Abstract
Efforts to develop ligands that distinguish between clinically relevant 5-HT2A and 5-HT2C serotonin receptor subtypes have been challenging, because their sequences have high homology. Previous studies reported that a novel aplysinopsin belonging to a chemical class of natural products isolated from a marine sponge was selective for the 5-HT2C over the 5-HT2A receptor subtype. Our goal was to explore the 5-HT2A/2C receptor structure-affinity relationships of derivatives based on the aplysinopsin natural product pharmacophore. Twenty aplysinopsin derivatives were synthesized, purified and tested for their affinities for cloned human serotonin 5-HT1A, 5-HT2A and 5-HT2C receptor subtypes. Four compounds in this series had >30-fold selectivity for 5-HT2A or 5-HT2C receptors. The compound (E)-5-((5,6-dichloro-1H-indol-3-yl)methylene)-2-imino-1,3-dimethylimidazolidin-4-one (UNT-TWU-22, 16) had approximately 2100-fold selectivity for the serotonin 5-HT2C receptor subtype: an affinity for 5-HT2C equal to 46 nM and no detectable affinity for the 5-HT1A or 5-HT2A receptor subtypes. The two most important factors controlling 5-HT2A or 5-HT2C receptor subtype selectivity were the combined R1, R3-alkylation of the imidazolidinone ring and the type and number of halogens on the indole ring of the aplysinopsin pharmacophore.
Keywords: 5-HT2C receptor selective ligand, 5-HT2C, serotonin receptor, 5-hydroxytryptamine, natural product analogue, aplysinopsin
1. Introduction
Aplysinopsins (Figure 1A) are a chemical class of natural product indole alkaloids, whose name derives from the marine sponge Thorecta aplysinopsis from which it was originally isolated.1 Aplysinopsins have also been purified from other sponges, including Verongia spengelii,2 Dercitus,3 Hyrtios erecta,4 Smenospongia aurea,5 and other Thorectandra, Smenospongia and Verongula species as well.6,7 Tissues from Cnidarians such as the corals Tubastraea faulkneri8 and Leptopsammia pruvoti9, and from the sea anemone Radiantius kuekenthali10 also contain aplysinopsins; however, in the latter case their presence was specifically attributed to an unidentified species of dinoflagellates symbiotic with the anemone10 raising the possibility that aplysinopsins are not endogenous to Porifera and Cnidaria, rather that they might be from a microbial source.
Figure 1.
A) Aplysinopsin pharmacophore and numbering. The original aplysinopsin reported had R1 = R3 = CH3, R2 = R4 = R5 = H.2 B) The structure of 6-bromo-2′-de-N-methylaplysinopsin or 6-bromo-R3-methylaplysinopsin reported by Hu5 (also known as Natural Product #6 in that report).
Although anti-tumor2 and anti-filarial11 activities have been ascribed to aplysinopsins, our interest relates to a previous discovery in our lab that a few aplysinopsins bind to human serotonin 5-HT2A and 5-HT2C receptors with moderate to low affinities. One of these structures identified as 6-bromo-2′-de-N-methylaplysinopsin ((Figure 1B), exhibited considerably higher affinity (>40-fold) for the 5-HT2C receptor than the 5-HT2A receptor5. This is of interest, because finding structurally simple small molecule pharmacophores that can distinguish between the closely related and clinically important 5-HT2A and 5-HT2C receptor subtypes has remained a challenge.12 Here we report on the synthesis of a library of novel aplysinopsin derivatives and their structure-affinity relationships for cloned human serotonin 5-HT2A and 5-HT2C receptors as well as another closely related and clinically important serotonin receptor subtype, the 5-HT1A receptor. The preference for 5-HT2A or 5-HT2C receptor subtypes can be controlled by changing the type and extent of halogenation on the aplysinopsin indole ring. Some of these modifications resulted in higher affinity compounds that preferentially bound either the 5-HT2A or 5-HT2C receptors. To avoid the use of “de-methyl” designations and for added clarity the aplysinopsin pharmacophore will be described here without the assumption that R1 and R3 are methylated as in the original aplysinopsin structure, rather alkyl substitutions at R1 and R3 will be explicitly defined in the simplified naming throughout.
2. Results
2.1. Synthesis of a library of aplysinopsin derivatives
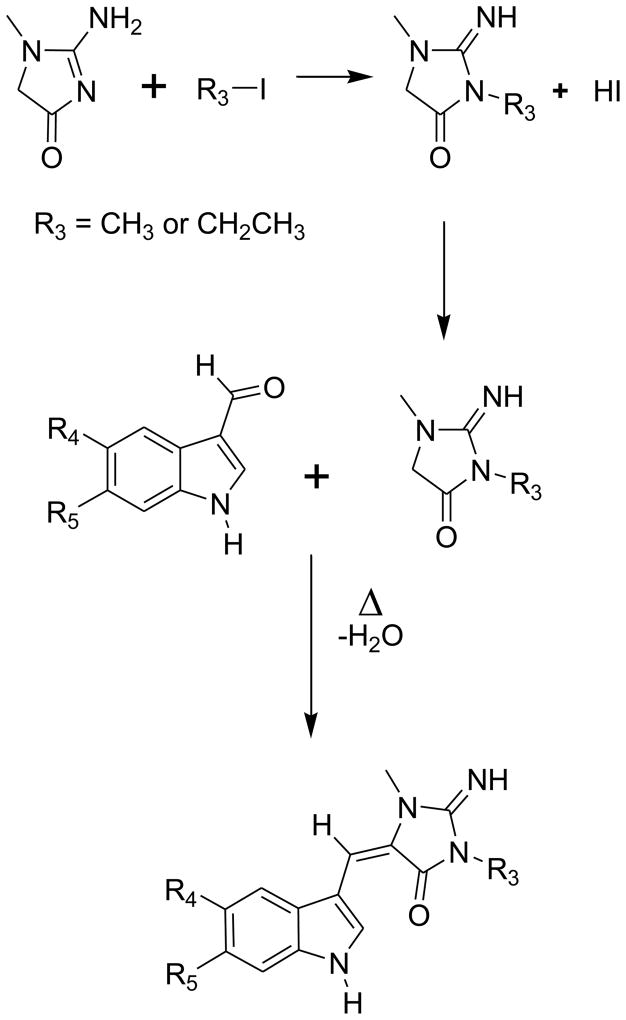
A library of aplysinopsin analogues were synthesized from indole aldehydes and differentially alkylated 2-imino-imidazolidin-4-ones utilizing a scheme similar to that described by Djura and Faulkner3 (Figure 2). The indole aldehydes (indole-3-carboxaldehydes) were either commercially available or were synthesized using a procedure described by Jiang et al13. The 2-imino-1,3-dimethylimidazolidin-4-one and 3-ethyl-2-imino-1-methylimidazolidin-4-one were synthesized by methylation or ethylation of creatinine (2-amino-1-methyl-2-imidazolin-4-one) utilizing a procedure by Kenyon and Rowley14 (Figure 2). Single crystal structures were determined for 1 and 2 and in both cases the double bond linking the indole and imidazolidine rings (C8-C1′) were in the E-configuration15. Compounds 1 and 3–8 can exist in two tautomeric forms: one where the imine double bond is endocyclic and the other where the imine double bond is exocyclic as shown.
Figure 2.
Synthesis of 2-imino-1,3-dimethylimidazolidin-4-one and 3-ethyl-2-imino-1-methylimidazolidin-4-one moieties and subsequent synthesis of aplysinopsin analogs.

The single crystal structure of 1 shows that the imidazolone ring exists in the endocyclic tautomeric form in the solid state. The 1H-NMR of 1 shows an absorption at 7.73 ppm integrating for two hydrogen atoms. This absorption must be due to the NH2 group, which demonstrates that 1 exists in DMSO-d6 solution in the endocyclic form.
2.2. Structure-affinity relationships for aplysinopsin derivatives
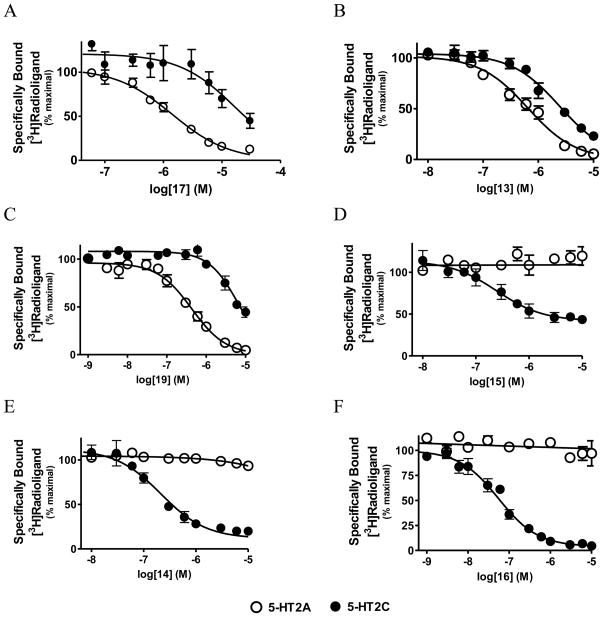
A series of twenty synthesized compounds possessing an aplysinopsin pharmacophore were examined at a high concentration (1–3 μM) for the ability to displace specifically bound radioligands from 5-HT1A, 5-HT2A and 5-HT2C receptors (Table 1). This initial screen revealed that compounds lacking R3-alkylation of the imidazolidine (1, 3–8) did not strongly interact with any of the three subtypes of serotonin receptor. With the exceptions of the 5-fluoro (2) and 5-bromo derivatives (9), all R1, R3-dimethyl and R1, R3-methyethyl derivatives (10–20) displaced at least 40% of specifically bound radioligand from one or more of the three receptor systems (Table 1). Of these eleven compounds, the monohalogenated 5-chloro (10), 5-bromo (11) and 5-iodo (12) derivatives were least able to distinguish between the three 5-HT receptor subtypes. In contrast, monohalogenation at the 6-position controlled preference for either 5-HT2A or 5-HT2C receptors over the other two receptor subtypes. For example, the 6-fluoro compound (13) preferentially interacted with the 5-HT2A receptor, while the 6-chloro (14) and 6-bromo (15) analogues preferred interactions with the 5-HT2C receptor (Figure 3-B, 3-E, 3-D). The higher selectivity ratio of 14 relative to 15 (>602-fold vs. >45-fold) is due to its higher affinity for the 5-HT2C receptor (Figure 3-D and 4-E, Table 2). Even though the 5-monochloro derivative 10 does not display a subtype preference (Table 1), the addition of a 5-chloro substituent to 14 to make the 5,6-dichloro derivative (UNT-TWU-22, 16) improves 5-HT2C selectivity further (>2170-fold for 16 compared to >602-fold for 14, Figure 3-E and 3-F, Table 2). This enhanced selectivity for 16 is due to its increased affinity for the 5-HT2C receptor.
Table 1.
Chemical structures and single point data for displacement of specifically bound radioligands from cloned serotonin 5-HT1A, 5-HT2A and 5-HT2C receptors. All compounds were screened at 3 μM, except 15, which due to poor solubility had to be screened at 1 μM. For all the structures shown in the table R1 = CH3 and R2 = H
 | ||||||
|---|---|---|---|---|---|---|
| Cmp No. | R3 | R4 | R5 | 1A | % displacement 2A | 2C |
| 1 | H | Br | H | 6±2 | 0±5 | 0±5 |
| 2 | CH3 | F | H | 4±3 | 10±1 | 0±6 |
| 3 | H | Cl | H | 9±3 | 9±1 | 0±6 |
| 4 | H | Cl | Cl | 4±2 | 0±4 | 10±5 |
| 5 | H | F | H | 0±3 | 0±1 | 19±6 |
| 6 | H | H | F | 0±4 | 6±4 | 1±8 |
| 7 | H | H | Cl | 1±2 | 0±3 | 0±6 |
| 8 | H | H | H | 0±6 | 0±2 | 6±10 |
| 9 | CH2CH3 | Br | H | 0±0 | 9±3 | 18±10 |
| 10 | CH3 | Cl | H | 9±2 | 22±4 | 43±11 |
| 11 | CH3 | Br | H | 18±2 | 37±1 | 64±5 |
| 12 | CH2CH3 | I | H | 15±1 | 35±2 | 46±6 |
| 13 | CH3 | H | F | 2±3 | 79±3 | 0±5 |
| 14 | CH3 | H | Cl | 3±3 | 0±5 | 49±5 |
| 15 | CH3 | H | Br | 1±3 | 8±1 | 46±8 |
| 16 | CH3 | Cl | Cl | 0±4 | 12±2 | 79±4 |
| 17 | CH3 | H | H | 0±1 | 47±3 | 0±7 |
| 18 | CH2CH3 | H | H | 0±3 | 50±2 | 0±5 |
| 19 | CH2CH3 | H | F | 0±1 | 86±1 | 0±5 |
| 20 | CH2CH3 | H | Br | 0±1 | 0±8 | 49±10 |
Figure 3.
Competition binding for selected compounds for cloned serotonin 5-HT2A (open circles) and 5-HT2C (closed circles) receptors (n = 3).
Table 2.
Compound affinities for cloned human serotonin 5-HT2A and 5-HT2C receptor subtypes (n =3).
| Name | Structure | Binding Affinity (Ki nM) | Ki Selectivity Ratio2 | |
|---|---|---|---|---|
| 5-HT2A±SEM | 5-HT2C±SEM | 5-HT2A/5-HT2C | ||
| 13 |  |
235±42 | 2114±203 | 0.11 |
| 14 |  |
N. D. | 166±55 | >602 |
| 15 |  |
N.D. | 2202±674 | >45 |
| 16 |  |
N.D. | 46±8.6 | >2170 |
| 17 |  |
598±53 | 14,451±5893 | 0.041 |
| 18 |  |
655±94 | 8323±2911 | 0.079 |
| 19 |  |
173±42 | 5230±858 | 0.033 |
ND = not detected at highest concentration tested.
For those affinity values that could not be detected at the highest concentration tested (see Figure 4), an affinity of >100,000 nM is assumed for the purpose of the selectivity calculations.
Aplysinopsin derivatives that either lack indole ring halogenation (17, 18) or have a 6-monofluoro substituent (13, 19) preferred interactions with 5-HT2A over 5-HT1A and 5-HT2C receptors (Figure 3 and Table 2). A methyl (17) to ethyl (18) substitution at R3 of the unhalogenated derivatives 17 and 18 or of the 6-fluoro derivatives 13 and 19 does not significantly affect the displacement profile for any of the three receptor subtypes (Table 1) or the affinity profile for 5-HT2A and 5-HT2C receptors (Table 2, 20 and 15Figure 3). A similar trend for ethylation versus methylation of R3 is observed for the 6-monobromo derivatives (Table 1). However, in the case of 5-monobromo derivatives the displacement profiles suggest that methylation of R3 (11) is preferred over ethylation (9) by all the serotonin subtypes (Table 1).
Interest in the aplysinopsin pharmacophore as a molecular scaffold with the potential to differentiate between the 5-HT2C and 5-HT2A receptor subtypes evolved from our previous work with aplysinopsins isolated from natural sources. One of those isolated aplysinopsins (natural Product #6, 6-bromo-2′-de-N-methylaplysinopsin) had reasonably good selectivity (>40-fold) for the 5-HT2C over the 5-HT2A receptor albeit its affinity for the 5-HT2C receptor was quite low (2.3 μM).5 Although we did not attempt to synthesize 6-bromo-2′-de-N-methylaplysinopsin due to its low affinity, we did synthesize the much less selective but higher affinity natural product #7 (6-bromoaplysinopsin)5 whose reported structure is identical to our 15. This allowed us to compare affinity values when 15 was derived from natural or synthetic sources. While 15 from both sources preferentially interacts with 5-HT2C over 5-HT2A receptors, the affinity values were quite different: 2.2 μM vs. 0.33 μM at 5-HT2C and not detectable vs. 2.0 μM at 5-HT2A when derived from synthetic versus natural sources, respectively. Although the isolated natural products were screened in a COS-7 cell line transient expression system and synthetic compounds were screened in an HEK293 cell line stable expression system, the other assay conditions were the same. One possibility for the discrepancy is that the isolated natural product samples, which were gummy in consistency, might have contained impurities with radioligand displacing activity.
3. Discussion
A key discovery is the versatility of the aplysinopsin pharmacophore in the context of the three 5-HT receptor subtypes investigated: 5-HT1A, 5-HT2A, 5-HT2C. Small molecule aplysinopsins can be created that preferentially interact with either 5-HT2C or 5-HT2A receptor subtypes. Based upon the elaborations examined in the series presented here, much higher levels of 5-HT2C selectivity (>2100-fold) could be achieved than 5-HT2A selectivity (>30-fold). As long as the 1 and 3 positions of the imidazolidinone ring are alkylated, selectivity for one or the other of these subtypes can be controlled by the type of halogenation at position 6 of the indole ring. Interactions with 5-HT2A over 5-HT2C receptors are favored when the indole ring contains a 6-fluoro substituent or is unhalogenated, while interactions with 5-HT2C over 5-HT2A receptors are favored for 6-chloro or 6-bromo derivatives.
Because the R1, R3-dimethyl-6-chloro-aplysinopsin (14) had the highest degree of 5-HT2C selectivity due to its higher affinity for this receptor subtype and lack of affinity for the 5-HT1A and 5-HT2A receptor subtypes, it was chosen as the best structure for investigating the effect of 5,6-dihalogenation of the indole. Addition of a 5-chloro to make the 5,6-dichloro-aplysinopsin (16) increased the affinity for 5-HT2C more than 3-fold beyond that of the 6-chloro congener (14) without increasing the affinities for the 5-HT1A or 5-HT2A receptor subtypes; the net effect was an increase in 5-HT2C selectivity from >602-fold for (14) to >2170-fold for (16). The critical role of alkylation at the R3-position of the imidazolidinone ring was also confirmed for the 5,6-dichloro derivative since removal of the R3-alkyl as in 4 results in a ligand with little or no apparent affinity for any of the three receptor subtypes.
The loss of affinity at all three serotonin receptor subtypes, when R3 lacks alkylation is likely due to the reduced probability of protonating the nitrogen attached to the 3′ carbon. This nitrogen is assumed to interact with the conserved aspartic acid approximately two helical turns from the extracellular side of the third transmembrane-spanning domain (Asp(116) for 5-HT1A, Asp(155) for 5-HT2A and Asp(134) for 5-HT2C; biogenic amine GPCR universal indexing D3.32). This is a reasonable assumption, because a protonatable amine is required for high affinity interactions in other G protein-coupled biogenic amine receptors16, and replacing the nitrogen attached to the 3′ carbon with an oxygen or sulfur results in no detectable affinity for any of the three 5-HT receptors tested (data not shown).
Although we cannot discount the effects of electronic factors, we speculate that the size of the halogen at position 6 most likely influences ligand selectivity via appropriately oriented hydrophobic bulk. If lipophilicity or ring electronegativity alone were the critical factors then 5-halogenated derivatives might be expected to have selectivity patterns similar to their 6-halogenated congeners, which was not the case. Unhalogenated (17–18) and 6-fluorinated (13, 19) derivatives had a preference for the 5-HT2A receptor; however, when chlorine or bromine occupies position 6 (14–16 and 20) interactions with the 5-HT2C receptor were preferred. This further suggests that subtype preference may depends upon the size of the substituent at the 6 position, because fluorine and hydrogen are similar in size, while chlorine and bromine are considerable larger.
Indole and indoline ring halogenation, while important for serotonin receptor affinity, has only occasionally led to compounds with high selectivity between the 5-HT2A and 5-HT2C receptor subtypes. In a study of racemic 2-(indol-1-yl)-1-methylethylamines mixtures, the 5-methyl derivative had a 13-fold preference for 5-HT2C over 5-HT2A receptors. Replacement of the 5-methyl group with a halogen (i.e., F, Cl or Br) increased ligand affinity for both 5-HT2A and 5-HT2C (Table 3).17 Since the improvement in affinity was somewhat more pronounced for the 5-HT2C receptor this led to an increased preference for 5-HT2C (3-fold improvement for the 5-bromo) (Table 3).17 The 6-fluoro derivative had an approximately 2-fold greater preference for 5-HT2C than did the 5-fluoro derivative (Table 3).17 While comparison is difficult, the most 5-HT2C selective enantiomerically pure 5,6-difluoronated derivative, the S-enantiomer, only had a 2-fold improvement over the racemic 6-fluoro derivative. This improvement was mostly due to an increased affinity for 5-HT2C (Table 3).17 In similar structures containing an indoline (i.e., 1-(1-indolinyl)-2-propylamines) instead of an indole, halogenation at the 6 or 5,6 positions results in no improved preference for 5-HT2C over 5-HT2A receptor as compared to the 6-thiomethyl, which has the highest preference in the series.18
Table 3.
Selected structures and affinity values from the literature highlighting the role of indole and indoline ring halogenated on compound selectivity for 5-HT2A and 5-HT2C receptors.
| Reference | Structure | R Group | Affinity (Ki, nM) | 2A/2C Ki Ratio | |
|---|---|---|---|---|---|
| 5-HT2A | 5-HT2C | ||||
| 17 |  |
5-Me (R, S) | 794 | 63 | 13 |
| 5-F (R, S) | 159 | 6.3 | 25 | ||
| 5-Cl (R, S) | 200 | 7.9 | 25 | ||
| 5-Br (R, S) | 159 | 4.0 | 40 | ||
| 6-F (R, S) | 251 | 4.0 | 63 | ||
| 5-F, 6-F (S) | 100 | 1.0 | 100 | ||
| 19 |  |
Unsubstituted | >10000 | 1259 | >8 |
| 5-Cl | 2512 | 79 | 32 | ||
| 6-Cl | 794 | 50 | 16 | ||
| 5,6-Cl | 251 | 7.9 | 32 | ||
| 4,5-Cl | 631 | 25 | 25 | ||
| 4-Br, 5-Me | 126 | 7.9 | 16 | ||
| 4-I, 5-Me | 2512 | 25 | 100 | ||
| 5-I, 6-Cl | 100 | 3.2 | 32 | ||
| 5-Cl, 6-Me | 398 | 10 | 40 | ||
| 5-Me, 6-Cl | 159 | 6.3 | 25 | ||
| 5-Me, 6-Br | 63 | 4.0 | 16 | ||
| 5-Me, 6-I | 63 | 3.2 | 20 | ||
| 6,7-Cl | 5012 | 794 | 6 | ||
| 21 |  |
Unsubstituted | >10000 | 100 | >100 |
| 5-F | >10000 | 40 | >251 | ||
| 5-Cl | >10000 | 6.3 | >1585 | ||
| 5-Br | 3981 | 3.2 | 1260 | ||
| 5-I | 1585 | 3.2 | 502 | ||
| 6-Cl | 316 | 4.00 | 80 | ||
| 5,6-Cl | 251 | 2.0 | 126 | ||
| 5-F, 6-Cl | 501 | 6.3 | 79 | ||
In the case of 1-(3-pyridylcarbamoyl)indolines, the unhalogenated parent compound has low affinity for 5-HT2C (1.3 μM) and no detectable affinity (>10 μM) for 5-HT2A at the highest concentration tested (~8 μM) (Table 3) 19. With the exception of a 6,7 dichloro derivative, all 1-(3-pyridylcarbamoyl)indolines with mono- or dihalogenated substitutions of the indoline ring (regardless of whether the indoline ring also contains a methyl substitution) exhibited moderately increased selectivity for 5-HT2C as a function of increased affinity at both 5-HT2A and 5-HT2C receptors (Table 3).19 Specifically, a 5-chloro or 6-chloro substitution resulted in a respective 32-fold and 16-fold increased selectivity for 5-HT2C over 5-HT2A receptors. Further improvements in selectivity were observed for 5,6-dichloro or 5-iodo, 6-chloro substitutions over the 6-chloro. Due to the undesirable interaction of “short” indoline structures with CYP4501A2,20 the extended 1-[2-[[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]indolines, were synthesized and tested.21 In comparison to the unhalogenated parent compound, substitution of any halogen at position 5 (i.e., F, Cl, Br, I) increased the selectivity for the 5-HT2C receptor by as much as 16-fold (Table 3).21 The 6-chloro derivative was considerably less 5-HT2C selective than the 5-chloro derivative due primarily to an increase in the affinity of the 6-chloro derivative for the 5-HT2A receptor (Table 3).21 No significant improvements were observed for the 5,6-dichloro or the 5-fluoro,6-chloro derivatives over the 5-monochlorinated derivative (Table 3).21
Here we report on a low molecular weight aplysinopsin pharmacophore that serves as a versatile chemical scaffold for synthesizing compounds capable of differentiating between cloned human 5-HT2C and 5-HT2A receptor subtypes. To our knowledge, the aplysinopsin derivatives are the only indole-containing structural class of compounds without a substituent bound to the indole nitrogen that display a significant degree of preference between 5-HT2C and 5-HT2A receptor subtypes. An additional finding is that the aplysinopsin pharmacophore can be uniquely tuned to select for 5-HT2A over 5-HT2C (13, 19) or for 5-HT2C over 5-HT2A (14–16, 20) through a halogen selectivity filter at the 6-position (or 5,6-position) of the indole ring. Selectivity for the 5-HT2C receptor subtype was more readily achievable in that compounds could be made that displayed essentially no detectable affinity for 5-HT2A or 5-HT1A subtypes. Future elaborations on this natural product pharmacophore are likely to produce selective compounds with higher affinities.
4. Experimental Section
4.1. Synthetic Procedures: General Methods
All chemicals used in this research were reagent grade and were used without purification unless specified. Melting points were determined in a OptiMelt Automated Melting Point System by Stanford Research Systems or a Mel-Temp melting point apparatus and are uncorrected. Column Chromatography was carried out using Silica 200–300 mesh from Natland International Corporation. Infrared spectra were determined on nujol mulls samples between sodium chloride plates using a Nicolet Magna 560 FTIR over the frequency range of 4000–600 cm−1. 1H NMR spectra were obtained in DMSO-d6 on a Varian Mercury 300 MHz spectrometer. Chemical shifts (δ) are given in parts per million relative to TMS and coupling constants (J) are given in hertz (Hz). The high resolution mass spectra were run at the University of Minnesota using either a Finnigan MAT 95 or Bruker BioTOF mass spectrometer. Compound purities were estimated to be >90% by NMR spectroscopy. When present, peaks representing the solvent DMF are noted.
4.2. Synthesis of Indole-3-carboxaldehydes
The procedures utilized for the synthesis of indole-3-carboxaldehydes and their halogenated derivatives were those reported previously by Jiang et al.13, Johnson et al.15, Kalir and Szara22, Noland and Reich23, James and Snyder24, and Somei et al.25. Briefly, Phosphorus (V) oxychloride (4.25 g, 0.028 mol) was added drop wise to DMF (6 mL) cooled in an ice bath. The mixture was kept at 0°C for approximately 30 min. A solution of the indole (3 g, 0.015 mol) in DMF (22 mL) was added drop wise, keeping the reaction mixture below 10°C. After 3 hr at 20°C the solution was poured into an ice-water mixture (180 g), neutralized with 1N NaOH, and left overnight at room temperature. The crude product was collected by filtration and recrystallized from ethanol/water to give the corresponding indole-3-carboxaldehyde. In all cases the descriptive and analytical values (e.g., melting points and NMR spectra) were identical to those reported previously. Additional data not cited in the initial reports were gathered for some of the indole-3-carboxaldehydes and are reported here: 5-fluoroindole-3-carboxaldehyde, Anal. Calcd for C9H6NFO: C, 66.26; H, 3.71; N, 8.59; F, 11.64; Found: C, 66.37; H, 3.83; N, 8.60; F, 11.89, and 5-iodoindole-3-carboxaldehyde, Anal. Calcd. For C9H6NIO: C, 39.88; H, 2.23; N, 5.17, I, 46.82; Found: C, 40.04; H, 2.36; N, 5.19; I, 46.61. Compounds 8 and 17 have been reported previously.3 Our 1H-NMR spectra were identical to the reported spectra.
4.3. Synthesis of 2-amino-1,3-dimethylimidazolidin-4-one
The procedure used for this synthesis was reported by Kenyon and Rowley.14
4.4. Synthesis of 2-amino-3-ethyl-1-methylimidazolidin-4-one
The procedure used for this synthesis was reported by Kenyon and Rowley.14
4.5. Reaction of indole aldehydes with creatinine or alkylated creatinine derivatives
The procedure for this reaction was originally reported by Djura and Faulkner.3 Indole-3-carboxaldehyde and creatinine (or methylated or ethylated creatinine derivatives) were placed in a 100 mL three-neck round-bottomed flask fitted with a nitrogen gas inlet, and a probe from a J-KEM 210 temperature controller for monitoring the temperature. The flask was then heated cautiously with a Bunsen burner and the temperature was kept at approximately 153 °C for 10 min. Considerable frothing of the mixture took place during the course of the reaction. The reaction mixture was allowed to cool to room temperature and then extracted with methanol (2×50mL). The remaining solid was recrystallized from DMF-water to give the desired product.
4.6. 2-amino-5-(5-bromo-1H-indol-3-ylmethylene)-1-methyl-1,5-dihydro-imidazol-4-one. (1)
This compound was synthesized and structurally characterized as reported by us recently15.
4.7. 5-(5-fluoro-1H-indol-3-ylmethylene)-2-imino-1,3-dimethyl-imidazolidin-4-one (2)
This compound was synthesized and structurally characterized as reported by us recently15.
4.8. (E)-2-amino-5-[(5-chloro-1H-indol-3-yl)methylene]-1-methyl-1H-imidazol-4(5H)-one (3)
5-chloroindole-3-carboxaldehyde (5.00 g, 0.028 mol) and creatinine (3.43 g, 0.030 mol) were reacted using the procedure described above to give 3. Recrystallization from DMF-water gave an orange solid (2.68 g, 54% yield), m.p. 208°C.
IR (cm−1): 3321, 3190, 3159, 1696, 1661 1627 cm−1.
1H-NMR: δ: 11.63 (s, 1H), 9.13 (d, J = 2.7 Hz, 1H), 8.05 (d, J = 2.1 Hz, 1H), 7.96 (s, DMF), 7.73 (s, 2H), 7.44 (d, J = 8.7 Hz, 1H), 7.26 [dd, J = 8.7 Hz and J = 1.8 Hz, 1H), 6.55 (s, 1H), 3.30 (s, 3H,), 2.86 (s, DMF), 2.70 (s, DMF).
HRMS m/z calcd for C13H12N4ClO = 275.0694 (M·+ + H); found 275.0691.
4.9. (E)-2-amino-5-[(5,6-dichloro-1H-indol-3-yl)methylene]-1-methyl-1H-imidazol-4(5H)-one (4)
5,6-dichloroindole-3-carboxaldehyde (1.00 g, 0.0046 mol) and creatinine (0.556 g, 0.0049 mol) were reacted using the procedure described above to afford 4 a red solid compound. (0.61g, 61% yield), m.p. 190 °C
IR (cm−1): 3457, 3173, 2719, 1675, 1639, 1608, 1531 cm−1
1H-NMR: δ: 11.68 (s, 1H), 9.15 (s, 1H), 8.28 (s, 1H), 7.67 (s, DMF), 6.54 (s, 1H), 3.29 (s, 3H).
HRMS m/z calcd for C13H11N4Cl2O, 309.0304 (M·+ + H); found 309.0303.
4.10. (E)-2-amino-5-[(5-fluoro-1H-indol-3yl)methylene]-1-methyl-1H-imidazol-4(5H)-one (5)
5-fluoroindole-3-carboxaldehyde (1.00 g, 0.0074 mol) and creatinine (0.759 g, 0.0071 mol) were reacted using the above procedure to give compound 5. (0.75g, 75% yield), m.p. 291–292°C.
IR (cm−1): 3375, 3266, 3151, 2729, 1650, 1621, 1564 cm−1
1H-NMR δ: 9.15 (d, J = 2.7 Hz), 7.96 (s, DMF), 7.77 (dd, J = 10.2 and 2.4 Hz, 1H), 7.44 (dd, J = 9.0 and 4.7 Hz, 1H), 7.01 (dd, J = 9.0 and 2.4 Hz, 1H), 3.29 (s, 3H), 2.89 (s, DMF), 2.74 (s, DMF).
HRMS m/z calcd for C13H12N4FO, 259.099 (M·+ + H); found 259.0992.
4.11. (E)-2-amino-5-[(6-fluoro-1H-indol-3yl)methylene]-1-methyl-1H-imidazol-4(5H)-one (6)
6-fluoroindole-3-carboxaldehyde (1.0 g, 0.0074 mol) and creatinine (0.84 g, 0.0074 mol) were reacted according to the above procedure to give 6. (0.61, 61% yield), m.p. 258–260°C.
IR (cm−1): 3231, 2725, 1667, 1589 cm−1.
1H-NMR: δ: 11.49 (d, J =1.8 Hz), 9.06 (d, J = 2.7 Hz, 1H), 7.95 (s, DMF), 7.91 (dd, J = 7.8 and 6.3 Hz, 1H), 7.71 (s, br, 2H), 7.21 (dd, J = 9.8 and 2.3 Hz), 6.97 (qd, J = 3.0, 2.4 and 0.9 Hz, 1H), 6.51 (s, 1H), 3.28 (s, 3H), 2.89 (s, DMF), 2.73 (s, DMF).
HRMS m/z calcd for C13H12N4FO, 259.099 (M·+ + H); found 259.0992.
4.12. (E)-2-amino-5-[(6-chloro-1H-indol-3-yl)methylene]-1-methyl-1H-imidazol-4(5H)-one (7)
6-chloroindole-3-carboxaldehyde (1.00 g, 0.0060 mol) and creatinine (0.685g, 0.0060 mol) were reacted using the above procedure to give 7. (0.44g, 44%yield), m.p. 300–302°C.
IR (cm−1): 3448, 3184, 3148, 3080, 1660, 1622 cm−1
1H-NMR: δ: 11.57 (s, 1H), 9.10 (d, J = 1.8 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.75 (s, 2H), 7.48 (d, J = 2.1 Hz, 1H), 7.12 (dd, J = 8.4 Hz and J = 1.8 Hz), 3.28 (s, 3H), 2.89 (s, DMF), 2.73 (s, DMF).
HRMS m/z calcd for C13H12N4FO, 289.085 (M·+ + H); found 289.0851.
4.13. (E)-5-((1H-indol-3yl)methylene)-2-amino-1-methyl-1H-imidazol-4(5H)-one (8)
The synthesis and structurally characterization of this compound has been reported previously3. Indole-3-carboxaldehyde (2.00 g, 0.014 mol) and creatinine (1.40 g, 0.012 mol) were reacted using the above procedure to give 8 (1.15g, 58% yield), m.p. 287–290°C.
IR (cm−1): 3360, 3184, 2714, 1675, 1650, 1613 cm−1.
1H-NMR: δ: 11.47 (s, 1H), 9.09 (d, J = 2.7, 1H), 7.89 (d, J = 7.2 Hz, 1H), 7.71 (s, 2H), 7.58 (d, J = 7.2, 1H), 7.13 (m), 6.55 (s, 1H), 3.29 (s, 2H). The NMR is identical to that reported by Djura and Faulkner, 1980.
HRMS m/z calcd 241.1087 (M·+ + H); found 241.1084.
4.14. (Z)-5-[(5-bromo-1H-indol-3yl)methylene]-3-ethyl-2-imino-1-methylimidazolidin-4-one (9)
5-bromoindole-3-carboxaldehyde (0.37 g, 0.0017 mol) and2-amino-3-ethyl-1-methylimidazolidin-4-one (0.233 g, 0.0017 mol) were reacted using the above procedure to give 9 (0.26g, 70% yield), m.p. 305–307°C.
IR (cm−1): 3318, 3194, 3148, 1716, 1666, 1612 cm−1.
1H-NMR: δ:12.19 (d, J = 8.7, 1H), 9.40 (s, 1H), 8.99 (d, J = 2.7, 1H), 8.39 (d, J = 1.5 Hz, 1H), 7.48 (d, J = 8.7, 1H), 7.36 (dd, J = 8.9 and 2.0 Hz, 1H), 7.34 (s, 1H), 3.79 (m, J = 7.5 Hz, 2H), 1.19 (t, J = 7.2, 3H).
HRMS m/z calcd for C15H16N4BrO, 347.502 (M·+ + H); found 347.508.
4.15. (E)-5-[(5-chloro-1H-indol-3-yl)methylene]-2-imino-1,3-dimethylimidazolidin-4-one (10)
5-chloroindole-3-carboxaldehyde (3.00 g, 0.017 mol) and 2-amino-1,3-dimethylimidazolidin-4-one (2.31g, 0.018 mol) were reacted according to the above procedure to give 10 1.79g, 60%yield, m.p. 245–246°C.
IR (cm−1): 3354, 3113, 2361, 1719, 1691, 1654, 1631 cm−1.
1H-NMR: δ: 11.64 (s, 1H), 8.75 (d, J = 1.2 Hz,1H), 8.039 (d, J = 1.2 Hz, 1H), 7.44 (d, J = 8.7 Hz, 1H), 7.15 (dd, J = 8.7 and 2.1 Hz, 1H), 3.27 (s, 3H), 3.066 (s, 3H).
HRMS m/z calcd for C14H14N4ClO, 289.0851 (M·+ + H); found 289.0841.
4.16. (E)-5-[(5-bromo-1H-indol-3yl)methylene]-2-imino-1,3-dimethylimidazolidin-4-one (11)
The synthesis and structurally characterization of this compound has been reported previously26. 5-bromoindole-3-carboxaldehyde (3.78 g, 0.017 mol) and 2-amino-1,3-dimethylimidazolidin-4-one (2.15 g, 0.017 mol) were reacted according to the above procedure to give 11 as a dark red solid. (1.33g, 35% yield), m.p. at 280–281°C.
IR (cm−1): 3353, 3251, 3121, 2722, 1722, 1653, 1630 cm−1
1H-NMR: δ: 11.62 (s, 1H), 8.74 (s, 1H), 8.18 [d, J = 2.1 Hz, 1H]], 7.39 [d, J = 8.7 Hz, 1H], 7.26 [dd, J = 8.4 and J = 1.8 Hz, 1H], 6.44 (s, 1H), 3.27 (s, 3H, N), 3.08 (s, 3H).
HRMS m/z calcd for C14H14N4BrO, 333.0345 (M·+ + H); found 333.0346.
4.17. (Z)-3-ethyl-2-imino-5-[(5-iodo-1H-indol-3yl)methylene]-1-methylimidazolidin-4-one (12)
5-iodoindole-3-carboxaldehyde (0.50 g, 0.0018 mol) and 2-amino-3-ethyl-1-methylimidazolidin-4-one (0.48 g, 0.0034 mol) were reacted using the procedure described previously to give 12 (0.39g, 78% yield) m.p. 290–292°C.
IR (cm−1): 3329, 3189, 2719, 1716, 1666, 1612 cm−1.
1H-NMR: δ:12.15 (s, 1H), 8.95 (d, J = 2.7 Hz, 1H), 8.53 (s, 1H), 7.51 (dd, J = 8.9 and J = 2.0 Hz, 1H), 7.36 (d, J = 8.7 Hz, 1H), 7.29 (s, 1H), 3.79 (q, J = 6.9 Hz, 2H), 1.19 (t, J = 6.9 Hz, 3H).
HRMS m/z calcd for C15H16N4IO, 395.0363 (M·+ + H); found 395.0385.
4.18. (E)-5-[(6-fluoro-1H-indol-3-yl)methylene]-2-imino-1,3-dimethylimidazolidin-4-one (13)
6-fluoroindole-3-carboxaldehyde ( 0.50 g, 0.0037 mol) and 2-amino-1,3-dimethylimidazolidin-4-one (0.467 g, 0.0037 mol) were reacted according to the above procedure to give 13. (0.22 g, 44% yield), m.p. 260–264°C.
IR (cm−1): 3359, 3124, 3072, 1721, 1650, 1633 1593 cm−1
1H-NMR: δ: 11.51 (s, 1H), 8.68 (d, J = 2.1, 1H), 7.89, (dd, J = 9.0 and 5.7 Hz, 1H), 7.21 (dd, J = 9.0 and 2.4 Hz, 1H), 6.96 (td, J = 9.0, 2.3 and 1.8 Hz, 1H), 6.42 (s, 1H), 3.25 (s, 3H), 3.06 (s, 3H).
HRMS m/z calcd for C14H14N4FO, 273.1146 (M·+ + H); found 273.1143.
4.19. (E)-5-[(6-chloro-1H-indol-3yl)methylene]-2-imino-1,3-dimethylimidazolidin-4-one (14)
6-chloroindole-3-carboxaldehyde (1.00 g, 0.0060 mol) and 2-amino-1,3-dimethylimidazolidin-4-one (0.77 g, 0.0061 mol) were reacted according to the above procedure to give 14. (0.74g, 74%yield), m.p. 278–280°C. IR (cm−1): 3340, 3128, 3063, 1723, 1646, 1632 cm−1
1H-NMR: δ: 11.57 (s, 1H), 8.70 (s, 1H), 7.91 (d, J = 8.7 Hz), 7.47 (d, J = 1.8), 7.17 (dd, J = 8.4 and J = 1.5 Hz, 1H), 6.75 (s, 1H), 6.40 (s, 1H), 3.34 (s, 3H), 3.25 (s, 3H).
HRMS m/z calcd for C13H12N4ClO, 289.085 (M·+ + H); found 289.0851.
4.20. 5-(6-bromo-1H-indol-3-ylmethylene)-2-imino-1,3-dimethyl-imidazolidin-4-one (15)
6-bromoindole-3-carboxaldehyde (1.00 g, 0.0045 mol) and 2-imino-1,3-dimethyl-imidazolidin-4-one (0.567 g, 0.0045 mol) were reacted using the procedure described above to give 15 as orange needles (0.550 g, 56%), m.p. 281–282 °C.
IR (cm−1): 3333, 3122 (w), 3065 (w), 1724, 1646, 1632 cm−1.
1H-NMR: δ: 11.58 (s, 1H), 8.69 (s, 1H), 7.87 (d, J = 9 Hz, 1H), 7.61 (d, J = 1.5 Hz), 7.23 (dd, J = 9 and J = 1.5 Hz, 1H), 6.75 and 6.54 (two s, br, 0.6H and 0.4H), 6.39 (s, 1H), 3.25 (s, 3H), 3.06 (s, 3H).
HRMSm/z calcd for C14H14N4BrO, 333.0345 (M·+ + H); found 333.0346.
Ultraviolet-visible spectrum: λmax 381 nm (ε = 4.50).
4.21. (E)-5-[(5,6-dichloro-1H-indol-3-yl)methylene]-2-imino-1,3-dimethylimidazolidin-4-one (16)
5,6-dichloroindole-3-carboxaldehyde (0.300 g, 0.0014 mol) and 2-amino-1,3-dimethylimidazolidin-4-one (0.1907 g, 0.0015 mol) were reacted using the above procedure to give 16 (0.15g, 50% yield) m.p. 214°C
IR (cm−1): 3341, 2719, 1723, 1698, 1655, 1632 cm−1.
1H-NMR: δ: 11.7 (s, 1H), 8.76, (d, J = 1.5 Hz, 1H), 8.28 (s, 1H), 7.95 (s, DMF), 7.68 (s, 1H), 6.41 (s, 1H), 3.26 (s, 3H), 3.05 (s, 3H), 2.89 (s, DMF), 2.73 (s, DMF).
HRMS m/z calcd for C14H13N4Cl2O, 323.0461 (M·+ + H); found 323.0462.
4.22. (E)-5-((1H-indol-3yl)methylene)-2-imino-1,3-dimethylimidazolidin-4-one (17)
The synthesis and structurally characterization of this compound has been reported previously3. Indole-3-carboxaldehyde (0.500 g, 0.0034 mol) and 2-amino-1,3-dimethylimidazolidin-4-one (0.437 g, 0.0034 mol) were reacted using the above procedure to give 17 (0.25g, 50%yield), m.p. 245–247°C.
IR (cm−1): 3417, 3303, 2729, 2662, 1712, 1634 cm−1.
1H-NMR: δ: 11.48, (s, 1H), 8.70 (d, J = 2.7 Hz, 1H), 7.88 (d, J = 7.2 Hz, 1H), 7.42 (dd, J = 7.3 and J = 1.4 Hz, 1H), 7.13 (m), 6.44 (s, 1H), 3.23 (s, 3H), 3.07 (s). NMR is identical to that reported by Djura and Faulkner, 1980.
HRMS m/z calcd 255.1246 (M·+ + H); found 255.124.
4.22. (Z)-5-((1H-indol-3yl)methylene)-3-ethyl-2-imino-1-methylimidazolidin-4-one (18)
Indole-3-carboxaldehyde (0.500 g, 0.0031 mol) and 2-amino-3-ethyl-1-methylimidazolidin-4-one (0.97g, 0.0069 mol) were reacted using the above procedure to give 18 (0.100g, 20%yield), m.p. 320–324°C.
IR (cm−1): 3450, 3238, 3189, 2725, 2662, 1729, 1663, 1662,, 1546 cm−1.
1H-NMR: δ: 12.08 (d, J = 2.4 Hz, 1H), 9.35, (s, 1H), 9.00 (d, J = 3 Hz, 1H), 8.08 (dd, J = 6.6 and J =2.5, 1H), 7.52 (dd, J = 6.3 and J= 2.4 Hz, 1H), 7.35 (s, 1H, 7.25 (m), 3.8 (q, J = 7.2 Hz, 2H), 1.20 (t, J = 7.2, 3H).
HRMS m/z calculated for C15H17N4O, 269.1397 (M·+ + H); found 269.1406.
4.23. (E)-3-ethyl-5-[(6-fluoro-1H-indol-3-yl)methylene]-2-imino-1methylimidazolidin-4-one (19)
6-fluoroindole-3-carboxaldehyde (0.500 g, 0.0037 mol) and 2-amino-3-ethyl-1-methylimidazolidin-4-one (0.43 g, 0.0031 mol) were reacted according to the above procedure to give compound 19 (0.27, 54% yield), m.p. 330–331°C.
IR (cm−1): 3230, 3193, 3070, 2713, 1731, 1664, 1624 cm−1.
1H-NMR: δ: 12.08 (s, 1H), 9.36 (s, 1H), 8.97 (d, J = 2.7 Hz, 1H), 8.11 (dd, J = 9.0 and 5.4 Hz, 1H), 7.33 (dd, J = 9.0 and 3.2 Hz, 2H), 7.12 (td, J = 3.0, 2.4 and 2.1 Hz), 3.80 (q, J = 7.2 Hz, 2H), 3.51 (s, 3H], 1.19 (t, J = 6.0 Hz, 3H).
HRMS m/z calcd for C15H16N4FO, 287.1303 (M·+ + H); found 287.1312.
4.24. (E)-5-[(6-bromo-1H-indol-3yl)methylene]3-ethyl-2-imino-1-methylimidazolidin-4-one (20)
6-bromoindole-3-carboxaldehyde (0.500 g, 0.0022 mol) and 2-amino-3-ethyl-1-methylimidazolidin-4-one (0.314 g, 0.0022 mol) were reacted as previously described to give compound 20 (0.12g, 24% yield), mp 310–315°C.
IR (cm−1): 3205, 3091, 2725, 1737, 1682, 1633 cm−1.
1H-NMR: δ:12.11 (s, 1H), 9.37 (s, 1H), 8.97 (d, J = 2.7, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.72 (d, J = 0.9, 1H), 7.37 (dd, J = 8.4 and 1.5 Hz, 1H), 7.31 (s, 1H), 3.79 (m, J = 7.2 Hz, 2H), 1.19 (t, J = 7.05, 3H).
HRMS m/z calcd for C15H16N4BrO, 347.0502 (M·+ + H); found 347.0505.
4.25. Preparation of Crude Membranes for Binding Assays
Cells derived from human embryonic kidney cells (HEK293) were employed as hosts for the expression of cloned human serotonin 5-HT1A, 5-HT2A and 5-HT2C receptors. As previously described,27 HEK293 cells transfected by calcium phosphate precipitation were put under selective drug pressure to select for the stable expression of membrane bound receptor. Briefly, 20 μg of purified plasmid DNA containing genes for the receptor of interest and for selection by drug resistance was mixed to a final volume of 1 mL calcium phosphate/HEPES buffered saline solution and then added drop wise to a 150 cm2 plate containing adherent HEK293 cells at 5–10% confluence in sterile growth medium (DMEM supplemented with 100 μM Sodium Pyruvate and 10% BCS). The transfection medium was replaced on the following morning (approx 12h post transfection) with fresh growth medium containing 2 mg/mL of the selection agent geneticin (G418). Cells were grown under constant G418 selection (medium changed every 3–4 days) with colony selection by sterile cylinder isolation after several pinhead-sized colonies were visually apparent and confirmed by microscopy (approximately 2 weeks). The expression level of the serotonin receptor subtype in individual clones was determined by radioligand saturation isotherm binding assays that utilized the standard rapid filtration techniques previously described by our lab.27
Cell membranes were prepared for radioligand binding assays through a standard laboratory procedure.27 Briefly, cells were detached by a 15 minute incubation in 10 mL of Dulbecco’s phosphate buffer saline (D-PBS) without Ca2+ and Mg2+. Detached cells were pipetted into a 50 mL conical centrifugation tube and the remainder of the tube volume filled with Earle’s balanced salts solution (EBSS). This cell suspension was pelleted in a Sorvall Legend RT centrifuge at 800 rpm (700×g) for 10 min. The cell pellet, after decantation, was resuspended in 10 mL of lysing buffer (5 mM MgCl2, 5 mM Tris, pH 7.4 at 4°C), homogenized using 8 full strokes of a Dounce glass homogenizer and then poured into a high speed centrifugation tube. This homogenate was balanced for centrifugation through the addition of approximately 25 mL of cold binding buffer (50 mM Tris, pH 7.4 at 4°C) before 30 minutes of centrifugation at 13,000 rpm (28,000×g) and 4°C. The resulting crude membrane pellet, after decantation, was resuspended in a small amount of cold binding buffer (approximately 6 mL), rebalanced and recentrifuged a final 30 minutes. The resulting membrane pellet was resuspended in an appropriate volume of cold binding buffer (1 data set = approx. 6 mL of 50 mM Tris, pH 7.4 at 4°C), homogenized by 4 strokes of the Dounce homogenizer, and kept on ice for same day use.
4.26. Radioligand Binding Assays
Membranes expressing cloned human 5-HT1A, 5-HT2A, or 5-HT2C receptors were assayed by saturation isotherm analysis of [3H]MPPF (4-(2′-Methoxy)-phenyl-1-[2′-(N-2″-pyridinyl)-p-fluorobenzamido]ethyl- piperazine) (79.8 Ci/mmol. Perkin-Elmer, NET-1109), [3H]methylspiperone (N-Methyl-8-[4-(4-fluorophenyl)-4-oxobutyl]-1-phenyl-1,3,8-triazaspiro-[4.5]decan-4-one) (84 Ci/mmol, Perkin-Elmer, NET-856) or [3H]mesulergine (N′-(1-methyl-6-methylergolin-8alpha-yl)-N,N-dimethylsulfamide) (80 Ci/mmol, GE Healthcare, TRK845) specific binding activities, respectively. The values for 5-HT1A, 5-HT2A and 5-HT2C radioligand affinities (KD ± S.D. (pM)) were 267 ± 36, 246 ±42 and 1803 ± 726 with corresponding receptor density (pmoles/mg membrane protein) values 3.1 ± 1.7, 4.2 ± 2.3 and 14 ± 7, respectively. Nonspecific binding activity was defined by 5 μM NAN-190 (1-(2-Methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine) for 5-HT1A or 5 μM mianserin (1,2,3,4,10,14b-Hexahydro-2-methyl-dibenzo[c,f]pyrazino[1,2-a]azepine) for 5-HT2A/2C receptors. A target concentration of approximately 500 pM radioligand was utilized for the competition binding experiments and actual concentrations of radioligand were determined by scintillation counting. Mirtazapine (1,2,3,4,10,14b-Hexahydro-2-methylpyrazino[2,1-a]pyrido[2,3-c][2]benzazepine) was used during the single point binding assessments as a high affinity 5-HT2A/2C reference compound (data not shown). As described previously,27 all radioligand assays were allowed at least 90 minutes to reach equilibrium at 25°C prior to rapid filtration through GF/C filters pre-soaked for 10 minutes in 0.5% polyethyleneimine. Radioligand-receptor complexes were rapidly filtered and washed with 3 × 3 mL of ice-cold binding buffer (50 mM Tris pH 7.4 at 2°C). The radioactivity of each sample was determined using a liquid scintillation counter (Perkin Elmer Tri-Carb 2800TR). Membrane protein concentrations were determined with a bicinchoninic acid assay using a bovine serum albumin (BSA) standard curve. The aplysinopsin analogs were prepared at an initial concentration of 3mM in DMSO with the exception of 15 which was prepared at a concentration of 1 mM in DMSO. These solutions were then diluted to 1:1000 v/v for the final assay solution. For the inhibition assays, stock solutions of test compounds were dissolved at the highest concentrations possible in either DMSO or 95:5 v/v of DMSO:0.01 N HCl, then diluted in binding buffer while not exceeding a 1:300 v/v ratio of non-aqueous solvent.
4.27. Calculations and Data Analyses
Each data point was sampled in triplicate and each experiment was repeated three to four times. Data are reported as the geometric mean of three to four experiments with standard deviations (tables) or standard errors (graphs). The inhibition constants (Ki) were calculated from IC50 values using the Cheng-Prusoff equation: Ki = IC50/(1 + [ligand]/KD). In cases where the displacement was >20% but less than 100% at the highest concentration of inhibitor, accurate IC50 values were calculated by setting the bottom of the curve fit to zero. A 95% confidence interval was employed for all curve-fitting procedures using Graphpad’s Prism software version 4.0.
Supplementary Material
Acknowledgments
Special thanks to Dr. Pilar Rodriguez-Loaiza for helpful insights and suggestions regarding the synthesis strategy. The authors wish to thank Greta D’Ambrosia for assistance in early phases of this work. This study was supported by the National Institutes of Health [Grants R01-MH063162; DBI-0649889 (J.A.S.) and R25-GM55380 (J.E.J.)], competitive institutional funds G67710 (J.A.S.), The Robert A. Welch Foundation M-200 (J.E.J.), and the Texas Woman’s University Research Enhancement Program (J.E.J.).
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT1A
Serotonin Receptor Subtype 1A
- 5-HT2A
Serotonin Receptor Subtype 2A
- 5-HT2C
Serotonin Receptor Subtype 2C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kazlauskas R, Murphy PT, Quinn RJ, Wells RJ. Tetrahedron Lett. 1977;18:61–64. [Google Scholar]
- 2.Hollenbeak KH, Schmitz FJ. Lloydia. 1977;40:479–481. [PubMed] [Google Scholar]
- 3.Djura P, Faulkner DJ. J Org Chem. 1980;45:735–737. [Google Scholar]
- 4.Aoki S, Ye Y, Higuchi K, Takashima A, Tanaka Y, Kitagawa I, Kobayashi M. Chem Pharm Bull (Tokyo) 2001;49:1372–1374. doi: 10.1248/cpb.49.1372. [DOI] [PubMed] [Google Scholar]
- 5.Hu JF, Schetz JA, Kelly M, Peng JN, Ang KK, Flotow H, Leong CY, Ng SB, Buss AD, Wilkins SP, Hamann MT. J Nat Prod. 2002;65:476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- 6.Segraves NL, Crews P. J Nat Prod. 2005;68:1484–1488. doi: 10.1021/np0501334. [DOI] [PubMed] [Google Scholar]
- 7.Kochanowska AJ, Rao KV, Childress S, El-Alfy A, Matsumoto RR, Kelly M, Stewart GS, Sufka KJ, Hamann MT. J Nat Prod. 2008;71:186–189. doi: 10.1021/np070371u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh EG, Sweatman H. J Exp Mar Bio Ecol. 2000;251:141–160. doi: 10.1016/s0022-0981(00)00222-7. [DOI] [PubMed] [Google Scholar]
- 9.Guella G, Mancini I, Zibrowius H, Pietra F. Helv Chim Acta. 1988;71:773–782. [Google Scholar]
- 10.Murata M, Miyagawa-Kohshima K, Nakanishi K, Naya Y. Science. 1986;234:585–587. doi: 10.1126/science.234.4776.585. [DOI] [PubMed] [Google Scholar]
- 11.Singh SN, Bhatnagar S, Fatma N, Chauhan PM, Chatterjee RK. Trop Med Int Health. 1997;2:535–543. doi: 10.1046/j.1365-3156.1997.d01-321.x. [DOI] [PubMed] [Google Scholar]
- 12.Lacivita E, Leopoldo M. Curr Top Med Chem. 2006;6:1927–1970. doi: 10.2174/156802606778522168. [DOI] [PubMed] [Google Scholar]
- 13.Jiang B, Smallheer JM, Amaral-Ly C, Wuonola MA. J Org Chem. 1994;59:6823–6827. [Google Scholar]
- 14.Kenyon GL, Rowley GL. J Am Chem Soc. 1971;93:5552–5560. doi: 10.1021/ja00750a038. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JE, Canseco DC, Doliver DD, Schetz JA, Fronczek FR. J Chem Crystallography. 2009;39:329–336. [Google Scholar]
- 16.Floresca CZ, Schetz JA. J Recept Signal Transduct Res. 2004;24:207–239. doi: 10.1081/rrs-200032088. [DOI] [PubMed] [Google Scholar]
- 17.Bos M, Jenck F, Martin JR, Moreau JL, Sleight AJ, Wichmann J, Widmer U. J Med Chem. 1997;40:2762–2769. doi: 10.1021/jm970030l. [DOI] [PubMed] [Google Scholar]
- 18.Bentley JM, Adams DR, Bebbington D, Benwell KR, Bickerdike MJ, Davidson JE, Dawson CE, Dourish CT, Duncton MA, Gaur S, George AR, Giles PR, Hamlyn RJ, Kennett GA, Knight AR, Malcolm CS, Mansell HL, Misra A, Monck NJ, Pratt RM, Quirk K, Roffey JR, Vickers SP, Cliffe IA. Indoline derivatives as 5-HT2C receptor agonists. Bioorg Med Chem Lett. 2004;14:2367–2370. doi: 10.1016/j.bmcl.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bromidge SM, Dabbs S, Davies DT, Duckworth DM, Forbes IT, Ham P, Jones GE, King FD, Saunders DV, Starr S, Thewlis KM, Wyman PA, Blaney FE, Naylor CB, Bailey F, Blackburn TP, Holland V, Kennett GA, Riley GJ, Wood MD. J Med Chem. 1998;41:1598–1612. doi: 10.1021/jm970741j. [DOI] [PubMed] [Google Scholar]
- 20.Bromidge SM, Dabbs S, Davies DT, Davies S, Duckworth DM, Forbes IT, Gaster LM, Ham P, Jones GE, King FD, Mulholland KR, Saunders DV, Wyman PA, Blaney FE, Clarke SE, Blackburn TP, Holland V, Kennett GA, Lightowler S, Middlemiss DN, Trail B, Riley GJ, Wood MD. J Med Chem. 2000;43:1123–1134. doi: 10.1021/jm990388c. [DOI] [PubMed] [Google Scholar]
- 21.Bromidge SM, Dabbs S, Davies S, Duckworth DM, Forbes IT, Jones GE, Jones J, King FD, Saunders DV, Blackburn TP, Holland V, Kennett GA, Lightowler S, Middlemiss DN, Riley GJ, Trail B, Wood MD. Bioorg Med Chem Lett. 2000;10:1863–1866. doi: 10.1016/s0960-894x(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 22.Kalir A, Szara S. J Med Chem. 1963;6:716–719. doi: 10.1021/jm00342a019. [DOI] [PubMed] [Google Scholar]
- 23.Noland WE, Reich C. J Org Chem. 1967;32:828–832. doi: 10.1021/jo01278a077. [DOI] [PubMed] [Google Scholar]
- 24.James PN, Snyder HR. In: Organic Synthesis. Rabjohn N, editor. John Wiley and Sons, Inc; New York, N. Y: 1963. p. 539. Coll. Vol. IV. [Google Scholar]
- 25.Somei M, Saida Y, Funamoto T, Ohta T. Chem Pharm Bull. 1987;35:3146–3154. [Google Scholar]
- 26.Tymiak Adrienne A, Rinehart Kenneth L, Jr, Bakus Gerald J. Constituents of morphologically similar sponges. Aplysina and Smenospongia species. Tetrahedron. 1985;41:1039–1047. [Google Scholar]
- 27.Ericksen SS, Cummings DF, Weinstein H, Schetz JA. J Pharm Exp Ther. 2008;328:40–54. doi: 10.1124/jpet.108.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kortagere S, Gmeiner P, Weinstein H, Schetz JA. Mol Pharmacol. 2004;66:1491–1499. doi: 10.1124/mol.104.001321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.