Abstract
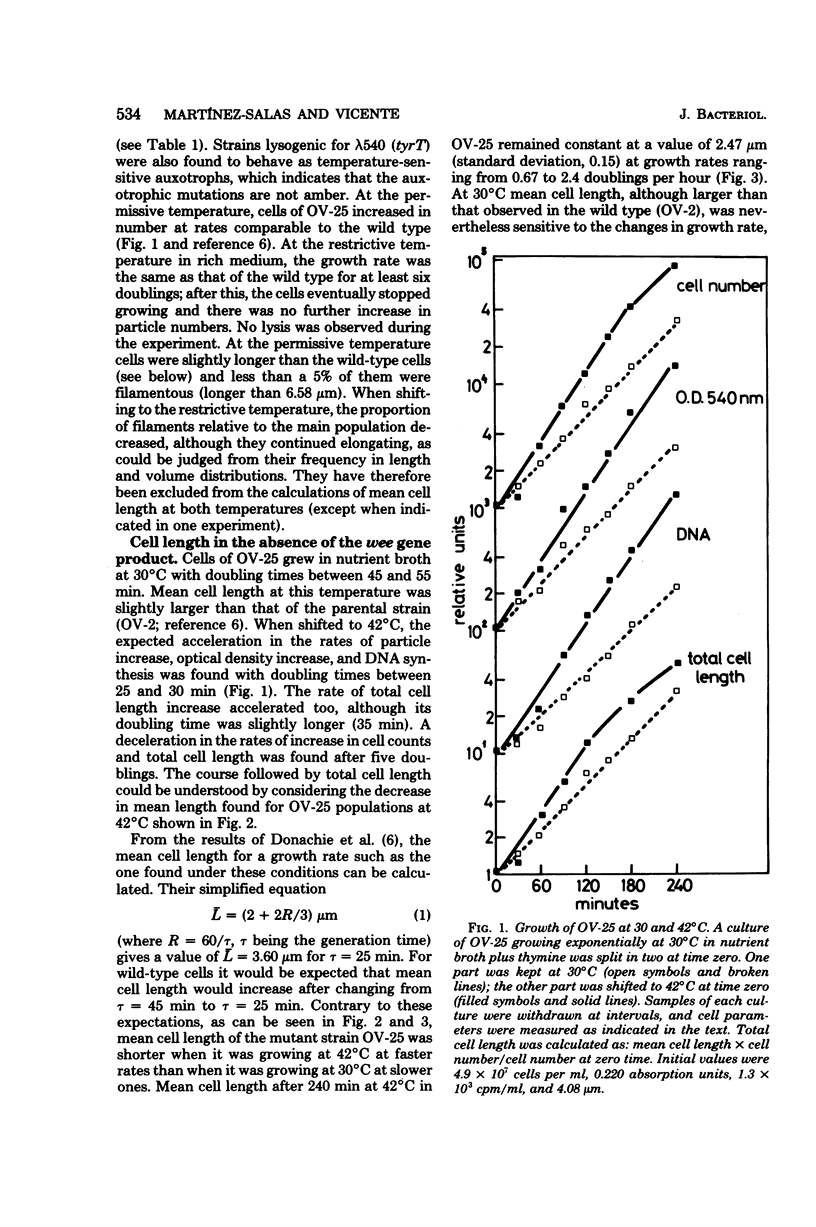
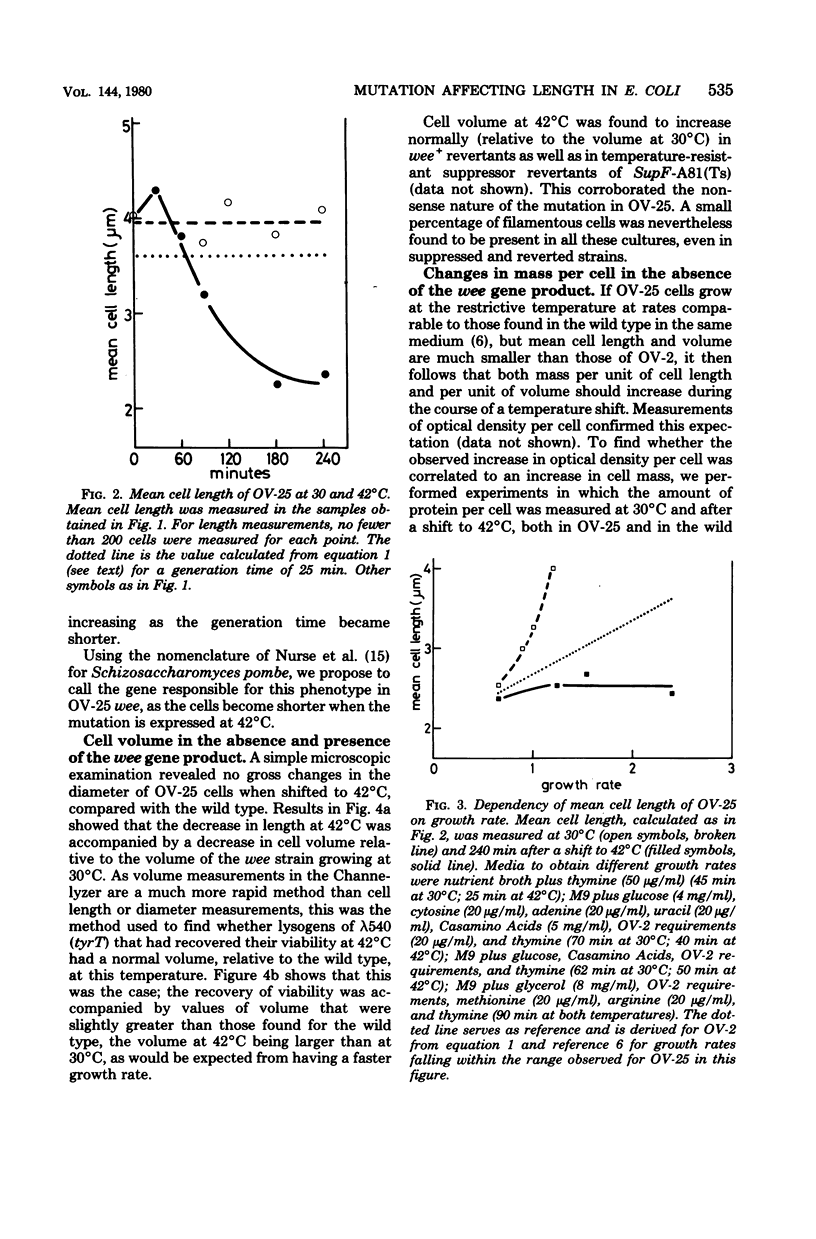
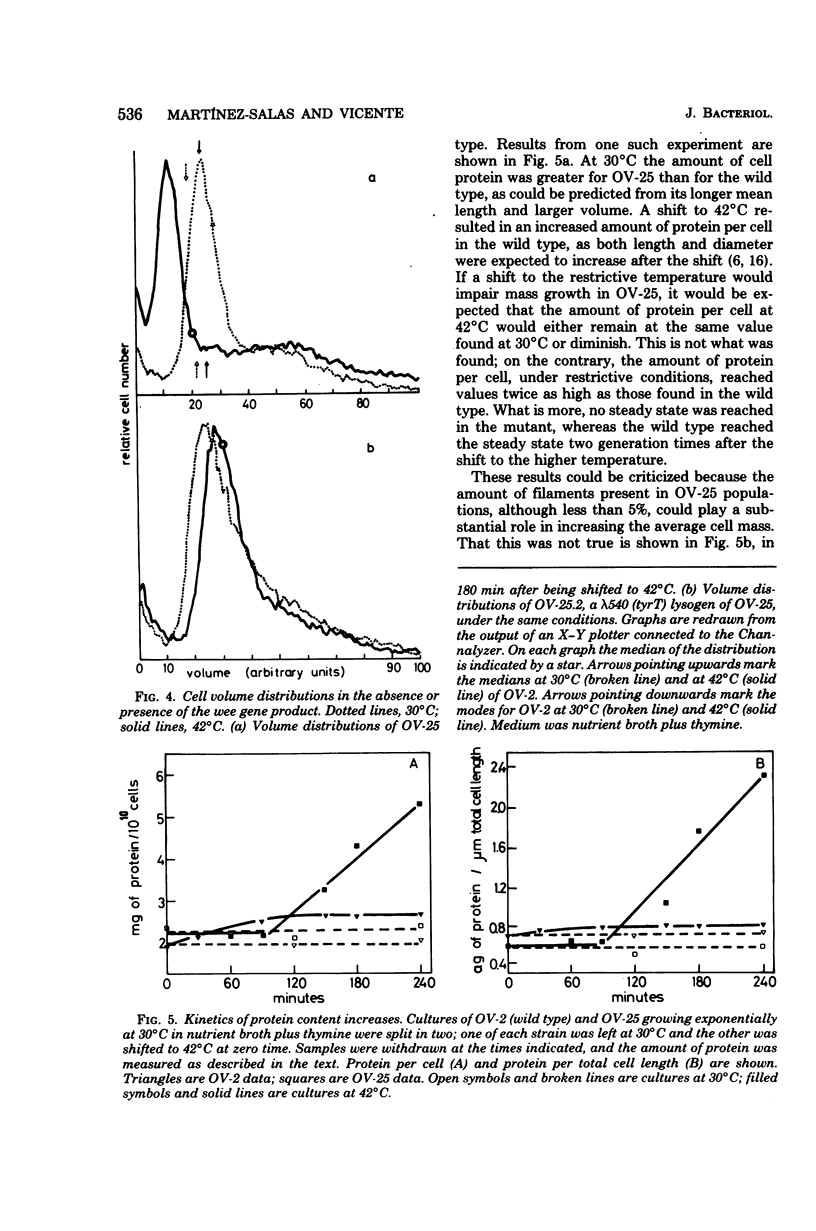
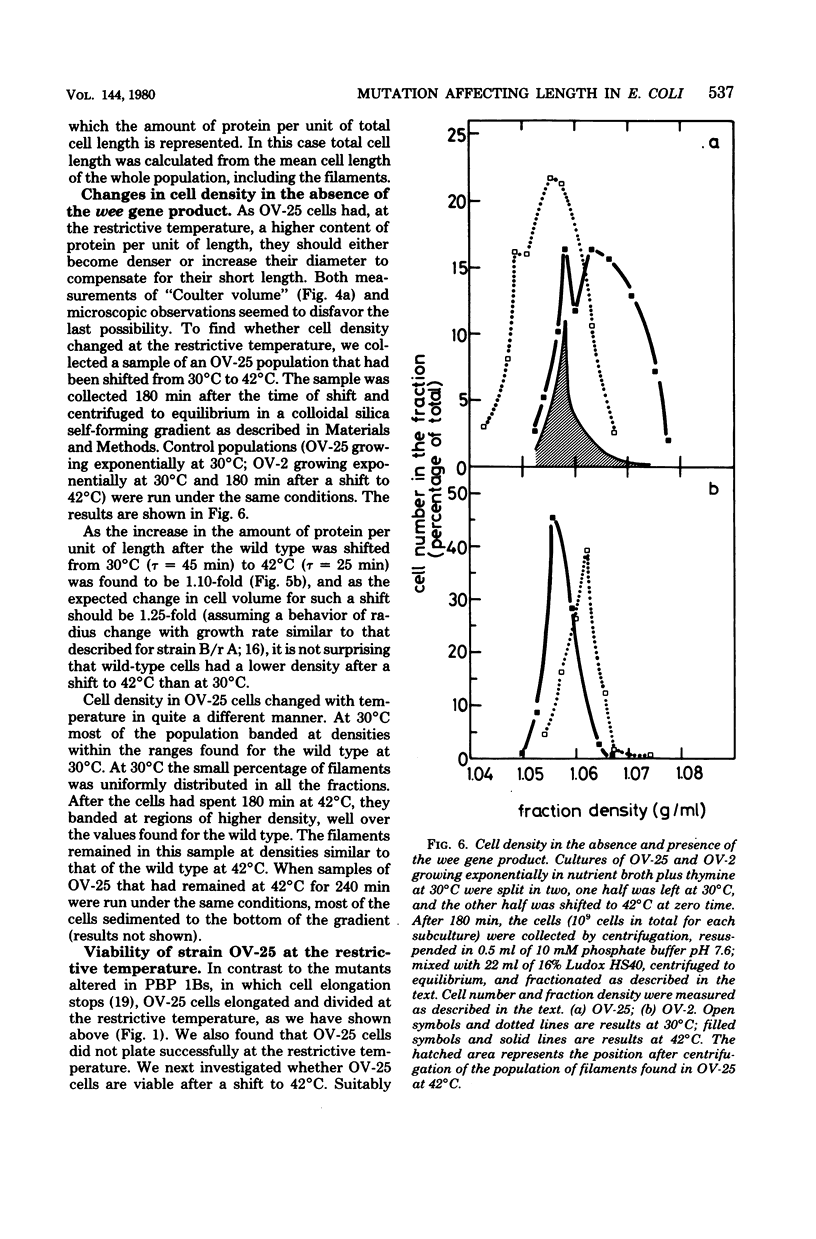
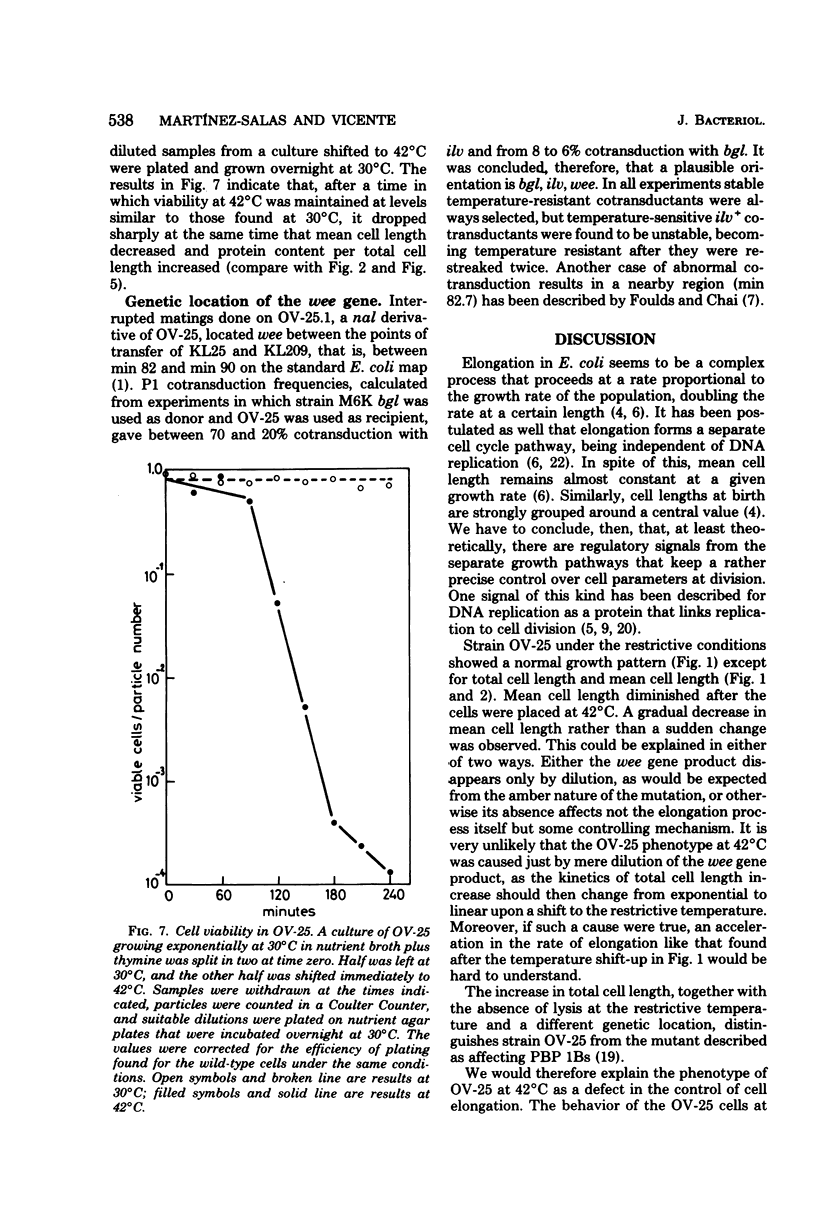
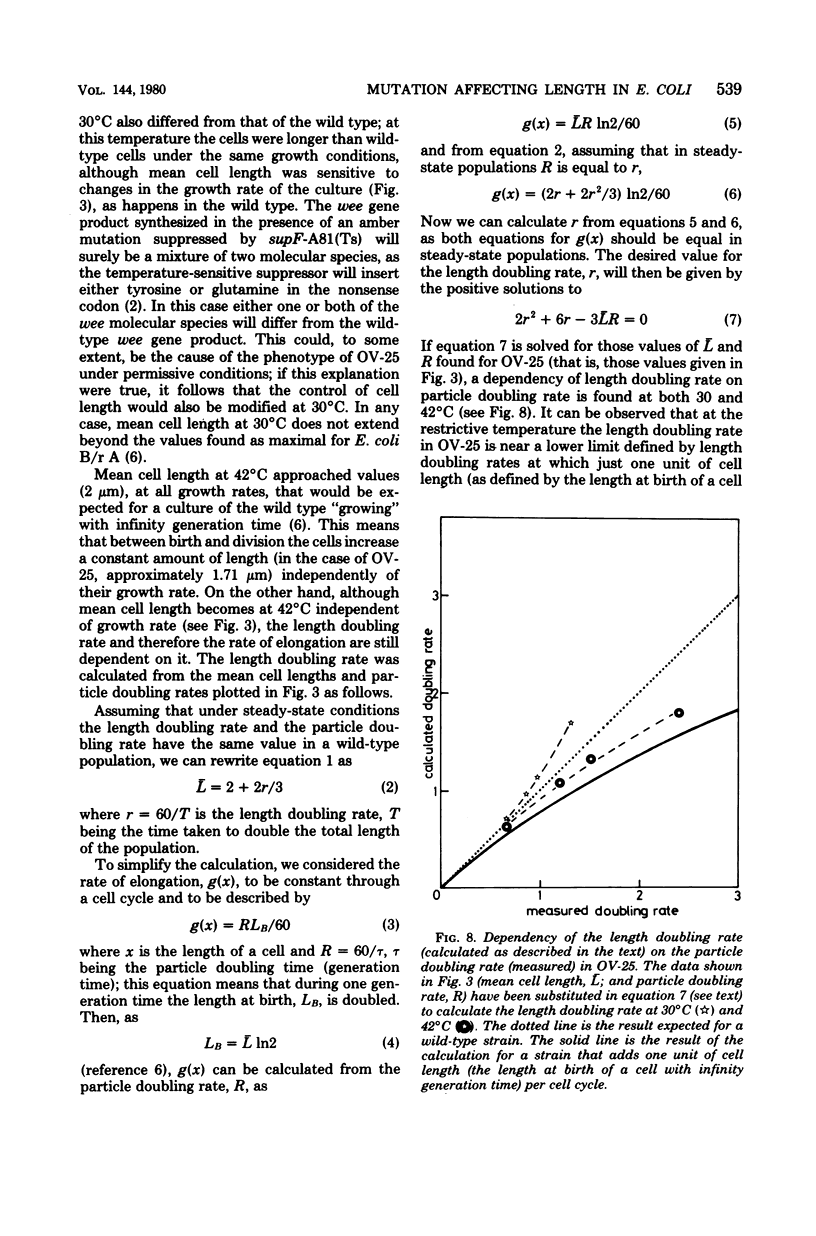
An amber mutation in a newly found gene (wee) of Escherichia coli has been isolated from strain OV-2, which harbors a temperature-sensitive suppressor. At 42 degrees C cells of the mutant, OV-25, increased in mass and deoxyribonucleic acid content and divided at normal rates, compared with the wild type under the same growth conditions. Total cell length increased under the restrictive conditions, although at a slightly lower rate. Values of mean cell length and cell volume, contrary to what would be expected from the increment in the rate of increase in particles, mass, and deoxyribonucleic acid, became at 42 degrees C smaller than those found in the wild type. A parallel increase in protein content per length and cell density and a loss of viability were found to occur after four generations at the restrictive temperature. The behavior of strain OV-25 in the absence of the wee gene product could be interpreted in terms of either a faulty regulation of the elongation processes or their abnormal coordination with the cell cycle. The genetic location of the wee gene has been found to be at 83.5 min on the E. coli genetic map.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Hooper M. L., Smith J. D. Amino acid acceptor stem of E. coli suppressor tRNA tyr is a site of synthetase recognition. Nat New Biol. 1973 Aug 29;244(139):261–264. doi: 10.1038/newbio244261a0. [DOI] [PubMed] [Google Scholar]
- Cullum J., Vicente M. Cell growth and length distribution in Escherichia coli. J Bacteriol. 1978 Apr;134(1):330–337. doi: 10.1128/jb.134.1.330-337.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Lutkenhaus J. F., Salmond G. P., Martinez-Salas E., Vincente M. Role of the ftsA gene product in control of Escherichia coli cell division. J Bacteriol. 1979 Nov;140(2):388–394. doi: 10.1128/jb.140.2.388-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Vicente M. Cell length, cell growth and cell division. Nature. 1976 Nov 25;264(5584):328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- Foulds J., Chai T. J. Chromosomal location of a gene (nmpA) involved in expression of a major outer membrane protein in Escherichia coli. J Bacteriol. 1978 Nov;136(2):501–506. doi: 10.1128/jb.136.2.501-506.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Kaiser A. D., Masuda T. Evidence for a prophage excision gene in lambda. J Mol Biol. 1970 Feb 14;47(3):557–564. doi: 10.1016/0022-2836(70)90322-0. [DOI] [PubMed] [Google Scholar]
- Koppes L. H., Woldringh C. L., Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978 May;134(2):423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masters M. The frequency of P1 transduction of the genes of Escherichia coli as a function of chromosomal position: preferential transduction of the origin of replication. Mol Gen Genet. 1977 Oct 20;155(2):197–202. doi: 10.1007/BF00393160. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Pierucci O. Dimensions of Escherichia coli at various growth rates: model for envelope growth. J Bacteriol. 1978 Aug;135(2):559–574. doi: 10.1128/jb.135.2.559-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K. Fluctuations in buoyant density during the cell cycle of Escherichia coli K12: significance for the preparation of synchronous cultures by age selection. J Gen Microbiol. 1977 Jan;98(1):177–186. doi: 10.1099/00221287-98-1-177. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Martínez-Salas E., Vicente M. Involvement of the ftsA gene product in late stages of the Escherichia coli cell cycle. J Bacteriol. 1980 Feb;141(2):806–813. doi: 10.1128/jb.141.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. A. The separation of cells and subcellular particles by colloidal silica density gradient centrifugation. Methods Cell Biol. 1975;10:85–104. doi: 10.1016/s0091-679x(08)60731-1. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L. Chromosome replication rate and cell shape in Escherichia coli: lack of coupling. J Bacteriol. 1978 Aug;135(2):581–587. doi: 10.1128/jb.135.2.581-587.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipkas D., Riley M. Simplified method for interruption of conjugation in Escherichia coli. J Bacteriol. 1976 Apr;126(1):559–560. doi: 10.1128/jb.126.1.559-560.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



