Abstract
Purpose
To determine whether there are regional differences in the age-related changes in peripapillary retinal nerve fiber layer thickness as measured by time-domain optical coherence tomography (OCT).
Methods
Fast peripapillary RNFL scans obtained with the Stratus™ time-domain OCT with nominal diameter of 3.46 mm centered on the optic disc were performed on 425 normal subjects over a wide age range. One eye was randomly selected for scanning or analysis. Average RNFL-, clock hour-, and quadrant-specific rates of RNFL thickness change were calculated and compared.
Results
The 425 study participants ranged in age from 18 to 85 years with mean (±SD) of 46 (±15) years. The mean (±SD) average measured RNFL thickness was 104.7 (±10.8) micrometers (μm). The decline in the average RNFL thickness was 2.4 μm per decade of age. Changes in RNFL thickness per decade of age ranged from -5.4 (p<0.001) at clock hour 12 to -0.9 (p=0.28) at clock hour 6. Similarly, the rate of thickness change per decade of age in the superior quadrant was -4.3 (p<0.001) versus -1.5 (p=0.006) in the inferior quadrant. The slopes of thinning superiorly and inferiorly were highly significantly different (p=0.001).
Conclusions
The age-related decline in normal RNFL measurements does not occur at equal rates around the disc and occurs mainly superiorly.
Introduction
Glaucoma is a slowly progressive optic neuropathy characterized by apoptosis of the retinal ganglion cells resulting in disappearance of their associated axons. The retinal nerve fiber layer (RNFL) contains the axons of the retinal ganglion cells, connecting their cell bodies to the lateral geniculate nucleus. Quigley's study of experimental glaucoma in non-human primates demonstrated that glaucoma causes progressive thinning of the RNFL.1 As retinal nerve fiber axons disappear because of glaucoma, the RNFL becomes progressively thinner and the optic cup becomes progressively larger. Pathologic studies have shown that 25 – 35% of retinal ganglion cell axons may be lost before any diagnostically characteristic defects appear within a single field examination by standard white-on-white automated perimetry.2-4 Clinically, structural abnormalities of the RNFL and optic cup may be recognized several years before an accompanying loss of function is recognized with standard white-on-white visual field testing in early glaucoma.5, 6 The loss of nerve fibers, expansion of the cup over time, and general reduction in visual sensitivity may be widespread early in glaucoma, and may be difficult to distinguish from the effects of age or cataract. However, as the disease worsens so that a substantial portion of the nerve fibers are damaged, the abnormalities appear in an uneven manner, but usually with preservation of central acuity until the end stage of the disease process despite loss of contrast sensitivity. In the earliest stages of glaucoma, there are presumably accelerated changes in the visual field or retinal nerve fiber layer or ganglion cell layer while these parameters are still in the “normal” range. However, aging is causing changes in these same parameters and the difficulty is sorting out the normal aging changes from those occurring as a result of glaucoma. Perhaps glaucoma might be recognized earlier if an accelerated rate of change in visual fields or other measurement is recognized to be faster than the normal rate of change expected with aging. Hence there is a need to know how each of the various diagnostic measurements changes with age in the absence of disease.
Retinal ganglion cell axons are in fact lost in a age-related manner that is slower than occurs with glaucomatous damage. This has been shown in normal individuals histologically7-10 as well as with scanning laser polarimetry 11-14 and optical coherence tomography (OCT).15-18 To assist in evaluating glaucoma, it is important to quantify the degree of thinning by age in the absence of glaucoma. It is also important to determine whether age-related loss is always uniform or has any tendency to be localized, as it often is in glaucoma, and might therefore be a confounding diagnostic feature. In a univariate model, Parikh and colleagues18 recently reported variable age-related rates of thinning in different regions around the optic nerve in a normal Indian-Asian population using time-domain OCT (Stratus™ OCT, Carl Zeiss Meditec, Dublin, CA). The currently reported study had been undertaken with a larger cohort and was designed with a multivariate model to evaluate the age-related differences in RNFL thickness of ethnically diverse subjects without glaucoma, while accounting for the effects of other factors known to influence RNFL thickness such as axial length, optic disc size, and ethnicity.17
Materials and Methods
The Institutional Review Board of each participating center approved the study. Informed consent was obtained for each subject. This study combined two cohorts of normal subjects recruited for studies designed to assess normal characteristics of the RNFL as measured by Stratus™ OCT. An exploratory analysis of a cohort of 108 normal subjects who participated in a study to determine the normal limits of interocular differences in RNFL thickness19 suggested regional differences in the age-related thinning of the RNFL. The second cohort consisted of the 328 individuals whose measurements comprise the Stratus™ OCT normative database.17 Results from 11 subjects who contributed measurements to both studies were included only once. This left us with 425 total subjects. In the former study, both eyes were scanned and only one eye randomly chosen for this analysis. In the latter study, one eye was randomly chosen for scanning at enrollment. Additional details of data collection are documented in the publications related to each of the two studies.
Inclusion criteria were the same for both studies. Males or females age 18 years or older who were able give consent and follow study instructions were included. Exclusion criteria included a contraindication to dilation or intolerance to topical anesthetics or mydriatics; an intraocular pressure (IOP) ≥ 22 mm Hg or glaucoma in either eye; a history of intraocular surgery in the study eye (except cataract or refractive surgery if performed more than 1 year prior to testing); a best corrected Snellen visual acuity worse than 20/40; evidence of diabetic retinopathy, diabetic macular edema, or other vitreoretinal disease in either eye; or evidence of optic nerve abnormality in either eye. Subjects were also excluded by the principal investigator if their Stratus™ OCT scans showed segmentation algorithm failure, determined by visual inspection of the lines that defined the boundaries of the RNFL and noting that the boundaries were placed in the incorrect location by the instrument. Each subject had a complete ophthalmologic evaluation, including Snellen visual acuity, slit lamp examination, intraocular pressure measurement, and dilated fundus examination. The assessment of optic disc size in the 328 subjects from the normative database was made in digitized stereo photographs as previously described.17 Optic discs with areas less than 2.0 mm2 were labeled small, 2.01 – 2.50 mm2 were labeled medium, and those greater than 2.50 mm2 were labeled large. All of the subjects in the normative database cohort underwent visual field testing with the SITA standard program of the Humphrey Visual Field Analyzer II and had normal and reliable visual fields. The classification of optic disc size for the second cohort of 108 subjects was made clinically with the direct ophthalmoscope technique described by Gross and Drance20 and divided into small, medium, and large discs. Cup:Disc (C:D) ratios were estimated by an experienced examiner using a condensing lens at the slit lamp. The determination of the lack of glaucoma in this second cohort was made by optic disc evaluation and subject report of no prior history of glaucoma; no visual fields were performed in this subgroup.
All subjects were tested with both the Standard and Fast scan algorithms of Stratus™ OCT. Only information from the Fast scans is reported here, because that is the algorithm most commonly used clinically. The Fast RNFL scan consists of 3 peripapillary scans, each consisting of 256 test points measured along a circle having a nominal diameter of 3.46 mm centered on the optic disc. The RNFL analysis averages the measurements at each of the 256 locations of the three peripapillary scans and produces 17 averages for each scan set. These include the average RNFL thickness of the complete circumference, 4 quadrant averages (temporal, superior, nasal, and inferior), and 12 clock-hours. For the analysis, the right to left clock hour averages were assumed to be mirror images of each other such that the 9:00 clock hour of the right eye was combined with the 3:00 clock hour of the left eye, 10:00 right with 2:00 left, and so forth.
All analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, IL). A Huynh-Feldt adjusted test of interaction in a repeated measures analysis of covariance was used to assess the statistical significance of the difference between rates of RNFL thinning in the twelve clock hours around the disc. To determine whether the rate of RNFL thinning changed with age, average RNFL data were plotted by age regression fits with linear, quadratic and non-parametric Loess fits, and these were compared.
Since an important aim of this study was to calculate rates of RNFL thinning with age after accounting for other factors known to influence RNFL thickness, regression methods were used to impute missing data. This was necessary for ethnicity of one individual (0.4%), spherical equivalent refraction of 17 eyes (7%), and disc size of 45 eyes (18%). These three variables plus age were included in the imputation procedure. With complete data for all 425 individuals, a regression model was used similar to that used in Budenz, Anderson, and Varma, et al.17 This model was used to obtain coefficients to adjust RNFL thickness to that expected for an eye of a Caucasian with medium disc size and spherical equivalent refraction of zero. These same adjustments were applied to all quadrants and clock hours.
Results
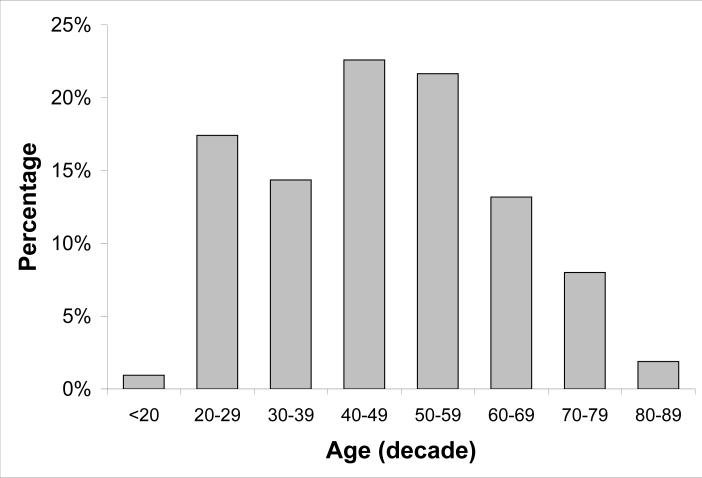
A total of 425 subjects ranging between age 18 and 85 years with a mean age (SD) of 46 years (15) were used in the analysis. Table 1 presents characteristics previously found to influence RNFL thickness for the two study cohorts. The cohorts were similar with respect to age and spherical equivalent refraction but different with respect to disc size and ethnicity. The difference between groups in optic disc size may have been due to the difference in assessment of optic disc size since cohort 1 was measured photographically and cohort 2 was measured clinically at the slit lamp. Figure 1 is a histogram of the age distribution of the entire group.
Table 1.
Characteristics of the two study cohorts found previously to influence RNFL thickness
| |
Cohort 1 (n=328) |
Cohort 2 (n=97) |
P-value1 |
|---|---|---|---|
| Mean (SD) Age [range] |
47 (16) [18, 85] |
45 (15) [20, 82] |
0.28 |
| Mean (SD) Spherical equivalent [range] |
-0.54 (1.88) [-11.75, 6.75] |
-0.90 (2.2) [-7.88, 4.50] |
0.14 |
| Disc Size | <0.001 | ||
| Small | 90 (30%) | 9 (12%) | |
| Medium | 122 (40%) | 67 (87%) | |
| Large | 91 (30%) | 1 (1%) | |
| Unspecified2 |
25 |
20 |
|
| Ethnicity | <0.001 | ||
| Hispanic | 80 (25%) | 42 (43%) | |
| Asian | 11 (3%) | 9 (9%) | |
| All others | 236 (72%) | 46 (47%) | |
| Unspecified2 | 1 | 0 |
Two-sample t-test for age and spherical equivalent, chi-square for disc size and ethnicity
The category, unspecified, was not included in the calculation of chi-square
Figure 1.
Distribution of study subject ages by decade.
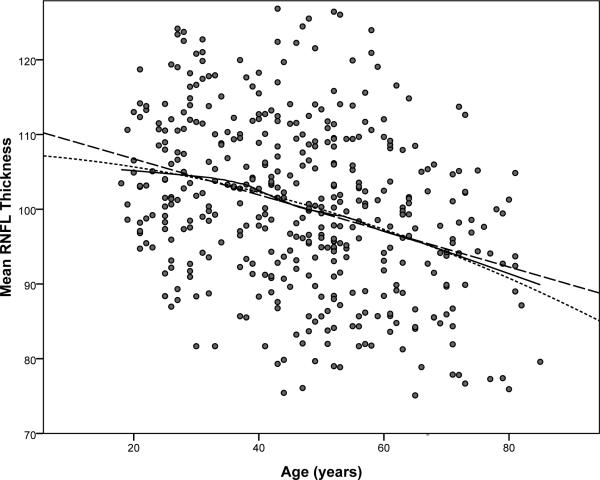
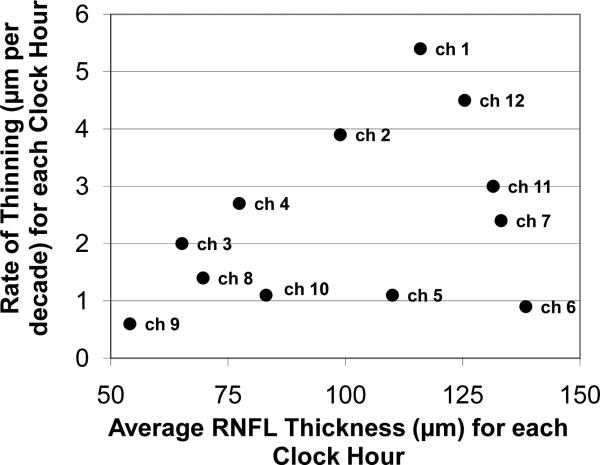
The mean (SD) average measured RNFL thickness was 104.7 (10.8) micrometers (μm). Although there was a wide range of normal thickness at every age, the mean RNFL thickness decreased, changing at a rate of -2.4 μm per decade (95% CI = -3.1,-1.8) by linear regression (P < 0.001), as greater thickness values became less frequent with age. There was no indication of a substantial departure from a simple linear relationship between mean RNFL thickness and age (Figure 2). However, there was a highly significant difference between the slopes of normal age related RNFL thinning around the 12 clock hours of the disc (p<0.001). Table 2 presents the slopes of age related thinning around the disc for the study cohorts separately and combined. There were no significant differences between the cohorts in the slopes of age related thinning at any disc location (all p>0.18). Figure 3 displays the relationship of the slopes of age related thinning to the average thickness for each of the twelve clock hours. While certain clock hours are different (note particularly difference in clock hours superiorly and inferiorly), the rate of thinning did not relate to the average normal thickness of the location except to the degree that a thinner region was truncated by virtue of a limit to how much thinning could occur. Rates of change with age were different among the four quadrants (p<0.001), with steeper slopes in superior and nasal quadrants and shallower slopes in temporal and inferior quadrants (Table 2). Changes in RNFL thickness per decade of age ranged from -5.4 (p<0.001) at clock hour 12 to -0.9 (p=0.28) at clock hour 6. Similarly, the rate of thickness change per decade of age in the superior quadrant was -4.3 (p<0.001) versus -1.5 (p=0.006) in the inferior quadrant. The slopes of thinning superiorly and inferiorly were highly significantly different (p=0.001).
Figure 2.
Relationship of average RNFL thickness to age. Quadratic and Loess fits are similar to linear fit. Addition of a squared term did not significantly improve the fit (p=0.31). Line key: dashed, linear fit; points, quadratic fit; solid, non-parametric Loess.
Table 2.
Slopes of RNFL change (μm) per decade by disc location
| Disc location | Cohort 1, N=328 | Cohort 2, N=97 | Combined Cohorts Slope (95% CI) | Parikh18 Slope (95% CI) N=187 |
|---|---|---|---|---|
| Average | -2.4 | -2.4 | -2.4 (-3.1, -1.8) | -1.6 (-2.9, -0.2) |
| Temporal | -0.9 | -1.2 | -1.0 (-1.8, -0.2) | -2.0 (-3.4, -0.7) |
| Superior | -4.1 | -5.2 | -4.3 (-5.3, -3.3) | -2.3 (-3.7, -0.9) |
| Nasal | -3.1 | -2.2 | -2.9 (-3.9, -1.8) | -1.1 (-2.5, 0.4) |
| Inferior | -1.4 | -0.12 | -1.5 (-2.5, -0.4) | -0.9 (-2.3, 0.6) |
| 9* | -0.5 | -0.7 | -0.6 (-1.2, 0.0) | -1.5 (-2.9, -0.1) |
| 10 | -1.0 | -1.1 | -1.1 (-2.1, 0.0) | -1.8 (-3.2, -0.4) |
| 11 | -3.0 | -0.28 | -3.0 (-4.3, -1.6) | -1.6 (-3.0, -0.2) |
| 12 | -4.2 | -5.6 | -4.5 (-6.0, -3.0) | -1.9 (-3.3, -0.5) |
| 1 | -5.0 | -7.1 | -5.4 (-6.8,-4.1) | -3.2 (-4.6, -1.8) |
| 2 | -3.9 | -3.9 | -3.9 (-5.2, -2.5) | -1.1 (-2.5, 0.4) |
| 3 | -2.1 | -1.3 | -2.0 (-3.0, -0.9) | -0.5 (-1.9, 1.0) |
| 4 | -3.2 | -1.2 | -2.7 (-3.9, -1.5) | -0.9 (-2.3, 0.5) |
| 5 | -1.2 | -0.5 | -1.1 (-2.6, 0.4) | -0.4 (-1.9, 1.0) |
| 6 | -0.8 | -0.5 | -0.9 (-2.4, 0.7) | -0.2 (-1.7, 1.2) |
| 7 | -2.3 | -2.7 | -2.4 (-3.8, -1.0) | -2.3 (-3.7, -0.8) |
| 8 | -1.4 | -1.7 | -1.4 (-2.3, -0.4) | -2.8 (-4.1, -1.4) |
Clock hours are labeled with respect to the right eye
Figure 3.
Relationship of rate of thinning to average thickness of each of the twelve clock hours (ch) around the disc. The superior clock hours appear to have a faster rate of decline with age than inferior clock hours.
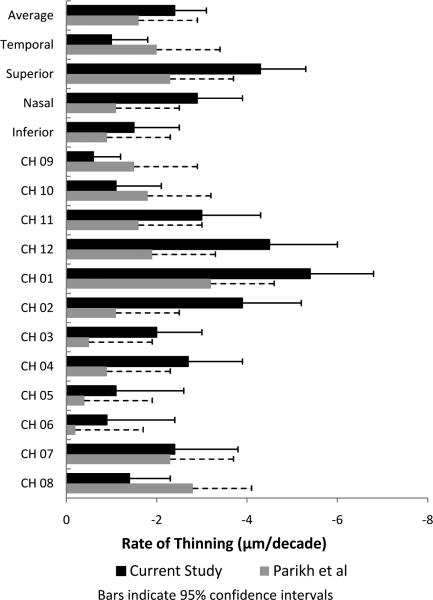
Regression coefficients for adjusting RNFL thickness to a standard of a Caucasian emmetropic eye with medium disc size were as follows: add 1.02 μm for each diopter of myopia, add 1.72 μm for a small disc, subtract 0.93 μm for a large disc, subtract 3.63 μm from Hispanic eyes, and subtract 9.11 μm for Asian eyes. The combined influence of all these variables was small, 7.7% of total variance in RNFL thickness, compared to 8.5% of total variance which is attributable to the effect of age. The remainder of the variance is attributable to individual variation, presumably genetic or developmental, not accounted for by the above demographic and clinical characteristics. Figure 4 summarizes the regression coefficients for each RNFL parameter and compares it to that of Parikh et al.18
Figure 4.
Rate of thinning (μ per decade) for retinal nerve fiber layer (RNFL) parameters measured by Stratus Oct for the current study and a previous study by Parikh et al.18
Discussion
When monitoring for glaucomatous progression, by whatever means, one must consider both the variability in the measurement and the change in measurement with normal aging. The former is addressed in studies of repeated measurements performed over a short period of time when presumably change has not occurred. The latter may be assessed in longitudinal studies of normal subjects, which are difficult to accomplish since the technologies seem to change so quickly, or cross sectional studies of normal subjects over a broad age range. The current study is of the latter type. While it is true that the normative databases of most instruments that measure parameters relevant to glaucomatous structure and function account for different ages of patients, these are incorporated for the purpose of diagnosing the disease by comparing patients with suspected disease to normal subjects of a similar age. The current research is addressing presumed rates of changes in a topographic parameter, retinal nerve fiber layer thickness, in different areas of the RNFL as a way of assessing change in an individual patient over time within the normal range. In addition, this rate of change might be assumed to be the rate of change attributable to aging in a glaucoma patient, separate from the glaucoma-related change that is occurring as a result of disease progression.
The progressive changes in glaucoma often affect some topographical regions or sectors more than others. Therefore, it is necessary to determine the topographical uniformity of age effects, which has been explored in only two OCT studies (Parikh et al18 and the current study). Parikh et al examined 187 eyes of 187 normal Asian-Indian subjects between age 5 and 75 years. Using cross sectional data, they postulated a decline in average RNFL thickness of 1.6 μm per decade, slightly lower than the postulated rate of decline found in the current study. Despite study protocol differences (ethnic composition of cohorts and our use of adjustment for refraction and disc size), the study by Parikh and the current study showed a similar topographical distribution of relative rates of thinning by clock hour (fastest age decline superiorly and slowest inferiorly), but we found slightly higher rates of loss superiorly, inferiorly, and temporally than they did. Longitudinal studies would provide better estimates of the age loss and topographical differences, but for now cross-sectional data must be used with adjustment for other factors known to affect RNFL thickness (such as ethnicity, axial length, and disc area)17 that might be unequally represented across the age spectrum in the cross-sectional study.
Previous cross-sectional histologic and clinical studies have shown that the number of axons declines with age. The estimated loss of axons in the optic nerves taken from autopsy or enucleation specimens of normal subjects is from 4,900 to 6,700 axons per year of age8 – 10, 23 in studies with a sufficient number of cases to show age related differences within the wide range of individual variation in axon counts in normal nerves, from 700,000 to 1,400,000.8 – 10, 23, 24 An approximate loss of 5,000 axons per year out of an approximate 1 million is about twice the rate of RNFL thinning per decade observed in this study, but is reasonable if it is taken into account that the RNFL layer also contains Muller cell components and astroglia. The single study that failed to find a statistically significant decline in axon count with increasing age24 failed to do so, as pointed out by the authors, because of the small number of subjects (N = 19) and high individual variability. Histologic studies of both age-related and glaucoma-related axon loss were recently reviewed by Frenkel and associates.25 They concluded that histological measurement of RNFL thickness may not be accurate because of artifacts introduced by the tissue swelling and autolysis that occurs between death and fixation, tissue shrinkage during fixation, differences in sectioning approaches for globes, variation in staining techniques, histological reconstruction of sections, and inadvertent oblique sectioning. Also, different sampling locations make it difficult to compare RNFL measurements between studies, because the RNFL is thicker near the optic disc margin than further away from the disc.
Recently developed methods such as scanning laser polarimetry and optical coherence tomography make it feasible to obtain in vivo cross-sectional measurements of the retinal layers. The artifacts of histology are thereby overcome, and a larger number of individuals can be studied to permit more power to discern small effects of variables (such as disc size or age) that contribute to the measurements. Moreover, as these instruments are used clinically, understanding the variables that contribute to the measurement, and the magnitude of the effects, become relevant to diagnosis and monitoring of chronic disease. Schuman and colleagues26 recently published results of RNFL measurements made in experimentally-induced glaucoma in monkeys with a prototype time-domain OCT unit and with histological methods They found that the OCT measured approximately 6 μm thicker than histologic sections, an exceptionally good correspondence considering the test-retest variability of OCT27 and the potential artifacts of histology pointed out by Frenkel and colleagues.25
There are currently two in vivo methods currently available for measuring RNFL thickness, scanning laser polarimetry and optical coherence tomography. In scanning laser polarimetry, the thickness of the RNFL is estimated from polarization (retardance) caused by birefringence in the RNFL. Poinoosawmy et al13 performed a cross sectional analysis of 150 normal subjects between the ages of 5 and 90 years using scanning laser polarimetry with a fixed corneal compensator. They found that RNFL birefringence, as a surrogate for thickness, was reduced in subjects of older age but they did not analyze any age change in different regions of the RNFL. From the youngest (< 10 years) to the oldest (>70 years) age group, RNFL changed from an average of 95.3 μm to 67.5 μm, or about 4 to 5 μm per decade. Chi and colleagues,11 also using scanning laser polarimetry with a fixed corneal compensator, estimated a 0.2 μm per year (2 μm per decade) decline in RNFL thickness in 75 normal subjects age 20 – 66. When examining individual quadrants, they found a decline with age only in the nasal and inferior areas and not the superior and temporal areas. Funaki and colleagues28 studied 60 normal subjects between age 23 and 75 using scanning laser polarimetry with a fixed corneal compensator. They failed to demonstrate any age related differences in RNFL thickness. Da Pozzo and colleagues14 measured RNFL birefringence using scanning laser polarimetry with a variable corneal compensator in a group of 324 normal subjects age 21 to 85 years and found reduced birefringence in older age groups. The change in RNFL thickness per year was found to be somewhat less, 0.08 μm per year, compared to previous studies performed using the scanning laser ophthalmoscope with fixed corneal compensation. The authors speculate that this is because the variable corneal compensator improves the accuracy of measurements of RNFL. When they analyzed the age related change in the superior and inferior quadrants, they found that the superior quadrant changed at a faster rate (0.16 μm per year) than the inferior quadrant (0.12μm per year), in agreement with the current study and that of Parikh and colleagues,18 but contrary to the study by Chi et al11 performed with an instrument with fixed corneal compensation. Thus, the studies to date using scanning laser polarimetry give an unclear picture of the amount of change in RNFL thickness with age or the topographic differences of these changes.
Optical coherence tomography measures RNFL thickness by interferometry. The interface between the vitreous and the interface between the posterior RNFL surface and retinal ganglion cell layer, are delineated, or “segmented”. The distance between the segmentation lines is calculated at various locations and reported as RNFL thickness. In keeping with the wide range in the number of axons in the healthy optic nerve, there is a wide range in the normal RNFL thickness, as well as the topographical distribution, presumably related to genetic or developmental factors in addition to the clinical and demographic factors already identified.17 Only in some well established cases is it possible to diagnose optic atrophy from glaucoma or other causes based on RNFL thickness alone. However, in the absence of other progressive non-glaucomatous optic neuropathies, only age and glaucoma have been found to cause thinning of the RNFL over time. Age related differences in RNFL thickness have been demonstrated using optical coherence tomography in several studies15 – 18 in addition to the present one. Whether the reduced measurements in older individuals is due to axon loss, rearrangement of glial elements, or other causes, the age-related alterations in the measurements apart from any glaucomatous effect should be taken into account when monitoring for glaucomatous progression. In this way, confirmed age-adjusted changes that exceed the limit of reproducibility of the measurement may indicate progressive glaucoma.27
To summarize, this and a few other studies with different methods and smaller cohorts of different composition mutually confirm a loss of axons and corresponding loss of nerve fiber layer thickness decrease and retardance with age. Moreover, the age-related progressive loss is most prominent in the superior quadrant, most importantly in comparison to the inferior quadrant. It is obvious that the thickness parameters themselves have a wide range at all ages (Figure 2), so that the lower boundary of average thickness is about the same at all ages. For this reason, rates of thickness change in emerging glaucoma may be the basis for diagnosis in some cases. In suspicious cases monitored for a few years to determine the presence of glaucoma, the rate of thinning must exceed that which might be attributed to age; that is, faster than the fastest rate within the 95% confidence limit for changes with age. Of particular note, glaucoma may be characterized by a certain generalized loss in all meridians at the beginning, but before long it is more prominent in some locations than others. Often this is in the inferior and superior quadrants. To conclude glaucomatous loss has occurred, the average thickness should decline more rapidly than the fastest rate of age-related decline of the average thickness at the 95% confidence limit. Based on the present data, to conclude that glaucomatous change is occurring, one would have to demonstrate that the rate of change is faster than that expected from the 95% confidence limit at a particular location or sector.
Acknowledgments
Supported by NIH center grant P30 EY014801, awarded by the National Eye Institute, Bethesda, Maryland and by an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York. Collection of the normative database used in this study was supported by a grant and instrumentation provided by Carl Zeiss Meditec, Dublin, CA.
Footnotes
Conflict of Interest Statement: Dr. Anderson is a consultant for Carl Zeiss, Meditec, Dublin, CA. Dr. Schuman receives royalties for intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec. Dr. Schuman received honoraria from Carl Zeiss Meditec, Heidelberg Engineering, and Optovue.
Presented, in part, as a poster at the annual meeting of the Association for Research in Vision and Ophthalmology, April, 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA. Examination of the retinal nerve fiber layer in the recognition of early glaucoma damage. Trans Am Ophhtalmol Soc. 1986;84:920–966. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 3.Mikelberg FS, Yidegiligne HM, Schulzer M. Optic nerve axon count and axon diameter in patients with ocular hypertension and normal visual fields. Ophthalmology. 1995;102:342–348. doi: 10.1016/s0161-6420(95)31019-6. [DOI] [PubMed] [Google Scholar]
- 4.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- 5.Pederson JE, Anderson DR. The mode of progressive optic disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980;98:490–5. doi: 10.1001/archopht.1980.01020030486010. [DOI] [PubMed] [Google Scholar]
- 6.Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol. 1993;111:62–5. doi: 10.1001/archopht.1993.01090010066028. [DOI] [PubMed] [Google Scholar]
- 7.Dolman CL, McCormick AQ, Drance SM. Aging of the optic nerve. Arch Ophthalmol. 1980;98:2053–8. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- 8.Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–6. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BM, Miao M, Sadun AA. Age-related decline of human optic nerve axon populations. Age. 1987;10:5–9. [Google Scholar]
- 10.Jonas JB, Muller-Bergh JA, Schlötzer-Schrehardt UM, Naumann GOH. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990;31:736–44. [PubMed] [Google Scholar]
- 11.Chi Q, Tomita G, Inazumi K, et al. Evaluation of ageing on the retinal nerve fiber layer thickness using scanning laser polarimetry. J Glaucoma. 1995;4:1–8. [PubMed] [Google Scholar]
- 12.Tjon-Fo-Sang MJ, de Vries J, Lemij HG. Measurement by nerve fiber analyzer of retinal nerve fiber layer thickness in normal subjects and patients with ocular hypertension. Am J Ophthalmol. 1996;122:220–7. doi: 10.1016/s0002-9394(14)72013-6. [DOI] [PubMed] [Google Scholar]
- 13.Poinoosawmy D, Fontana L, Wu J X, Fitzke F W, Hitchings R A. Variation of nerve fibre layer thickness measurements with age and ethnicity by scanning laser polarimetry. Br J Ophthalmol. 1997;81:350–4. doi: 10.1136/bjo.81.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Pozzo S, Iacono P, Marchesan R, Minutola D, Ravalico G. The effect of ageing on retinal nerve fibre layer thickness: an evaluation by scanning laser polarimetry with variable corneal compensation. Acta Ophthalmologica Scandinavica. 2006;84:375–9. doi: 10.1111/j.1600-0420.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 15.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 16.Varma R, Bazzaz S, Lai M. Optical tomography--measured retinal nerve fiber layer thickness in normal Latinos. Invest Ophth Vis Sci. 2003;44:3369–73. doi: 10.1167/iovs.02-0975. [DOI] [PubMed] [Google Scholar]
- 17.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–52. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007;114:921–6. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Budenz DL. Symmetry between the right and left eyes of the normal retinal nerve fiber layer measured with optical coherence tomography. Trans Am Ophthalmol Soc. 2008;106:272–275. [PMC free article] [PubMed] [Google Scholar]
- 20.Gross PG, Drance SM. Comparison of a simple ophthalmoscopic and planimetric measurement of glaucomatous neuro-retinal rim areas. J Glaucoma. 1995;4:314–6. [PubMed] [Google Scholar]
- 21.Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–27. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- 22.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic optic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–46. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 23.Mikelberg FS, Drance SM, Schulzer M, Yidegiligne HM, Weis MM. The normal optic nerve: axon count and axon diameter distribution. Ophthalmology. 1989;96:1325–8. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- 24.Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- 25.Frenkel S, Morgan JE, Blumenthal EZ. Histologic measurement of retinal nerve fibre layer thickness. Eye. 2005;19:491–8. doi: 10.1038/sj.eye.6701569. [DOI] [PubMed] [Google Scholar]
- 26.Schuman JS, Pedut-Klotzman T, Pakter H. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Invest Ophthalmol Vis Sci. 2007;48:3645–54. doi: 10.1167/iovs.06-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budenz DL, Fredette M-J, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with the Stratus™ OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–6. doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Funaki S, Shirakashi M, Abe H. Relation between size of optic disc and thickness of retinal nerve fibre layer in normal subjects. Br J Ophthalmol. 1998;82:1242–5. doi: 10.1136/bjo.82.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]