Abstract
In brain tissue, astrocytes play defensive roles in central nervous system integrity by mediating immune responses against pathological conditions. Type I phosphatidylinositol 4-phosphate 5-kinase α (PIP5Kα) that is responsible for production of phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2) regulates many important cell functions at the cell surface. Here, we have examined whether PIP5Kα is associated with astrocyte inflammatory responses. Gangliosides are releasable from damaged cell membranes of neurons and capable of inducing inflammatory responses. We found that treatment of primary cultured astrocytes with gangliosides significantly enhanced PIP5Kα mRNA and protein expression levels. PI(4,5)P2 imaging using a fluorescent tubby (R332H) expression as a PI(4,5)P2-specific probe showed that ganglioside treatment increased PI(4,5)P2 level. Interestingly, microRNA-based PIP5Kα knockdown strongly reduced ganglioside-induced transcription of proinflammatory cytokines IL-1β and TNFα. PIP5Kα knockdown also suppressed ganglioside-induced phosphorylation and nuclear translocation of NF-κB and the degradation of IκB-α, indicating that PIP5Kα knockdown interfered with the ganglioside-activated NF-κB signaling. Together, these results suggest that PIP5Kα is a novel inflammatory mediator that undergoes upregulation and contributes to immune responses by facilitating NF-κB activation in ganglioside-stimulated astrocytes.
Keywords: astrocytes, brain, gangliosides, inflammation, NF-κB, 1-phosphatidylinositol-4-phosphate 5-kinase
Introduction
Astrocytes are the most abundant glial cells in the brain and play crucial roles as immune effector cells against brain insult (Farina et al., 2007). Reactive astrocytes produce inflammatory mediators, including proinflammatory cytokines such as IL-1β and TNFα, reactive oxygen species, and nitric oxide (Pawate et al., 2004; Jou et al., 2006; Farina et al., 2007). Under pathological conditions such as neurodegenerative disorders, astrocyte immune function exerts a neuroprotective role, but is also recognized to cause the progression of neuronal damage (Teismann and Schulz, 2004; Schwab and McGeer, 2008).
Phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2), generated from phosphatidylinositol 4-phosphate by type I phosphatidylinositol 4-phosphate 5-kinase (PIP5K) family members and also from phosphatidylinositol 5-phosphate by type II phosphatidylinositol 5-phosphate 4-kinase (PIP4K) family members, is enriched in the plasma membrane (Rameh et al., 1997; Doughman et al., 2003). Synthesis of PI(4,5)P2 is catalyzed mainly by type I PIP5K because phosphatidylinositol 4-phosphate is more abundant than phosphatidylinositol 5-phosphate in mammalian cells (Di Paolo and De Camilli, 2006). PIP5K and PI(4,5)P2 are critical regulators of a variety of cellular processes involving membrane signaling and trafficking, and actin dynamics (Takenawa and Itoh, 2001; Doughman et al., 2003; Di Paolo and De Camilli, 2006).
Gangliosides, sialic acid-containing glycosphingolipids, are enriched in neuronal cell membranes and released into the extracellular space upon brain injury and play a significant role in various molecular events related to brain pathology and physiology (Blennow et al., 1992; Sonnino and Chigorno, 2000; Ariga et al., 2008; Posse de Chaves and Sipione, 2010). Recently, gangliosides were shown to affect proliferation of mouse embryonic stem cells and differentiation of neural precursor cells (Jung et al., 2009). Furthermore, treatment of astrocytes and microglia (another important glial cell type that mediates brain inflammation) with gangliosides stimulates the activation of multiple signaling proteins, such as NADPH oxidase, PKC, and p42/44 MAPK, and the transcription factor NF-κB, which are major participants in glial inflammatory responses (Pyo et al., 1999; Min et al., 2004; Jou et al., 2006).
Here, we investigated the potential link between PIP5Kα (an isoform of PIP5K) and ganglioside-induced astrocyte inflammatory responses. Our findings revealed that gangliosides enhanced PIP5Kα expression, which led to an increase in PI(4,5)P2, and that gene knockdown of PIP5Kα attenuated the ganglioside-induced activation of NF-κB signaling and inflammatory responses in rat primary cultured astrocytes.
Results
Ganglioside treatment of primary astrocytes enhances PIP5Kα expression
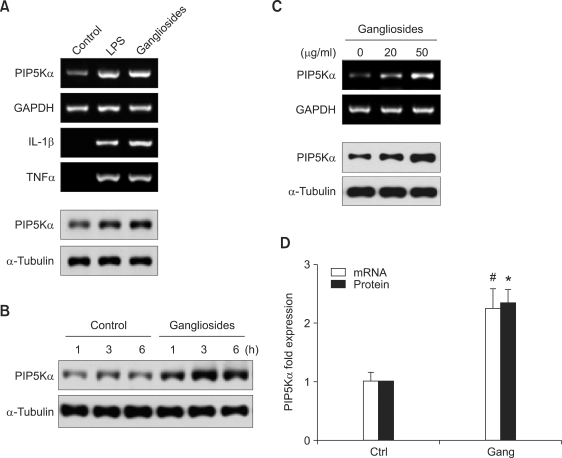
As a first step to examine whether PIP5Kα is involved in ganglioside-induced astrocyte inflammatory responses, we analyzed PIP5Kα expression in primary culture of rat astrocytes. PIP5Kα mRNA (Figures 1A and 1C) and protein (Figures 1A-1C) expression, determined by RT-PCR and Western blot analysis, respectively, was detectable in primary astrocytes. We next examined whether ganglioside treatment altered PIP5Kα expression levels. The expression of PIP5Kα mRNA and protein was significantly enhanced after stimulation with gangliosides for 3 h (Figure 1A). Under the same assay conditions, LPS was similarly effective in inducing the expression of PIP5Kα mRNA and protein (Figure 1A). As expected, both inflammatory stimulators induced increases in IL-1β and TNFα transcription (Figure 1A). Upregulation of PIP5Kα protein expression occurred within 1 h after ganglioside stimulation and continued for up to 3 h, followed by a slight decrease at 6 h, while basal PIP5Kα protein levels remained almost constant in the resting conditions (Figure 1B). Gangliosides enhanced the expression of PIP5Kα mRNA and protein in a dose-dependent manner (Figure 1C). On average, gangliosides (50 µg/ml, 3 h) increased the mRNA, which was determined by quantitative real-time RT-PCR (qRT-PCR) analysis, and protein expression levels of PIP5Kα by approximately 2.3-fold with some quantitative variances among experiments (Figure 1D).
Figure 1.
Effects of gangliosides on PIP5Kα expression in primary astrocytes. Primary cultured rat astrocytes were serum-starved overnight before treatment. Primary astrocytes were incubated in the presence or absence of 100 ng/ml LPS or 50 µg/ml gangliosides for 3 h (A), 50 µg/ml gangliosides for the indicated times (B), or the indicated concentrations of gangliosides for 3 h (C). Changes in the mRNA expression levels of IL-1β and TNFα (A), and PIP5Kα (A, C) were analyzed by RT-PCR. Changes in the protein expression levels of PIP5Kα (A-C) were assayed by Western blot analysis. As a loading control for RT-PCR and Western blot analysis, GAPDH (A, C) and α-tubulin (A-C), respectively, were included. (D) After stimulation with 50 µg/ml gangliosides (Gang) for 3 h, PIP5Kα mRNA levels were determined by qRT-PCR. Under the same condition, PIP5Kα protein levels in (A-C) were measured. Both expression levels were quantified as fold-expression over the unstimulated control (Ctrl). Values are presented as mean ± SEM. *P < 0.01 and #P < 0.05 compared with the control.
Ganglioside treatment increases PI(4,5)P2 levels in primary astrocytes
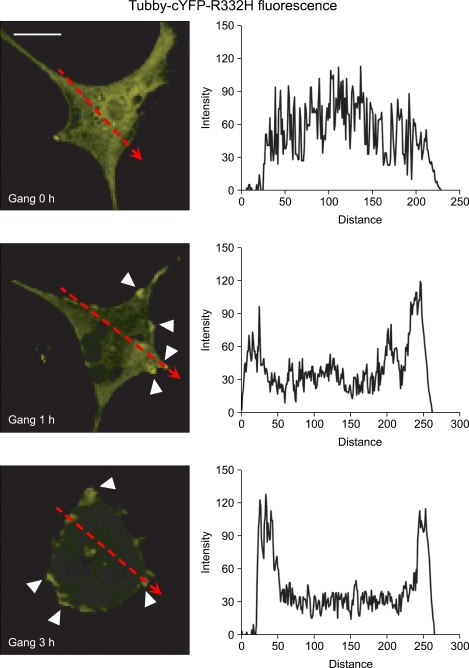
PI(4,5)P2 is synthesized mainly by PIP5K. Because treatment with gangliosides enhanced PIP5Kα expression, we hypothesized that ganglioside stimulation augments the production of PI(4,5)P2. To test this, we measured PI(4,5)P2 levels before and after ganglioside treatment by transfection and imaging of the tubby mutant (R332H) fused to yellow fluorescent protein (YFP) at the C-terminus (tubby-cYFP-R332H) (Quinn et al., 2008). The C-terminal tubby domain of the transcription factor tubby specifically binds to plasma membrane PI(4,5)P2 (Santagata et al., 2001). Fluorescent tubby constructs such as tubby-cYFP-R332H have been used to monitor changes in the PI(4,5)P2 level that are reflected by differences in relative tubby fluorescence intensities between the plasma membrane and the cytoplasm (Santagata et al., 2001; Quinn et al., 2008; Szentpetery et al., 2009). Confocal images of the expressed tubby-cYFP-R332H fusion protein showed a diffuse pattern of YFP signals throughout the cytoplasm in the resting condition (zero-time) (Figure 2). After 1 or 3 h stimulation with gangliosides, YFP signals in the plasma membrane increased and YFP signals in the cytoplasm decreased in a time-dependent manner. The changed intensity profiles obtained from image analysis indicated that gangliosides triggered PI(4,5)P2 production in the plasma membrane (Figure 2).
Figure 2.
Monitoring changes in PI(4,5)P2 levels induced by gangliosides in primary astrocytes. Primary astrocytes were transfected with a tubby-cYFP-R332H expression construct, a PI(4,5)P2-specific probe, using Amaxa Nucleofection. At 48 h posttransfection, cells were serum- starved and treated with 50 µg/ml gangliosides (Gang) for 0, 1, or 3 h. YFP fluorescence in the FITC channel was visualized using an LSM 710 confocal microscopy. Arrowheads indicate the localization of the tubby protein in the plasma membrane. Scale bar, 20 µm. YFP fluorescence intensities along the dotted line arrows in the cell images were analyzed using the Zeiss ZEN 2009 software. Their intensity profiles in the graphs show the translocation of tubby protein between the membrane and the cytosol.
PIP5Kα knockdown attenuates ganglioside-induced inflammatory responses in primary astrocytes
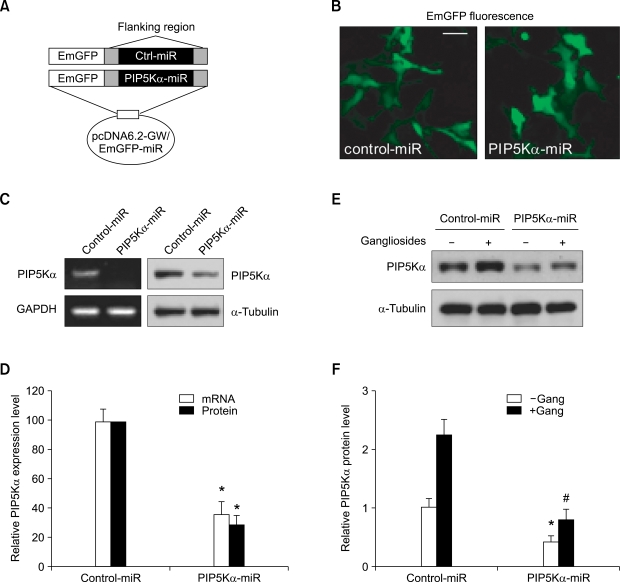
Because PIP5Kα and PI(4,5)P2 are important regulators of diverse cellular functions (Doughman et al., 2003; Di Paolo and De Camilli, 2006), we investigated whether PIP5Kα is involved in regulating ganglioside-induced inflammatory responses. To explore this possibility, we first developed a method of PIP5Kα knockdown using a vector-based microRNA (miRNA) expression system. Complementary oligonucleotides harboring the PIP5Kα target sequence (1,844-1,864 bp) were cloned into a pcDNA™6.2-GW/EmGFP-miR expression vector that contains Emerald green fluorescent protein (EmGFP), a variant of enhanced GFP, as a reporter (Figure 3A). This construct was efficiently expressed in primary astrocytes by Amaxa Nucleofection, as assessed by the presence of EmGFP (~70%) in fluorescence microscopy (Figure 3B). As a negative control that does not target mammalian genes, a pcDNA™6.2-GW/EmGFP-miR-neg control plasmid, which was supplied by the manufacturer, was expressed in the same manner (Figures 3A and 3B). qRT-PCR and Western blot analysis demonstrated that the expression of PIP5Kα miRNA significantly reduced PIP5Kα mRNA (27-45%) and protein (21-36%) levels compared to the expression levels (set as 100%) produced by the control miRNA (Figures 3C and 3D). As expected, ganglioside-induced upregulation of PIP5Kα protein levels was observed in negative control cells (Figures 3E and 3F). In contrast, gangliosides did not substantially upregulate PIP5Kα protein levels in PIP5Kα-knockdown cells (Figures 3E and 3F).
Figure 3.
PIP5Kα knockdown by miRNA expression and its effect on ganglioside-induced PIP5Kα expression. (A) The diagram shows a brief map of plasmids expressing PIP5Kα miRNA or negative control miRNA. A PIP5Kα target sequence was cloned into Invitrogen's pcDNA™6.2-GW/EmGFP-miR expression vector containing EmGFP as a reporter. (B-E) Primary astrocytes were transfected with the plasmids expressing PIP5Kα miRNA or negative control miRNA using Amaxa Nucleofection for 48 h. (B) Cells were visualized by fluorescence microscopy for the detection of EmGFP. Scale bar, 50 µm. (C) PIP5Kα mRNA and protein expression levels were examined by RT-PCR and Western blot analysis, respectively. GAPDH or α-tubulin was included as a corresponding loading control. (D) PIP5Kα mRNA levels were determined by qRT-PCR analysis and PIP5Kα protein levels in (C) were measured. PIP5Kα mRNA and protein expression levels in the PIP5Kα miRNA were quantified as a percentage of those in negative control miRNA. Values are mean ± SEM. *P < 0.01 compared with the control miRNA. (E) After serum starvation overnight, cells were treated with or without 50 µg/ml gangliosides for 3 h. PIP5Kα protein expression levels were monitored by Western blotting. (F) The PIP5Kα protein levels in (E) were quantified relative to that in unstimulated negative control miRNA. Values are mean ± SEM. *P < 0.01 and #P < 0.05 compared with the unstimulated control miRNA.
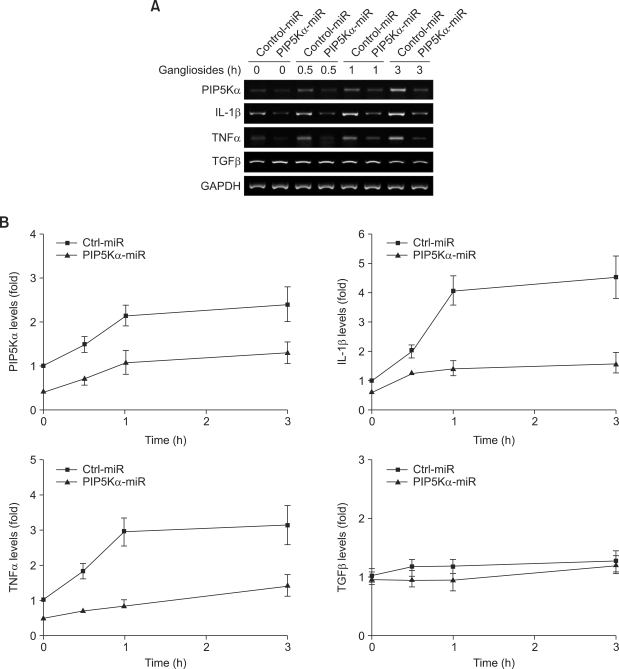
We next investigated whether the expression of PIP5Kα miRNA affected astrocyte immune function by comparing the time courses of ganglioside-induced transcriptional changes in PIP5Kα, IL-1β, TNFα, and TGFβ between negative control cells and PIP5Kα-knockdown cells using RT-PCR and qRT-PCR analysis. Transcriptional increases in PIP5Kα induced by gangliosides occurred time-dependently for up to 3 h in negative control cells, but were remarkably weakened in PIP5Kα-knockdown cells over the same time period (Figures 4A and 4B). Similarly, IL-1β and TNFα transcription induced by gangliosides were reduced in PIP5Kα-knockdown cells compared to negative control cells at all time points examined (Figures 4A and 4B). TGFβ mRNA levels were not affected by gangliosides in negative control cells and were also unaffected by PIP5Kα knockdown (Figures 4A and 4B). These results indicate that PIP5Kα knockdown exerts inhibitory effects on astrocyte inflammatory responses that are stimulated by gangliosides.
Figure 4.
Effects of PIP5Kα knockdown on ganglioside-induced transcription of inflammatory cytokines. (A, B) Primary astrocytes were transfected with PIP5Kα miRNA or negative control miRNA expression plasmids using Amaxa Nucleofection for 48 h. Following serum starvation overnight, cells were incubated in the presence or absence of 50 µg/ml gangliosides for the indicated times. (A) mRNA expression levels of PIP5Kα, IL-1β, TNFα, TGFα, and GAPDH (a loading control) were assayed by RT-PCR analysis. (B) The time-dependent transcriptional changes in each gene were analyzed by qRT-PCR and quantified as fold-expression over the negative control miRNA at the zero-time.
PIP5Kα knockdown interferes with ganglioside-activated NF-κB signaling in primary astrocytes
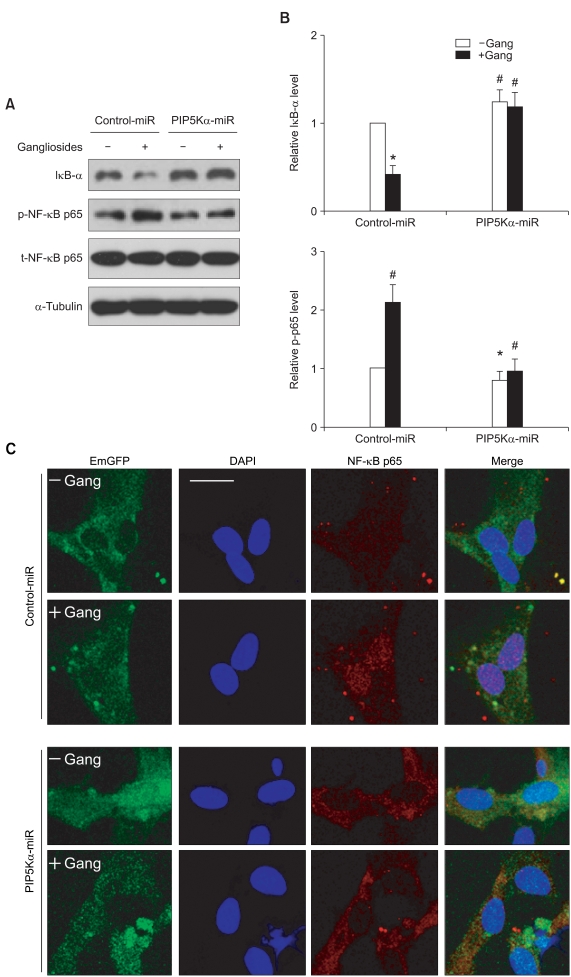
NF-κB is a major transcription factor responsible for the induction of proinflammatory cytokines in response to inflammatory stimuli in various types of immune cells (Li and Verma, 2002). The strong inhibition of PIP5Kα knockdown on the stimulating effects of gangliosides on IL-1β and TNFα transcription (Figure 4) suggested that PIP5Kα knockdown might interfere with ganglioside-triggered NF-κB activation. Activation of the NF-κB signaling pathway involves the degradation of IκB through the ubiquitin-proteasome system, which induces nuclear translocation of NF-κB (Li and Verma, 2002). In addition, the serine 536 residue in the NF-κB p65 (a member of the NF-κB family) transactivation domain is phosphorylated by IκB kinase β in response to LPS or TNFα, leading to its transcriptional activity (Sakurai et al., 1999; Yang et al., 2003). We tested whether PIP5Kα knockdown perturbs such molecular changes in IκB and NF-κB p65 induced by gangliosides in primary astrocytes. Stimulation of negative control cells with gangliosides for 15 min induced both the degradation of IκB-α and the phosphorylation of NF-κB p65 at serine 536, compared to the unstimulated condition (Figures 5A and 5B). In contrast, in PIP5Kα-knockdown cells, IκB-α levels remained relatively high and NF-κB p65 remained unphosphorylated (Figures 5A and 5B).
Figure 5.
Effects of PIP5Kα knockdown on ganglioside-stimulated NF-κB signaling. (A, C) Primary astrocytes were transfected with the miRNA expression plasmids and treated as described above. (A) After treatment with or without 50 µg/ml gangliosides for 15 min, cell lysates were prepared and changes in protein levels of IκB-α, phosphorylated and total NF-κB p65, and α-tubulin (a loading control) were examined by Western blot analysis using their specific antibodies. (B) IκB-α and phosphorylated NF-κB p65 levels in (A) were quantified relative to those in unstimulated negative control miRNA. Values are mean ± SEM. *P < 0.01 and #P < 0.05 compared with the unstimulated control miRNA. (C) Cells were stimulated with gangliosides under the same condition as (A) and then immunostained with a specific primary antibody against NF-κB p65, followed by an Alexa Fluor 594-conjugated secondary antibody. The transfected cells and the nuclei were visualized by EmGFP and DAPI staining, respectively. Cell images were obtained by an LSM 710 confocal microscopy under the laser filter sets (FITC, Rhodamine, and DAPI). Scale bar, 20 µm.
Under the same assay conditions, we examined the subcellular localization of NF-κB p65 immunocytochemically using an anti-NF-κB p65 antibody. Confocal images revealed that NF-κB p65 was distributed in the cytosol in both unstimulated negative control and PIP5Kα-knockdown cells (Figure 5C). The ganglioside-dependent nuclear translocation of NF-κB p65 was observed in negative control cells, but not in PIP5Kα-knockdown cells (Figure 5C), consistent with the results of the Western blot analysis (Figure 5A). Together, these results indicate that PIP5Kα positively regulates the ganglioside-induced activation of NF-κB signaling pathway.
Discussion
Accumulating evidence indicates that the PIP5K-driven production of PI(4,5)P2 regulates signal transduction, membrane trafficking, actin cytoskeletal reorganization, and ion channel modulation (Takenawa and Itoh, 2001; Suh and Hille, 2005; Di Paolo and De Camilli, 2006). Despite the essential pleiotropic functions of PIP5K and PI(4,5)P2, however, little is known about their roles in glial inflammatory responses in the brain. The findings of the present study indicate that PIP5Kα undergoes transcriptional and translational upregulation in response to gangliosides in brain astrocytes. The concomitant induction of IL-1β and TNFα by gangliosides provides novel evidence that PIP5Kα is an inflammatory mediator that participates in astrocyte inflammatory responses. PI(4,5)P2 level is regulated by alterations in expression level or catalytic activity of PIP5K. PIP5K-mediated PI(4,5)P2 synthesis is achieved by changes in the catalytic activity of PIP5K through various regulatory mechanisms, such as protein-protein interactions and protein phosphorylation (van den Bout and Divecha, 2009). PI(4,5)P2 levels are also under the control of changes in the PIP5K expression level, as demonstrated by the effects of gene knockdown or overexpression of PIP5K as a means of manipulating PI(4,5)P2 levels (Yamamoto et al., 2001; Padron et al., 2003). In addition, inflammatory responses are accompanied by extensive changes in metabolic pathways, based on the induction of gene expression and the production of inflammatory mediators. Hence, the increase in PI(4,5)P2 induced by gangliosides is, at least in part, likely due to the upregulation of PIP5Kα expression, although we cannot at this time exclude the possibility that stimulation of PIP5K by gangliosides increases PI(4,5)P2.
PI(4,5)P2 levels in primary astrocytes were evaluated by PI(4,5)P2 imaging with an expression construct of tubby-cYFP-R332H. The transcription factor tubby localizes to the plasma membrane due to its interaction with PI(4,5)P2 (Santagata et al., 2001). Following ligand stimulation of G-protein-coupled receptors, activated phospholipase C-β degrades PI(4,5)P2 and the resulting decrease in PI(4,5)P2 releases tubby from the plasma membrane to cytoplasm and nucleus (Santagata et al., 2001). The expression of tubby-cYFP-R332H sensitively reflected the PI(4,5)P2-dependent tubby translocation (Quinn et al., 2008). Taking advantage of this property of the tubby mutant, we found that gangliosides induced the translocation of tubby-cYFP-R332H from the cytoplasm to the plasma membrane. The tubby imaging results support that the increased PI(4,5)P2 production induced by gangliosides occurs specifically at the plasma membrane.
To evaluate the role of ganglioside-induced PIP5Kα upregulation in primary astrocytes, we knocked down PIP5Kα expression using a miRNA expression system. We demonstrated that PIP5Kα knockdown inhibited the gangliosides-induced increase in the transcription of IL-1β and TNFα as well as the ganglioside-induced activation of NF-κB signaling. Ganglioside stimulation of primary astrocytes and microglia activates NF-κB signaling (Pyo et al., 1999; Min et al., 2004; Jou et al., 2006). Thus, it is likely that the attenuated transcription of IL-1β and TNFα in PIP5Kα-knockdown cells is due to the suppression of NF-κB signaling by PIP5Kα knockdown. Taken together, our results suggest that increased PIP5Kα expression contributes to ganglioside-induced cytokine production by facilitating the NF-κB signaling pathway.
Previously, it was reported that Toll-like receptor 4 (TLR4) knockdown by small interfering RNA or transfection of dominant negative TLR4 in primary astrocytes strongly inhibits ganglioside-stimulated IL-1β and TNFα transcription, NF-κB activation, and IL-6 promoter activity, suggesting an essential role of TLR4 in ganglioside-induced astrocyte inflammatory responses (Jou et al., 2006). Interestingly, PI(4,5)P2 has an important role in the LPS/TLR4 signaling pathway in bone marrow-derived macrophages (Kagan and Medzhitov, 2006). Upon LPS stimulation, PI(4,5)P2 levels are increased and subsequently recruit TIRAP (a Toll/IL-1 receptor domain-containing adaptor protein), required for myeloid differentiation factor 88 (MyD88)-dependent TLR4 signaling pathway (Fitzgerald et al., 2001; Horng et al., 2002), to the plasma membrane by mediating its specific interaction with TIRAP (Kagan and Medzhitov, 2006). The resulting PI(4,5)P2-dependent concentration of TIRAP in the plasma membrane was further suggested to promote formation of the TLR4-TIRAP-MyD88 complex, thereby inducing production of a proinflammatory cytokine IL-6 (Kagan and Medzhitov, 2006). We found that LPS also upregulates PIP5Kα expression levels. The TLR4 signaling pathways and downstream effector NF-κB are well conserved among innate immune cells, including astrocytes (Akira and Takeda, 2004; Farina et al., 2007). Based on the similarities between these previous results and the present findings, PIP5Kα may participate in such PI(4,5)P2-mediated TLR4 signaling in brain astrocytes. Whether PI(4,5)P2-mediated recruitment of TIRAP to the cell surface TLR4 is involved in inflammatory responses to gangliosides, however, remains to be determined.
While we have focused on PIP5Kα in this study, role of other type I PIP5K isoforms, PIP5Kβ and PIP5Kγ, in astrocyte inflammatory responses needs to be examined. PIP5Kβ and/or PIP5Kγ may play a redundant role in astrocyte immune function. However, PIP5Ks can also be differentially regulated, thereby controlling spatiotemporal pattern of PI(4,5)P2 production (Mao and Yin, 2007; van den Bout and Divecha, 2009). Mouse PIP5Kα deficiency accompanying decrease in PI(4,5)P2 levels resulted in hypersensitive allergic inflammation and mast cell degranulation upon cross-linking of Fcε receptor-I by antigen (Sasaki et al., 2005). Protein expression of mouse PIP5Kβ was elevated in LPS-stimulated BV2 microglial cells (Lund et al., 2006). A plasma membrane PI(4,5)P2 pool was present in the lipid rafts that are enriched in gangliosides (Szymanska et al., 2009; Posse de Chaves and Sipione, 2010). Upon activation of Fcγ receptor IIA in BHK cells, PIP5Kα was recruited to the PI(4,5)P2-rich lipid rafts and colocalized with the receptor, which was suggested to contribute to Fcγ receptor IIA-mediated signaling (Szymanska et al., 2009). These cell type-specific PIP5K responses raise a possibility that PIP5Ks may differentially modulate immune responses in a microenvironment-dependent manner. All three PIP5Ks are expressed in the brain tissue (Ishihara et al., 1996, 1998). Thus, it will be necessary to monitor alterations of in vivo expression and localization of each PIP5K during astrocyte immune responses.
In summary, we report that PIP5Kα expression is enhanced in ganglioside-stimulated astrocytes. Our results also suggest that the upregulation of PIP5Kα contributes to NF-κB signaling and inflammatory responses that are activated by gangliosides. To our knowledge, these findings are the first demonstration of a potential link between PIP5Kα and astrocyte inflammatory responses. As such, this study provides new insight into the roles of PIP5Kα and PI(4,5)P2 in innate immunity mediated by brain astrocytes.
Methods
Materials
An ammonium salt form of purified gangliosides was purchased from Matreya (Pleasant Gap, PA). Most research chemicals including LPS and anti-α-tubulin antibody were purchased from Sigma (St. Louis, MO). MEM, FBS, and penicillin/streptomycin were obtained from Hyclone (Logan, UT). An anti-PIP5Kα antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against NF-κB p65, phospho-NF-κB p65 (Ser536), and IκB-α were purchased from Cell Signaling Technology (Beverly, MA).
Cell culture and treatment
Primary astrocytes were cultured from the cerebral cortices of 1-day-old Sprague-Dawley rats (Samtako, Osan, Korea) as described previously (Min et al., 2004; Jou et al., 2006). Briefly, the cortices were triturated into single cells, plated into T-75 flasks, and grown in MEM containing 10% FBS and penicillin/streptomycin at 37℃ in a humidified atmosphere of 5% CO2 and 95% air. After culture for 2 weeks, primary microglial cells were removed by mild shaking. Adherent primary astrocytes were washed with PBS, trypsinized, and resuspended in MEM containing 5% FBS and penicillin/streptomycin. After replating into 60-mm dishes (1 × 106 cells/dish) for 2 days, cells were serum-starved overnight and then treated with gangliosides or LPS under the indicated conditions. The purity of primary cultures was tested by immunostaining for glial fibrillary acidic protein, a marker protein for astrocytes. Confocal microscopy observations confirmed that primary cultures were more than 90% pure.
Reverse transcription-polymerase chain reaction
Astrocytes were washed with ice-cold PBS and harvested in a TRIzol lysis reagent (Invitrogen, Carlsbad, CA) and total mRNA was isolated following the supplier's instructions. cDNA was synthesized from isolated total mRNA using avian myeloblastosis virus reverse transcriptase (Takara, Japan) according to the manufacturer's instructions. cDNA was amplified by PCR using the following oligonucleotide primers (Bioneer, Daejeon, Korea): (sense) 5'-CTGAGGGACCTTATGCCTCTC-3' and (antisense) 5'-GAAGAGGGAACCGCTAGCTC-3' for rat PIP5Kα (NM_001042621, 546 amino acids); and (sense) 5'-GAGAGCCCTGGATACCAACTACTG-3' and (antisense) 5'-GTGTGTCCAGGCTCCAAATGTAG-3 for rat TGFβ. In this study, the previous mouse and rat PIP5Kβ is referred to as PIP5Kα, and vice versa, according to the revised nomenclature in the current GenBank database. The primers used to amplify IL-1β, TNFα, and GAPDH were previously described (Min et al., 2004). Amplified PCR products were separated by electrophoresis on 1.5% agarose gels and detected under ultraviolet light. Gel images were obtained using the Gel Doc molecular imaging system (Bio-Rad Laboratories, Hercules, CA).
Quantitative real-time PCR
cDNA was prepared as describe above and qRT-PCR was performed on an ABI PRISM 7000 real-time PCR machine (Applied Biosystems, Foster City, CA) using a SYBR Premix Ex Taq II reagent (Takara, Japan) according to the manufacturer's protocol. The following specific primers (Bioneer) were used: (sense) 5'-TGACTACGAAGGCGGAAGTG-3' and (antisense) 5'-AAACTGGGACCAGGAACAGG-3' for PIP5Kα; (sense) 5'-TGATGTTCCCATTAGACAGC-3' and (antisense) 5'-GAGGTGCTGATGTACCAGTT-3' for IL-1β; (sense) 5'-CGTCTACTCCTCAGAGCCCC-3' and (antisense) 5'-TCCACTCAGGCATCGACATT-3' for TNFα; and (sense) 5'-GGCCAAAAGGGTCATCATC-3' and (antisense) 5'-GTGATGGCATGGACTGTGG-3' for GAPDH (a housekeeping gene). qRT-PCR primers for TGFα were the same as those described above. Generation of a single gene-specific PCR product was confirmed by melting curve analysis. All PCR reaction samples were prepared in triplicate for each gene. The relative mRNA expression levels were determined by the 2-ΔΔCt methods [ΔCt = Ct (target gene) - Ct (GAPDH), ΔΔCt = ΔCt (gangliosides) - ΔCt (control) or ΔCt (PIP5Kα-knockdown cells) - ΔCt (control cells)].
Western blot analysis
Astrocytes were scraped into a lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM Na3VO4, 5 mM NaF, and 1% Triton X-100) containing protease inhibitor cocktail tablets (Roche, Mannheim, Germany) and cell lysates were cleared by centrifugation (15,000 × g, 20 min, 4℃). Protein concentration was determined using BCA protein assay reagents (Pierce, Rockford, IL), and 30 to 40 µg of cell lysates were separated by SDS-PAGE on 8% to 10% resolving gels, and transferred to nitrocellulose membranes (Schleicher & Schuell Bioscience, Germany). Following blocking with 5% nonfat milk solution, membrane blots were probed with anti-PIP5Kα or anti-α-tubulin antibodies, washed four times with Tris-buffered saline containing 0.1% Tween-20, and further probed with horseradish peroxidase-conjugated secondary antibodies (Zymed Laboratories, San Francisco, CA). Phosphorylated and total NF-κB p65, and IκB-α were detected using corresponding antibodies following the manufacturer's protocol. The resulting immune complexes were visualized using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology).
PIP5Kα knockdown
We made an expression construct encoding PIP5Kα miRNA using BLOCK-iT Pol II miR RNAi Expression Vector Kits (Invitrogen). The following 64-bp oligonucleotides harboring the rat PIP5Kα sequences were synthesized (Genotech, Daejeon, Korea): (top strand) 5'-TGCTGAACAGGTGAACCCTCACTTATGTTTTGGCCACTGACTGACATAAGTGAGTTCACCTGTT-3', where 21-bp antisense and 19-bp (2 bp removed) sense sequences are indicated by the underlines, respectively, and (bottom strand) 5'-CCTGAACAGGTGAACTCACTTATGTCAGTCAGTGGC CAAAACATAAGTGAGGGTTCACCTGTTC-3'. According to the manufacturers instructions, the single-stranded oligos were annealed and ligated into the pcDNA™6.2-GW/EmGFP-miR expression vector. After transformation, spectinomycin-resistant Escherichia coli clones were isolated and identified by DNA sequencing (Genotech). PIP5Kα miRNA expression plasmids were purified using an EndoFree Plasmid Maxi Kit (Qiagen, Hilden, Germany) and transiently transfected into primary astrocytes by Amaxa Nucleofection using a rat astrocyte Nucleofector Kit (Amaxa Biosystems, Cologne, Germany) according to the manufacturer's instructions. Briefly, primary astrocytes (2 × 106 cells) were mixed with a Nucleofector solution plus 5 µg plasmid. Following electroporation using the T-20 program, the cell suspension was immediately diluted with prewarmed growth medium and seeded into 60-mm dishes or 12-well plates (5 × 104 cells/cm2). At 48 h posttransfection, cells were analyzed or serum-starved overnight for treatment with gangliosides.
NF-κB immunocytochemistry
Primary astrocytes were fixed and permeabilized with ice-cold methanol for 5 min, and washed with PBS. Cells were incubated with an anti-NF-κB p65 antibody diluted in PBS containing 1% BSA for 1 h at 37℃ and then with an Alexa Fluor 594-conjugated secondary antibody (Molecular Probes). VECTASHIELD mounting medium supplemented with 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) was used to mount samples. Images were collected on a laser scanning confocal microscope (LSM 710, Carl Zeiss Microimaging, Göttingen, Germany) equipped with C-Apochromat 40x/1.20 water immersion objective and acquired with ZEN 2009 software (Carl Zeiss Microimaging).
Tubby imaging
PI(4,5)P2 was visualized by the ectopic expression of a membrane-bound transcription factor tubby that has been used as a specific probe for PI(4,5)P2 (Santagata et al., 2001; Szentpetery et al., 2009). A mutant (R332H) plasmid of the full-length tubby subcloned into the eYFP-N1 vector expressing YFP at the C-terminus was provided by Andrew Tinker (University College London, UK) (Quinn et al., 2008). Primary astrocytes were transfected with the fluorescent tubby construct using Amaxa Nucleofection and seeded onto coverslips in 12-well plates (1 × 105 cells/well). At 48 h posttransfection, cells were further treated with gangliosides and YFP fluorescent images in the FITC channel were obtained using an LSM 710 confocal microscope.
Statistical analysis
Band intensities of Western blots were measured using NIH Image J software (National Institutes of Health). Statistical significance of Western blot and real-time PCR results shown in the graphs was determined using an unpaired t-test (Graphpad Software, San Diego, CA) and data are presented as mean ± SEM.
Acknowledgements
We thank Dr. Andrew Tinker for providing the tubby expression construct. This work was supported by research grants from the Korea Science and Engineering Foundation through the Chronic Inflammatory Disease Research Center at Ajou University (R13-2003-019), the Korea Research Foundation (KRF-2008-331-C00238), and Ajou University School of Medicine.
Abbreviations
- EmGFP
emerald green fluorescent protein
- miRNA
microRNA
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PIP5K
phosphatidylinositol 4-phosphate 5-kinase
- YFP
yellow fluorescent protein
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease--a review. J Lipid Res. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blennow K, Davidsson P, Wallin A, Fredman P, Gottfries CG, Mansson JE, Svennerholm L. Differences in cerebrospinal fluid gangliosides between "probable Alzheimer's disease" and normal aging. Aging (Milano) 1992;4:301–306. [PubMed] [Google Scholar]
- 4.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 5.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 6.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 8.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara H, Shibasaki Y, Kizuki N, Katagiri H, Yazaki Y, Asano T, Oka Y. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1996;271:23611–23614. doi: 10.1074/jbc.271.39.23611. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara H, Shibasaki Y, Kizuki N, Wada T, Yazaki Y, Asano T, Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J Biol Chem. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- 11.Jou I, Lee JH, Park SY, Yoon HJ, Joe EH, Park EJ. Gangliosides trigger inflammatory responses via TLR4 in brain glia. Am J Pathol. 2006;168:1619–1630. doi: 10.2353/ajpath.2006.050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung JU, Ko K, Lee DH, Ko K, Chang KT, Choo YK. The roles of glycosphingolipids in the proliferation and neural differentiation of mouse embryonic stem cells. Exp Mol Med. 2009;41:935–945. doi: 10.3858/emm.2009.41.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 15.Lund S, Christensen KV, Hedtjarn M, Mortensen AL, Hagberg H, Falsig J, Hasseldam H, Schrattenholz A, Porzgen P, Leist M. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Mao YS, Yin HL. Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases. Pflugers Arch. 2007;455:5–18. doi: 10.1007/s00424-007-0286-3. [DOI] [PubMed] [Google Scholar]
- 17.Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- 18.Padron D, Wang YJ, Yamamoto M, Yin H, Roth MG. Phosphatidylinositol phosphate 5-kinase Ibeta recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J Cell Biol. 2003;162:693–701. doi: 10.1083/jcb.200302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 20.Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–1759. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Pyo H, Joe E, Jung S, Lee SH, Jou I. Gangliosides activate cultured rat brain microglia. J Biol Chem. 1999;274:34584–34589. doi: 10.1074/jbc.274.49.34584. [DOI] [PubMed] [Google Scholar]
- 22.Quinn KV, Behe P, Tinker A. Monitoring changes in membrane phosphatidylinositol 4,5-bisphosphate in living cells using a domain from the transcription factor tubby. J Physiol. 2008;586:2855–2871. doi: 10.1113/jphysiol.2008.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 25.Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001;292:2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki J, Sasaki T, Yamazaki M, Matsuoka K, Taya C, Shitara H, Takasuga S, Nishio M, Mizuno K, Wada T, Miyazaki H, Watanabe H, Iizuka R, Kubo S, Murata S, Chiba T, Maehama T, Hamada K, Kishimoto H, Frohman MA, Tanaka K, Penninger JM, Yonekawa H, Suzuki A, Kanaho Y. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I {alpha} J Exp Med. 2005;201:859–870. doi: 10.1084/jem.20041891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 28.Sonnino S, Chigorno V. Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim Biophys Acta. 2000;1469:63–77. doi: 10.1016/s0005-2736(00)00210-8. [DOI] [PubMed] [Google Scholar]
- 29.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Szentpetery Z, Balla A, Kim YJ, Lemmon MA, Balla T. Live cell imaging with protein domains capable of recognizing phosphatidylinositol 4,5-bisphosphate; a comparative study. BMC Cell Biol. 2009;10:67. doi: 10.1186/1471-2121-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szymanska E, Korzeniowski M, Raynal P, Sobota A, Kwiatkowska K. Contribution of PIP-5 kinase Ialpha to raft-based FcgammaRIIA signaling. Exp Cell Res. 2009;315:981–995. doi: 10.1016/j.yexcr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533:190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 33.Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 34.van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Hilgemann DH, Feng S, Bito H, Ishihara H, Shibasaki Y, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin stress-fiber formation and inhibits membrane ruffling in CV1 cells. J Cell Biol. 2001;152:867–876. doi: 10.1083/jcb.152.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]