFA levels and inflammatory cytokines, reduced circulating adiponectin levels and enhanced leptin resistance may all contribute to the decrease in lipid oxidation in other insulin sensitive organs, thereby triggering ectopic accumulation of lipids, lipotoxicity and insulin resistance (5). (Figure 1)
Figure 1. Chronically Positive Energy Balance Gradually Progresses to Insulin Resistance.
In obesity, increased presence of hypertrophic adipocytes is positively correlated with the frequency of adipocyte death, which facilitates the infiltration of macrophages, thereby sustaining and perpetuating adipose inflammation. Insulin-resistant adipocytes also demonstrate increased release of free fatty acids (FFA) due to low liposynthetic capacity and high lipolytic capacity. FFA activates toll-like receptor 4 (TLR4)-induced IkappaB kinase (IKKβ)/c-jun N- terminal kinase (JNK) signaling, which further stimulates chemokine and cytokine release, amplifies insulin resistance, lipolysis and inflammation in adipose tissue. Thus, increased circulating FFA levels and inflammatory cytokines, reduced circulating adiponectin levels and enhanced leptin resistance lead to decreased lipid oxidation in other insulin sensitive tissues, thereby triggering ectopic accumulation of lipids, lipotoxicity and insulin resistance in distant organs.
2. Adipose “Talks” to Distant Organs in the Regulation of Insulin Sensitivity
Collective evidence suggests that adipose may affect liver and muscle insulin sensitivity. Adipose-selective targeting of the glucose transporter (GLUT4) gene impaired insulin action in muscle and liver (14), may be due to the effects of chronic hyperinsulinaemia, or via changes in the release of an as yet unidentified adipocyte-derived molecule that affects insulin action in other tissues, since the circulating level of FFA, leptin and TNFα did not change in mice with adipose selective deficiency of GLUT4 (G4A−/−). It is postulated that insulin resistance in adipose results in the redistribution of triglycerides to the liver and skeletal muscle; this leads to insulin resistance in distant organs, since the insulin action is normal in the skeletal muscle of G4A−/− mice ex vivo, but not in vivo (14).
Various cytokines and hormones produced by adipose tissue play a major role in the regulation of insulin sensitivity, however, until very recently, adipose selective ablation of genes of interest has been presented to provide direct evidence that adipose signals to distant organs to affect insulin sensitivity through the secretion of adipokines. A recent study suggested that JNK1 deficiency in adipose tissue suppressed HFD–induced insulin resistance in the liver; JNK1-dependent secretion of the inflammatory cytokine IL-6 by adipose tissue upregulated expression of the liver suppressor of cytokine signaling-3 (SOCS3), a protein that induces hepatic insulin resistance (15).
Furthermore, increased release of free fatty acids from adipose tissue has long been linked to muscle insulin resistance (16) and lipogenesis and steatosis in liver (17). Using quantitative lipidomic analyses and mice deficient in adipose tissue lipid chaperones aP2 and mal1, Cao et al. elucidated the link between metabolic alterations in adipose tissue and whole-body metabolism through lipid signals. A robust increase in de novo lipogenesis enabled the adipose tissue of these mice to be resistant to the deleterious effects of dietary lipid exposure. Systemic lipid profiling also identified C16:1n7-palmitoleate as an adipose tissue-derived lipid hormone that strongly enhances muscle insulin action and suppresses hepatosteatosis (4). Although the adipose-generated fatty acid metabolites, such as palmitoleate may serve as novel circulatory factors that regulate insulin actions of liver and muscle in the murine model, one of the major concerns is that the capacity of human adipocytes for de novo biosynthesis of fatty acids is remarkably less than that in rodent models (18). Despite this, palmitoleate acts as regulatory signal that participates in the maintenance of a beneficial metabolic profile through its systemic effects and has the potential to serve as a lipid signal that mediates communications between adipose and other insulin sensitive tissues by acting as a “lipokine” (4).
In addition to muscle and liver, the central nervous system (CNS) also plays crucial role in the regulation of energy homeostasis (19). Hypothalamus acts as a primary regulatory center of the brain responsible for mediating the effects of adipokines and other circulating metabolic hormones (19). Specifically, the arcuate nucleus (ARC) is the area that lacks the blood brain barrier thus allowing for the entry of peptides and proteins such as certain adipokines from the circulation (19). There is increased interest in the central effects of adipokines on peripheral glucose metabolism. In Lepob/ob mice with leptin deficiency, intracerebroventricular leptin administration suppressed hepatic glucose production (20–21), causing a significant reduction in food intake, body weight and epididymal fat (21). Similarly, intracerebroventricular adiponectin injection resulted in increased thermogenesis, weight loss and reduction in serum glucose and lipid levels (22). Adiponectin also potentiated the effect of leptin on thermogenesis and lipid levels, suggesting an interactive association in their central effects (22). On the contrary, central delivery of resistin induced hepatic insulin resistance and increased endogenous glucose production during hyperinsulinemic-euglycemic clamp (23). As the list of adipokines continues to grow, the central effects of other adipokines on energy homeostasis, peripheral insulin resistance, and beta-cell function and mass warrants further attention.
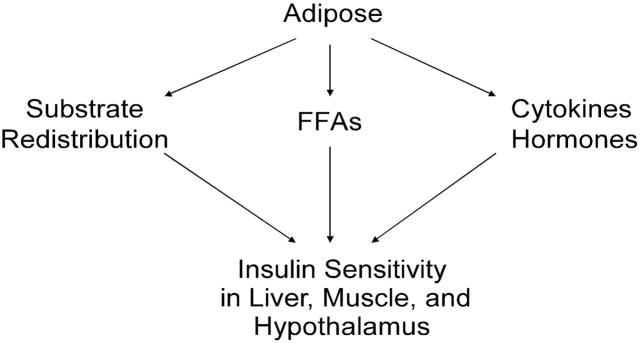
In summary, adipose-derived cytokines, hormones and lipokines may serve as circulatory signal molecules to affect the insulin sensitivity in distant organs. Further studies are needed to identify signaling molecules in adipose, which exert profound protective effects against HFD-induced insulin resistance. This will aid in elucidating how adipose communicates with liver and muscle, as well as the hypothalamus in the regulation of insulin sensitivity. (Figure 2)
Figure 2. Adipose May “Talk” to Distant Organs via Three Proposed Pathways.
Adipose derived FFA, cytokines and hormones may act as circulatory factors to regulate the insulin sensitivity of other insulin sensitive organs. Substrate redistribution caused by impaired adipose insulin sensitivity promotes lipotoxicity and insulin resistance in distant organs.
3. Perivascular Adipose Regulates Vascular Function and Insulin Sensitivity
Adipocytes and perivascular adipose tissue (PVAT) are now recognized as regulators of vascular function and insulin sensitivity (24). Characterization of the biological and pathological functions of various adipose-derived factors over the past decade has expanded our understanding of the communication between PVAT and blood vessels (25). Here we summarize the relaxing and contracting effects, as well as proinflammatory effects of PVAT, and how PVAT affects vascular function and insulin sensitivity through endocrine/paracrine actions.
3.1 The Relaxing and Contracting Effects of Perivascular Adipose
Most biological regulatory systems maintain homeostasis through feedback mechanisms that recognize hyper- or hypo- conditions triggering appropriate counteracting factors to return the system to a balanced state. The modulation of vascular function by PVAT includes secreting both relaxing and contracting factors that appear to operate in this way.
Recently, several studies have reported that PVAT is a modulator of vascular tone and that PVAT lowers the response to a broad spectrum of vasoconstricting agonists by releasing a transferable substance with direct vasodilator properties (26). PVAT exerts anti-contractile effects through two distinct mechanisms: 1) by releasing transferable relaxing factors that induce endothelium-dependent relaxation through nitric oxide (NO) release and subsequent calcium-activated potassium channel (KCa) activation, and 2) by endothelium-independent mechanisms involving H2O2 and subsequent activation of soluble guanylyl cyclase (sGC) (27). The chemical nature of adipocyte-derived relaxing factor (ADRF) is still undetermined and, as with endothelial-derived hyperpolarizing factor (EDHF), the mechanism of action as well as the potency of the dilator response varies significantly among species and vascular beds.
PVAT of normotensive rats releases a transferable factor that induces relaxation by opening voltage-dependent K+ (Kv) channels. The anticontractile effects of perivascular fat were reduced in aged-matched spontaneously hypertensive rats (SHR) mesenteric artery rings compared with Wistar Kyoto rats (WKY) (28). PVAT in human internal thoracic arteries releases a transferable relaxation factor that acts through the activation of KCa channels. PVAT is often removed in coronary artery bypass grafting. Accordingly, retaining PVAT might be helpful in reducing the occurrence of vasospasm of the graft vessels (29). PVAT may also be a source of NO, since endothelial NO synthase (eNOS) expression and activity in perivascular fat were confirmed by immunohistochemistry, western blot and citrulline assay. Perivascular fat-derived NO plays a beneficial role in saphenous veins harvested traumatically and used as grafts in patients undergoing coronary artery bypass surgery (30). Lee et al examined the alterations of PVAT function in rat aorta incubated with 22 mmol/l D-glucose for 30 min, and in aorta from streptozotocin (STZ)-induced diabetic rats (31). Under both acute and chronic conditions, hyperglycemia enhanced the relaxation response of the vessels mediated by PVAT (31). In obese subjects, the local inflammation and hypoxia abolish the protective anticontractile effects of perivascular fat (32). When human arteries with intact PVAT were exposed to TNFα, there was a reduction in the anticontractile function of PVAT, which resulted in a diseased obese phenotype (33). PVAT also enhanced the contractile response elicited by perivascular nerve activation by electrical field stimulation (EFS), and this enhancement involved the activation of tyrosine kinase and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways by superoxide generated in PVAT (34).
3.2 The Proinflammatory Effects of Perivascular Adipose
Adipose tissue depots originating from various precursor cells are functionally diverse, and mediate disease processes in a depot-specific manner (35). The functional properties of perivascular adipocytes, and their influence on disease of the blood vessel wall, remain to be investigated (35). Human coronary perivascular adipocytes demonstrate a reduced state of adipocytic differentiation when compared with adipocytes derived from subcutaneous and visceral adipose depots (35). Secretion of anti-inflammatory adiponectin is markedly reduced, but that of proinflammatory cytokines (IL-6, IL-8, and MCP-1) is considerably increased in perivascular adipocytes (35). Murine aortic arch perivascular adipose tissues also express lower levels of adipocyte-associated genes as compared with subcutaneous and visceral adipose tissues (35). Moreover, 2 weeks of HFD feeding caused further reductions in adipocyte-associated gene expression while concomitantly upregulating proinflammatory gene expression in perivascular adipose tissues (35). Thus, perivascular adipocytes exhibit a reduced differentiation and enhanced proinflammatory state. Dysfunction of perivascular adipose tissue induced by a HFD indicates that this unique adipose depot may link metabolic signals to inflammation in the blood vessel wall. The sensitivity of perivascular adipose tissue to the harmful influences of excess dietary fat may serve as a driving force for inflammatory cell recruitment to the vascular wall, which plays a primary role in development of adventitial inflammation, as well as atherosclerotic lesions (35).
3.3 The Endocrine/Paracrine Effects of Perivascular Adipose
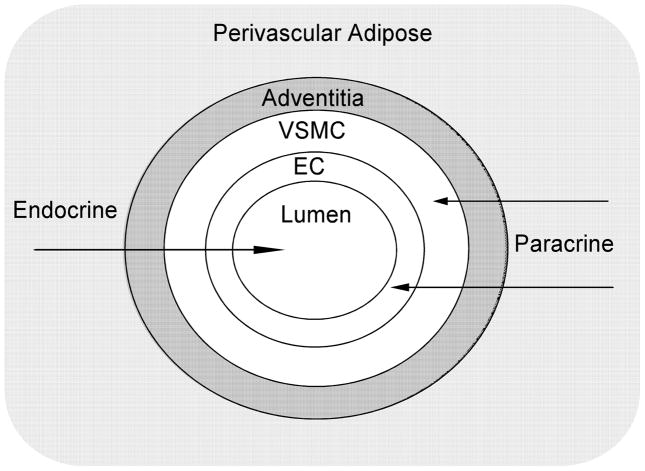
Endocrine and paracrine actions of adipocytes, especially those in the vicinity of cardiovascular organs, are the key factors that most likely link obesity with cardiovascular disease (36). Two areas of research that may provide insights into this linkage involve the vasoactive properties of PVAT and the inflammatory changes that occur in fat as obesity progresses. Perivascular fat may evolve from primordial cells in the adventitia or from circulating precursors migrating through the arterial wall (37). Once deposited periarterially, adipose tissue locally releases many adipokines that can potentially interact with the arterial wall. In addition, perivascular fat, per se, may attract circulating monocytes through the release of chemokines such as MCP-1 (37). Some of the macrophages traversing the arterial wall to the perivascular fat may be redirected and eventually populate the arterial wall itself, thereby enhancing vascular inflammation (37). (Figure 3)
Figure 3. The Endocrine/Paracrine Effects of Perivascular Adipose.
Endocrine and paracrine actions of adipose, especially those in the vicinity of cardiovascular organs, make them prime suspects for linking obesity to cardiovascular disorders. Perivascular fat may locally release many cytokines, hormones, and adipocyte-derived relaxing factors that can potentially interact with the arterial wall. In addition, perivascular fat may attract monocytes in the circulation through the release of chemokines such as monocyte chemoattractant protein-1 (MCP-1). Some of the macrophages traversing the arterial wall to the perivascular fat may be redirected and eventually populate the arterial wall itself, thereby accelerating vascular inflammatory processes. EC, endothelial cells; VSMC, vascular smooth muscle cells.
In vitro approaches in which coculture systems or conditioned media were used, demonstrated that human adipocytes increased intercellular adhesion molecule-1 (ICAM-1) and platelet endothelial cell adhesion molecule (PECAM) expression in endothelial cells (38). Mature adipocytes release hydrosoluble protein growth factor(s) for smooth muscle cells (SMCs). Perivascular adipose tissue stimulates SMC proliferation, which is enhanced in aged WKY and in HFD-induced obesity but not in leptin receptor-deficient obese Zucker rats (39). These tantalizing findings indicate that understanding of these and other factors will help define the linkage between adipose and cardiovascular diseases and provide better remediation strategies.
3.4 The Effects of Perivascular Adipose in Regulating Insulin Sensitivity
Different ectopic fat depots, such as visceral fat, are known to affect whole body insulin sensitivity. Hypothetically, PVAT around resistance vessels may also contribute to insulin resistance, possibly via direct vascular effects leading to reduced capillary cross-sectional area in the muscle, which in turn affects muscular blood flow and glucose uptake (40). A recent study (40) examined whether PVAT around conduit arteries (i.e. the brachial artery) influences NO bioavailability, expressed as flow-mediated dilation (FMD), or insulin sensitivity in humans in vivo. PVAT was negatively correlated with insulin sensitivity: the association between PVAT and insulin sensitivity was independent of age, sex, visceral adipose tissue, liver fat, body mass index (BMI) and further cardiovascular risk factors. No correlation could be detected between PVAT and local endothelial function. However, there was an independent association between PVAT and post-ischemic increase in blood flow (40). Therefore, PVAT may play an independent role in the pathogenesis of insulin resistance. This may be due to direct vascular effects affecting muscular blood flow.
In summary, accumulating evidence suggests perivascular adipose plays a role in the regulation of vascular function and insulin sensitivity. We recommend that longitudinal studies, including multiple time point samples, be dynamically undertaken in obesity bound animals to detect and document progressive changes in the function of perivascular and other adipose tissue depots that ultimately manifest in profound obesity-related changes such as atherosclerosis, endothelial dysfunction, and hypertension.
4. TLR4 Acts as a Crosslink between Inflammation and Insulin Resistance
Inflammatory response is associated with insulin resistance and obesity. TLR4 is abundant in adipose tissue and differentiated adipocytes and plays a crucial role in inflammation and immunity (41). Since it is activated by dietary saturated fatty acids, TLR4 may be a candidate participating in the crosstalk between inflammatory and metabolic signals. mRNA level of TLR4 was greatly enhanced in epididymal fat pads of male (2-fold) db/db mice compared to those of db/+ littermates. Interestingly, mRNA expression of TLR4 was increased in adipocytes of db/db mice compared to those of lean mice, while the expression level of TLR4 mRNA in the stromal vascular cell fraction was not increased in db/db mice (41). C3H/HeJ mice, which have a loss-of-function mutation in TLR4, are protected against the development of HFD-induced insulin resistance. More importantly, a loss of function mutation in TLR4 protects against HFD-induced decrease in serum adiponectin, and increase in serum TNFα and IL-6 (12). Inflamed adipose tissue constitutes the main source of adipose-derived cytokines, which are released from the depot and are thereby able to signal systemically (42–43). For example, IL-6-dependent insulin resistance is mediated, at least in part, by induction of SOCS3 in insulin target cells, which inhibits tyrosine phosphorylation of IRS (44–45); TNFα is a potent activator of JNK, which in turn phosphorylates IRS-1 at Ser 307 (46); adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1 (47). Thus, Adipose TLR4 may initiate and perpetuate insulin resistance in distant organs by modulating the production and circulatory levels of adipose-derived cytokines and hormones; this warrants further investigation, which would be aided by development of an adipose selective deletion of TLR4 in the murine model.
In summary, TLR4 appears to be an important mediator of obesity and insulin resistance and a potential target for the therapy of these highly prevalent and debilitating medical conditions.
4. Future Perspectives
A strong correlation is observed between obesity and insulin resistance. However, the existence and nature of a communication/linkage between adipose tissue and distant organs in the regulation of insulin action and vascular function needs further clarification. Future studies may better elucidate the mechanisms of this communication by delving into these aspects: 1) Clarify the primary event in adipose inflammatory cell infiltration and resultant change in the expression profile of various adipose-derived factors, which perpetuate adipose insulin resistance; 2) Direct and specific evidence is needed to determine if/how adipose communicates with other insulin sensitive organs, including liver and muscle, to regulate whole body insulin sensitivity through various adipose-derived cytokines, hormones and lipid signals; 3) The role of adipose, as peripheral tissue, results in altered levels of systemic regulators of appetite. Therefore, the CNS may be involved in appetite regulation and energy metabolism, thereby affecting the insulin resistance in peripheral tissues. Adipose/CNS signaling warrants further study. 4) Finally, studies are needed to examine the local pathogenic effects of perivascular fat compared to the systemic effects of obesity on vascular structure and function.
5. Concluding Remarks
Recent studies have identified adipose tissue as a critical site for whole body metabolic regulation. Growing evidence supports the concept that cytokines, peptides, hormones and lipid signals produced within adipose tissue constitute an important component of the endocrine effects of this site on systemic carbohydrate and lipid homeostasis, as well as the paracrine effects on vascular function. Although the challenge to fully understand the etiology of obesity, insulin resistance and vascular dysfunction continues, the research outlined in this review illustrates how a better understanding of the role of adipose insulin resistance and inflammation in the regulation of insulin action in distant organs may facilitate the development of rational approaches to the treatment of metabolic disease. These findings may lead to identifying new therapeutic targets to fight obesity and type 2 diabetes by affecting the adipokine profile and lipid metabolism.
Figure 4. TLR4 Acts as a Crosslink among Inflammation and Insulin Resistance.

Inhibition of TLR4 signaling in genetically modified mice demonstrates increased adiponectin level, and decreased IL-6 and TNFα level in the serum, as well as enhanced insulin signaling in adipose tissue, muscle, liver, and hypothalamus compared with control mice when both are fed a high fat diet.
Acknowledgments
This study was supported by grants from American Heart Association Scientist Development Grant (110350047A), Pfizer Atorvastatin Research Award (2004-37) and NIH grants (RO1-HL077566 and RO1-HL085119) to Dr. Cuihua Zhang.
Abbreviations
- ADRF
adipocyte-derived relaxing factor
- Akt
protein kinase B
- BMI
body mass index
- CNS
central nervous system
- EC
endothelial cells
- EDHF
endothelial-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- FFA
free fatty acids
- FMD
flow-mediated dilation
- GLUT
glucose transporter
- HFD
high fat diet
- ICAM-1
intercellular adhesion molecule-1
- IFNγ
interferon-gamma
- IKK
IkappaB kinase
- IL
interleukin
- IRS
insulin receptor substrate
- JNK
c-jun N-terminal kinase
- KCa
calcium-dependent K+ Channels
- Kv
voltage-gated K+ channels
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- NO
nitric oxide
- PECAM-1
platelet endothelial cell adhesion molecule
- PVAT
perivascular adipose tissue
- sGC
soluble guanylyl cyclase
- SHR
spontaneous hypertensive rat
- SOCS3
suppressor of cytokine signaling-3
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor-alpha
- VSMC
vascular smooth muscle cells
- WAT
white adipose tissue
- WKY
Wistar Kyoto rats
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 3.MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab. 2007;6:159–61. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–44. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis. 2009;19:146–52. doi: 10.1016/j.numecd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Nakatani Y, Kaneto H, Kawamori D, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 7.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita A, Soga Y, Iwamoto Y, et al. Macrophage-adipocyte interaction: marked interleukin-6 production by lipopolysaccharide. Obesity (Silver Spring) 2007;15:2549–52. doi: 10.1038/oby.2007.305. [DOI] [PubMed] [Google Scholar]
- 10.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–10. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 11.Rocha VZ, Folco EJ, Sukhova G, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–76. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells. 2006;21:174–85. [PubMed] [Google Scholar]
- 14.Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 15.Sabio G, Das M, Mora A, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–43. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–6. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 2006;14 (Suppl 1):41S–9S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- 18.Olefsky JM. Fat talks, liver and muscle listen. Cell. 2008;134:914–6. doi: 10.1016/j.cell.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610. [PubMed] [Google Scholar]
- 20.Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55 (Suppl 2):S145–54. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Hong SM, Sung SR, Jung HK. Long-term effects of central leptin and resistin on body weight, insulin resistance, and beta-cell function and mass by the modulation of hypothalamic leptin and insulin signaling. Endocrinology. 2008;149:445–54. doi: 10.1210/en.2007-0754. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 23.Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–32. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engeli S. Is there a pathophysiological role for perivascular adipocytes? Am J Physiol Heart Circ Physiol. 2005;289:H1794–5. doi: 10.1152/ajpheart.00762.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and type 2 diabetes: evidence of an adipose-vascular loop. Am J Biomed Sci. 2009;1:133–42. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–53. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–31. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvez B, de Castro J, Herold D, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 29.Gao YJ, Zeng ZH, Teoh K, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005;130:1130–6. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J Vasc Res. 2007;44:175–81. doi: 10.1159/000099833. [DOI] [PubMed] [Google Scholar]
- 31.Lee RM, Lu C, Su LY, Werstuck G, Gao YJ. Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J Hypertens. 2009;27:118–31. doi: 10.1097/HJH.0b013e3283163cc9. [DOI] [PubMed] [Google Scholar]
- 32.Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 33.Wimalasundera R, Fexby S, Regan L, Thom SA, Hughes AD. Effect of tumour necrosis factor-alpha and interleukin 1beta on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br J Pharmacol. 2003;138:1285–94. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao YJ, Takemori K, Su LY, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–73. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–9. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 37.Stern N, Marcus Y. Perivascular fat: innocent bystander or active player in vascular disease? J Cardiometab Syndr. 2006;1:115–20. doi: 10.1111/j.1559-4564.2006.05510.x. [DOI] [PubMed] [Google Scholar]
- 38.Curat CA, Miranville A, Sengenes C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–92. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 39.Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–13. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 40.Rittig K, Staib K, Machann J, et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia. 2008;51:2093–9. doi: 10.1007/s00125-008-1128-3. [DOI] [PubMed] [Google Scholar]
- 41.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–45. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 43.Andersson CX, Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab Res Rev. 2008;24:595–603. doi: 10.1002/dmrr.889. [DOI] [PubMed] [Google Scholar]
- 44.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–8. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 45.Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–6. doi: 10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 46.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Mao X, Wang L, et al. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282:7991–6. doi: 10.1074/jbc.M700098200. [DOI] [PubMed] [Google Scholar]