Abstract
Angiogenesis is a multi-step process that involves the activation, proliferation, and migration of endothelial cells. We have recently shown that TGF-β1 can induce mouse macrophages to produce VEGF, a potent angiogenic factor. In the present study, we explored whether TGF-β1 has a similar effect on mouse dendritic cells. First, we show that under hypoxic conditions, TGF-β1 induced the expression of VEGF transcripts in bone marrow-derived dendritic cells. Overexpression of Smad3/4 further augmented TGF-β1-induced VEGF transcription, while overexpression of DN-Smad3 decreased VEGF transcription in DC2.4 cells, a mouse dendritic cell line. We also show that TGF-β1 and Smads are involved in the induction of VEGF protein secretion. Interestingly, under the same conditions, the expression of VEGF receptor 1 (Flt-1) was also elevated at both the transcriptional and protein levels. Additionally, we found that the TGF-β1-induced VEGF secretion in activated DC2.4 cells has wound-healing properties. Finally, Smad7 and Smurf1 negatively regulated the TGF-β1-induced and Smad3/4-mediated VEGF expression. Taken together, these results indicate that TGF-β1 can enhance the expression of VEGF and Flt-1 through the typical Smad pathway in mouse dendritic cells.
Keywords: dendritic cells; neovascularization, physiologic; Smad3 protein; transforming growth factor β1; vascular endothelial growth factor A; vascular endothelial growth factor receptor-1
Introduction
Angiogenesis, the growth of new blood vessels, is a multi-step process characterized by endothelial cell migration, proliferation, survival, proteolytic activity, and tube formation. Among professional APCs, macrophages secrete various angiogenic factors such as VEGF and Bv8, in addition to producing matrix metallopeptidase-9, which increases the bioavailability of VEGF and potentially other proangiogenic molecules (Shojaei et al., 2008). The role of VEGF in angiogenesis is well documented. VEGF expression is regulated by pathological conditions such as hypoxia and hypoglycemia (Shweiki et al., 1992; Stein et al., 1995; Song et al., 2009), as well as by cytokines and growth factors such as PDGF, TNF-α, IL-1, IL-6 (Ryuto et al., 1996; Finkenzeller et al., 1997; Salven et al., 2002; Wei et al., 2003). Similarly, it has been shown that dendritic cells (DCs) under the influence of anti-inflammatory molecules such as calcitriol, PGE2, or IL-10 selectively secrete VEGF isoforms VEGF165 and VEGF121 (Riboldi et al., 2005). Thus, DCs are increasingly being recognized as important regulators of angiogenesis and lymphangiogenesis (Riboldi et al., 2005; Webster et al., 2006).
We have previously demonstrated that TGF-β1 activate mouse macrophages and induce the expression of angiogenic mediators, including VEGF and Flk-1 (Jeon et al., 2007). However, it is not known whether TGF-β can induce mouse DCs to produce VEGF and, if so, what mechanisms are involved. The present study demonstrates that TGF-β1-activated DCs produce VEGF, and that Smad3/4 mediates this response. TGF-β1/Smad3/4 also increased the expression of VEGF receptor 1 (Flt-1). These results indicate that TGF-β1-activated mouse DCs contribute to angiogenesis via the production of angiogenic factors, including VEGF and Flt-1, and that the typical Smad pathway is involved in this response.
Results and Discussion
Effect of TGF-β1 on the expression of VEGF in mouse dendritic cells
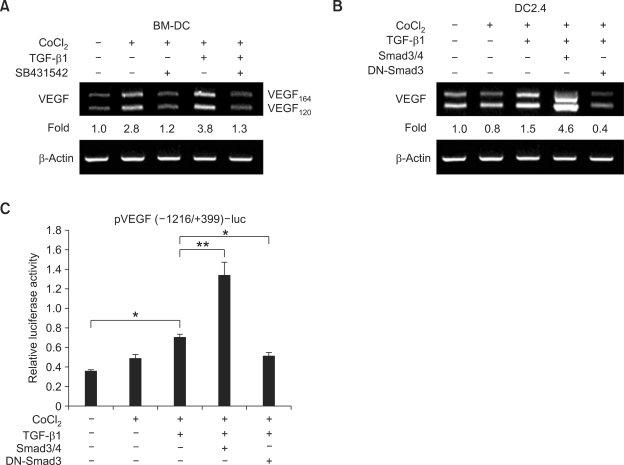
We have previously shown that treatment with TGF-β1 increases VEGF expression by mouse macrophages under hypoxic conditions (Jeon et al., 2007). Although DCs are also known to produce VEGF and contribute to angiogenesis in humans and mouse models (Riboldi et al., 2005; Webster et al., 2006), it is not known whether TGF-β1 can induce mouse DCs to express VEGF. In the present study, we first measured the levels of VEGF mRNA expression in freshly isolated bone marrow-derived DCs in the presence of TGF-β1 and CoCl2 (a chemical inducer of HIF-1). As shown in Figure 1A, treatment of bone marrow-derived DCs with CoCl2 increased the expression of VEGF mRNA by 2.8-fold. This expression was increased further by the addition of TGF-β1. Treatment with SB431542, an inhibitor of TGF-β receptor type I, abolished the induction of VEGF mRNA by CoCl2 and TGF-β1. As Smad3 and Smad4 are well known signal transducers and transcription factors in the TGF-β signaling pathway, we explored involvement of these molecules in the induction of VEGF mRNA expression by DC2.4 cells (a mouse DC cell line). Overexpression of Smad3/4 markedly increased TGF-β1-induced VEGF expression, while overexpression of DN-Smad3 reduced the expression of VEGF (Figure 1B). Similarly, treatment with TGF-β1 substantially increased the promoter activity of the VEGF gene under hypoxic conditions, and this was augmented by overexpression of Smad3/4, but expression decreased in response to overexpression of DN-Smad3 (Figure 1C). We note that the sole effect of CoCl2 vary in Figures 1A, 1B, and 1C. This discrepancy seemed due to the intrinsic features of cell types (normal DC vs. DC cell line) and assays (endogenous VEGF transcripts vs. exogenous VEGF promoter activity) adopted in this study. In addition, not shown here, TGF-β1 alone did not increase VEGF mRNA expression and VEGF promoter activity. These results indicate that, under hypoxic conditions, treatment with exogenous TGF-β1 causes mouse DCs to increase expression of VEGF, and that this increase is mediated by Smad3/4.
Figure 1.
TGF-β1 and Smad3 induces VEGF transcription in mouse dendritic cells. (A) Effect of TGF-β1 on VEGF mRNA expression by mouse bone marrow-derived dendritic cells (BM-DC). Freshly isolated bone marrow stem cells were differentiated into BM-DC as described in Methods. Cells (2 × 106 cells/well) were incubated with CoCl2 (50 µM), TGF-β1 (1 ng/ml), and SB431542 (10 µM) for 24 h. VEGF mRNA was measured by RT-PCR. (B and C) The role of Smad 3 and 4 in TGF-β1-induced VEGF transcription. DC2.4 cells were incubated with TGF-β1 (1 ng/ml) and CoCl2 (50 µM) for 24 h. (B) Levels of endogenous VEGF transcripts were measured by RT-PCR. DC2.4 cells were transfected with reporter [2.5 µg of pVEGF(-1216/+399)-luc] and expression vector coding for Smad3, Smad4, and DN-Smad3 (2 µg each). (C) Transcriptional activity of pVEGF(-1216/+399)-luc was measured with a luciferase assay. Data represent average luciferase activities of three independent transfections with SEM (bars). *P < 0.05; **P < 0.01.
TGF-β1-stimulated DCs secrete biologically active VEGF
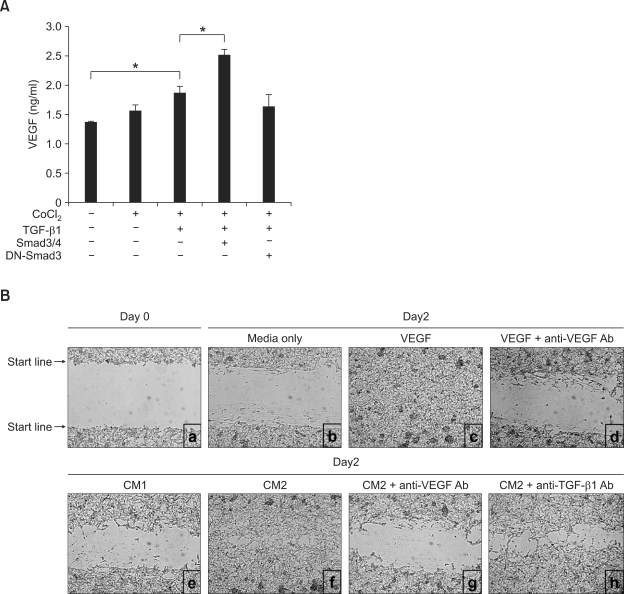
We determined that TGF-β1 causes DCs to express VEGF at the transcriptional level. We then sought to show whether TGF-β1 causes DCs to make VEGF at the protein level. Consistent with the pattern of transcription, TGF-β1 caused DCs to increase VEGF secretion under hypoxic conditions. The level of secretion was further augmented by the overexpression of Smad3/4, while the overexpression of DN-Smad3 abolished the VEGF secretion increase induced by TGF-β1 treatment (Figure 2A). VEGF has been shown to promote angiogenesis and plays an important role in wound healing (Byrne et al., 2005; Carmeliet, 2005; Bao et al., 2009). To test the biological activity of the VEGF secreted by the TGF-β1-stimulated DC2.4 cells, we performed an in vitro wound-healing assay using B16-F1 cells. As shown in Figure 2B, conditioned medium 2 (CM2) from the culture of DC2.4 cells transfected with Smad3/4 in the presence of TGF-β1 and CoCl2 markedly enhanced cell migration, a process indicative of wound-healing activity. The increase in cell migration was comparable to the migration that occurred in response to 10 ng/ml VEGF, and the wound-healing activity by CM2 was virtually abolished by treatment with anti-VEGF Ab. It is well known that TGF-β1 itself also possesses wound-healing activity (Peinado et al., 2003; Wang et al., 2006). To test the effect of any residual TGF-β1 retained in CM2, we pretreated the CM2 with anti-TGF-β1 Ab, which caused a moderate inhibition of the cell migration induced by CM2. Thus, the inhibitory activity of anti-TGF-β1 Ab was far less than that by anti-VEGF Ab. These results indicate that the VEGF present in CM2 is biologically active and largely responsible for cell migration.
Figure 2.
TGF-β1 stimulates dendritic cells to secrete biologically active VEGF. (A) DC2.4 cells (5 × 105/well) were transfected with Smad3/4 or DN-Smad3 (1 µg each). Cells were cultured with TGF-β1 (1 ng/ml) and CoCl2 (50 µM). After 2 days of culture, supernatants were collected and VEGF production was measured by ELISA. Data are means of triplicate samples ± SEM. *P < 0.05. (B) Wound-healing assay in which conditioned media 1 (CM1) was prepared from cultured DC2.4 cells transfected with pcDNA3 and CM2 was prepared from DC2.4 cells transfected with Smad3/4 treated with TGF-β1 (1 ng/ml) and CoCl2 (50 µM). Confluent B16-F1 cells were scraped by using P200 tips and cultured with various stimuli as indicated. To neutralize VEGF and TGF-β1, conditioned media (CM2) was pre-incubated with the corresponding neutralizing Abs. Wound healing was determined with a light microscope (×100) after 2 days incubation.
Smad7 and Smurf1 inhibit TGF-β1-induced Smad3/4-activated VEGF transcription
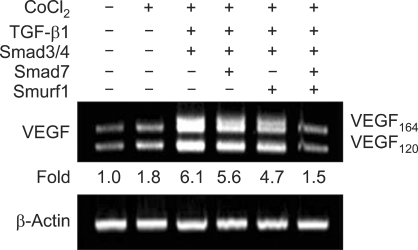
To study the role of VEGF expression induced by the TGF-β signaling pathway in DCs, we examined the roles of Smad7 and Smad ubiquitination regulatory factor 1 (Smurf1) in TGF-β1-induced VEGF transcription. Smad7, a TGF-β-inducible antagonist of TGF-β signaling, inhibits Smad3 phosphorylation by competing with Smad3 for binding to the activated TGF-β type I receptor in the cytoplasm (Hayashi et al., 1997; Nakao et al., 1997). Smad7 also recruits Smurf1, an E3-ubiquitin ligase, to the activated TGF-β type I receptor, which results in receptor ubiquitination and degradation, and ultimately reduced TGF-β signaling (Ebisawa et al., 2001). We show that, under hypoxic conditions, TGF-β1-induced, Smad3/4-mediated VEGF transcription was partially inhibited by overexpression of either Smad7 or Smurf1. Furthermore, VEGF transcription was virtually abolished by overexpression of both of these molecules (Figure 3). These results indicate that the typical Smad pathway is engaged in TGF-β1-induced VEGF expression in murine DCs, and that this process involves the negative regulators Smad7 and Smurf1.
Figure 3.
Effects of Smad7 and Smurf1 on TGF-β1-induced, Smad3/4-mediated VEGF transcription. DC2.4 cells (2 × 105/well) were transfected with Smad3/4, Smad7, and Smurf1 (1 µg each). Cells were incubated with CoCl2 (50 µM) and TGF-β1 (1 ng/ml) for 24 h. Total RNA was isolated and levels of endogenous VEGF transcripts were measured by RT-PCR.
Smad3/4 mediates TGF-β1-induced VEGF receptor 1 expression
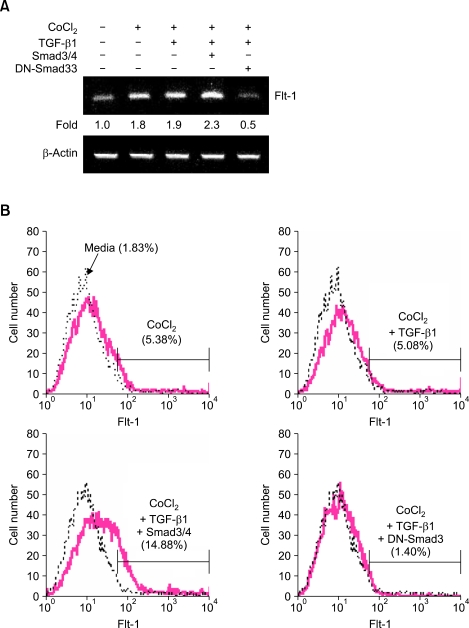
VEGF binds to three different VEGF receptors, which belong to the family of receptor tyrosine kinases. These receptors are VEGFR-1 (Flt-1), VEGFR-2 (Flk-1), and VEGFR-3 (Flt-4). Other studies have shown that TGF-β1 induces the upregulation of Flk-1 expression in mouse mammary epithelial cells (Breier et al., 2002). We have previously shown that TGF-β1 specifically stimulates mouse macrophages to express Flk-1 through Smad3/4-mediated VEGF expression under hypoxic conditions (Jeon et al., 2007). To expand on these studies, we assessed the effect of TGF-β1 and Smad3/4 on the expression of VEGF receptors in DCs. Unexpectedly, treatment with TGF-β1 and overexpression of Smad3/4 did not affect expression of Flk-1 mRNA (data not shown). However, overexpression of Smad3/4 in conjunction with CoCl2 and TGF-β1 markedly enhanced Flt-1 mRNA expression, whereas overexpression of DN-Smad3 abolished this increase (Figure 4A). Similar patterns of surface Flt-1 expression were also observed in response to TGF-β1 treatment and overexpression of Smad3/4 (Figure 4B). We note that the present report does not assess the TGF-β1/Smad-mediated Flt-1 expression at the level of the promoter. It remains to be determined whether Smad-binding elements exist within the promoter region of the Flt-1 gene.
Figure 4.
Effects of TGF-β1 and Smad3/4 on the expression of VEGF receptor 1 (Flt-1). DC2.4 cells (2 × 106) were transfected with Smad3/4 and DN-Smad3 (1 µg each); cells were then incubated with CoCl2 (50 µM) and TGF-β1 (1 ng/ml). (A) After 24 h, Flt-1 mRNA was measured by RT-PCR. (B) After 48 h of culture, cells were stained with FITC-labeled anti-Flt-1 Ab and analyzed by FACS.
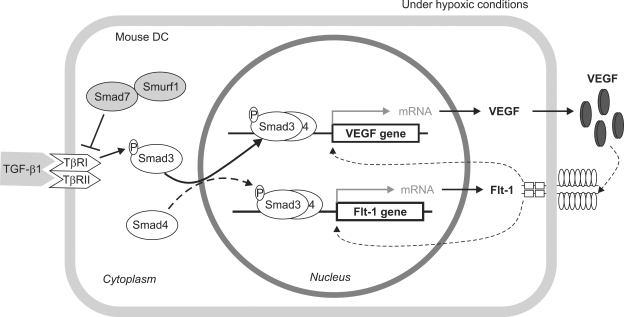
In summary, our results reveal that TGF-β1 induces the expression of VEGF and Flt-1 in mouse dendritic cells under hypoxic conditions, as illustrated in Figure 5. Upon being stimulated by TGF-β1, Smad3 becomes phosphorylated by the activated TGF-β receptors and forms a complex with Smad4. Under hypoxic conditions, this Smad complex translocates into the nucleus, where it binds Smad-binding elements in the VEGF promoter, thereby activating transcription of the VEGF gene and presumably the Flt-1 gene. The subsequently secreted VEGF protein may enhance the expression of VEGF and Flt-1 via binding to Flt-1. The fact that macrophages stimulated with TGF-β1 express VEGF and Flk-1 proteins (Jeon et al., 2007) indicates a TGF-β1-mediated role for macrophages and dendritic cells in angiogenesis and lymphangiogenesis. However, TGF-β1 is produced by a variety of cells including macrophages, T cells, and tumor cells (Roberts and Sporn, 1987). Thus, particularly in the context of tumor progression, one should assess carefully if the angiogenic property of TGF-β1-activated VEGF is favorable to host or tumors.
Figure 5.
Proposed mechanism by which TGF-β1 induces expression of VEGF and Flt-1 in mouse dendritic cells.
Methods
Experimental materials and mice
TGF-β1, VEGF, GM-CSF, and IL-4 were purchased from R&D System (Minneapolis, MN). SB431542, an inhibitor of TGF-β type I receptor, was purchased from Sigma Chemical Co. (St. Louis, MO). BALB/c mice were purchased from Orient. Co., Ltd. (Gyeonggido, Korea) and maintained on an 8:16 h light:dark cycle in an animal environmental control chamber (Myung Jin Inst. Co., Seoul, Korea). Animal care was conducted in accordance with the institutional guidelines of Kangwon National University.
Cell culture
Cells of the mouse dendritic cell line DC2.4 were cultured in DMEM (Sigma) supplemented with 10% fetal bovine serum (HyClone Labs, Logan, UT), 5 mM HEPES, 2 mM L-glutamine, and penicillin (100 U/ml)/streptomycin (100 µg/ml). Cells were cultured at 37℃ in an incubator (Sanyo, Osaka, Japan) with a humidified atmosphere containing 5% CO2. Bone marrow stem cells were isolated from BALB/c mouse femurs and cultured with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml). After 7 days, of the presence of dendritic cells was determined by staining the cells with mouse anti-CD11c mAb (R&D Systems, Inc., Minneapolis, MN), and analyzing their phenotype by FACS. This method of culture resulted in a population that was approximately 70% CD11c positive.
Mouse VEGF ELISA
VEGF produced in DC2.4 cell cultures was detected by ELISA as previously described (Jeon et al., 2007). Briefly, anti-mouse VEGF antibody (R&D Systems, Minneapolis, MN) was added at 0.4 µg/ml in 0.05 M bicarbonate buffer (pH 9.3) to 96-well, U-bottom, polyvinyl microplates (Becton Dickinson and Co., Oxnard, CA). After incubation overnight at 4℃, the plates were washed and blocked with 1% gelatin for 1 h. Samples (50 µl) or standard protein (mouse recombinant VEGF, R&D Systems) diluted in 0.5% gelatin were added to the wells. After incubation for 1 h at 37℃, the plates were washed again, and 50 ng/ml biotinylated anti-mouse VEGF antibody (R&D Systems) was added for 1 h at 37℃. The plates were then washed and incubated with streptavidin-HRP for 1 h at 37℃. After washing, 0.2 mM ABTS (Sigma Chemical Co.) was added to the wells, and after 10 min, the colorimetric reaction was measured at 405 nm with an ELISA reader VERSAmax (Molecular Devices, Sunnyvale, CA).
RT-PCR
RNA preparation, reverse transcription, and PCR were performed as previously described (Park et al., 2001). Primers for PCR were synthesized by Bioneer Corp. (Seoul, Korea). The primers for mouse VEGF were: forward primer, 5'-CAGGCTGCTCTAACGATGAA-3'; reverse primer, 5'-CAGGAATCCCAGAAACAACC-3'. Primers spanning the mouse VEGF gene amplified three mRNA variants, VEGF120, VEGF164, VEGF188, as the expected 522-, 636-, and 708-bp products, respectively. The primer for Flt-1 were: forward primer, 5'-CCACCACTCAAGATTACTCC-3'; reverse primer, 5'-GTAGAGCCACTGATGGAGAC-3'. All reagents for conducting RT-PCR were purchased from Promega Corp. (Madison, WI). PCR reactions for the detection of β-actin were performed in parallel in order to normalize cDNA concentrations within each set of samples. PCR products were separated on 2% agarose gel and photographed.
Preparation of plasmids
A luciferase reporter construct containing the mouse VEGF promoter between -1216 and +399 [pVEGF(-1216/+339)-luc] was made as previously described (Jeon et al., 2007). Genes encoding Smad3 (Zhang et al., 1996), Smad4 (Hahn et al., 1996), and Smad7 (Nakao et al., 1997) subcloned into Flag-pcDNA3 (Imamura et al., 1997) were provided by Dr. M. Kawabata (The Cancer Institute, Tokyo, Japan). Smurf1 (Ebisawa et al., 2001) subcloned into Flag-pcDNA3 was provided by Dr. K. Miyazono (The Cancer Institute, Tokyo, Japan). The dominant-negative Smad3 expression plasmid (Smad3D407E) (Goto et al., 1998) was provided by Dr. M. Kato (The Cancer Institute, Tokyo, Japan).
Transfection and luciferase assays
The reporter plasmids were co-transfected with the expression plasmids and pCMVβgal (Stratagene, La Jolla, CA) into DC2.4 cells by using a MicroPorator™ MP-100 (Digital Bio Technology, Seoul, Korea); cotransfection was performed according to the manufacturer's instructions. Briefly, 2 × 106 cells were mixed with the reporter plasmid and the expression plasmid and pulsed twice at 1000 V for 30 ms. Following a 24 h incubation, luciferase and β-galactosidase assays were performed as described previously (Park et al., 2001).
Flow cytometry
To stain cells for FACS analysis, cultured cells were resuspended in DMEM, with 5% FBS and 0.1% NaN3, at a density of 2 × 106 cells/well. Rabbit anti-mouse Flt-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was added to the cell suspension and the cells were incubated at 4℃ for 30 min. After washing three times with HBSS, FITC-conjugated goat anti-rabbit IgG (Sigma) was added the cell suspension, which was incubated at 4℃ for 30 min. The cells were then washed and resuspended in PBS/1% formalin. Flow cytometric analysis was performed with a FACS (Becton Dickinson, Mountain View, CA).
In vitro wound-healing assay
An in vitro wound-healing assay was used to assess cell migration in the mouse melanoma cell line, B16-F1. Cells were placed in 24-well plates (Becton Dickinson, San Jose, CA) at a concentration of 3 × 105 cells/well. After the cells had grown to confluence, wounds were made using sterile P200 pipette tips, the plates were washed twice with PBS to remove detached cells, and then the cells were incubated with DMEM containing 1% FBS or conditioned medium. The medium was replaced with fresh conditioned medium every 24 h. To neutralize VEGF and TGF-β1 proteins, conditioned medium was pre-incubated with 0.3 µg/ml of anti-VEGF Ab (R&D Systems) and 5 µg/ml of anti-TGF-β Ab (R&D Systems) for 1 h at 37℃. After 48 h, the plates were washed and wound healing was assessed with a light microscope (×100).
Statistical analysis
Statistical differences between experimental groups were determined by analysis of variances. Values of P < 0.01 or P < 0.05 by unpaired two-tailed Student's t tests were considered significant.
Acknowledgements
This work was supported by the Regional Core Research Program funded by the Korea Ministry of Education, Science, and Technology (Medical & Bio-material Research Center), a Vascular System Research Center grant from the Korea Science and Engineering Foundation, and by the second stage of the Brain Korea 21 program. Experiments were performed in the facilities of the Vascular System Research Center and the Institute of Bioscience and Biotechnology at Kangwon National University.
Abbreviations
- DC
dendritic cells
- Flt-1
fetal liver tyrosine kinase-1
- HIF
hypoxia-inducible factor
References
- 1.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breier G, Blum S, Peli J, Groot M, Wild C, Risau W, Reichmann E. Transforming growth factor-beta and Ras regulate the VEGF/VEGF-receptor system during tumor angiogenesis. Int J Cancer. 2002;97:142–148. doi: 10.1002/ijc.1599. [DOI] [PubMed] [Google Scholar]
- 3.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 5.Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 6.Finkenzeller G, Sparacio A, Technau A, Marme D, Siemeister G. Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene. 1997;15:669–676. doi: 10.1038/sj.onc.1201219. [DOI] [PubMed] [Google Scholar]
- 7.Goto D, Yagi K, Inoue H, Iwamoto I, Kawabata M, Miyazono K, Kato M. A single missense mutant of Smad3 inhibits activation of both Smad2 and Smad3, and has a dominant negative effect on TGF-beta signals. FEBS Lett. 1998;430:201–204. doi: 10.1016/s0014-5793(98)00658-9. [DOI] [PubMed] [Google Scholar]
- 8.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 10.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 11.Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, Kim NS, Yie SW, Byeon WH, Eom SH, Ha KS, Kim YM, Kim PH. Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J Leukoc Biol. 2007;81:557–566. doi: 10.1189/jlb.0806517. [DOI] [PubMed] [Google Scholar]
- 12.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 13.Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001;31:1706–1715. doi: 10.1002/1521-4141(200106)31:6<1706::aid-immu1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 15.Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 16.Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–2792. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- 17.Roberts AB, Sporn MB. Transforming growth factor-beta: potential common mechanisms mediating its effects on embryogenesis, inflammation-repair, and carcinogenesis. Int J Rad Appl Instrum B. 1987;14:435–439. doi: 10.1016/0883-2897(87)90020-1. [DOI] [PubMed] [Google Scholar]
- 18.Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, Kuwano M. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 19.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1473. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 20.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 22.Song IS, Wang AG, Yoon SY, Kim JM, Kim JH, Lee DS, Kim NS. Regulation of glucose metabolism-related genes and VEGF by HIF-1alpha and HIF-1beta, but not HIF-2alpha, in gastric cancer. Exp Mol Med. 2009;41:51–58. doi: 10.3858/emm.2009.41.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 25.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–117. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 26.Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF- beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]