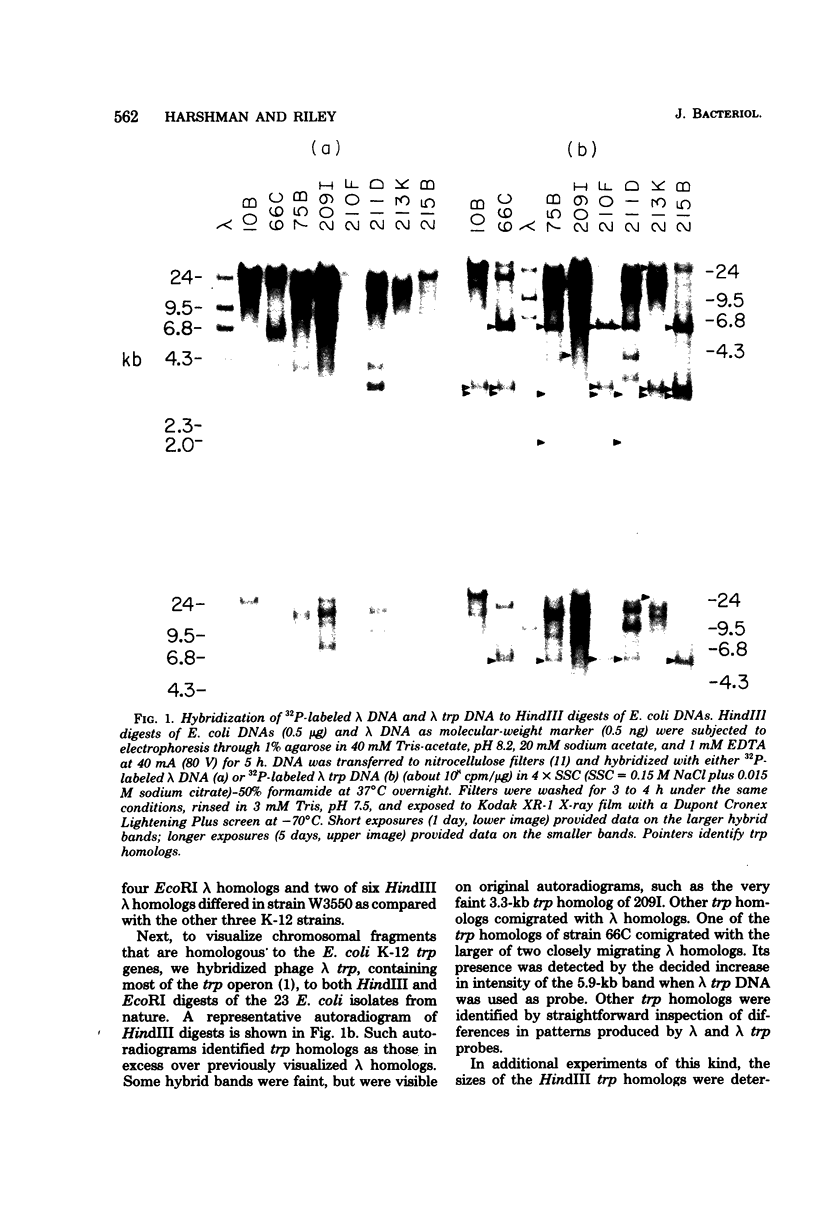
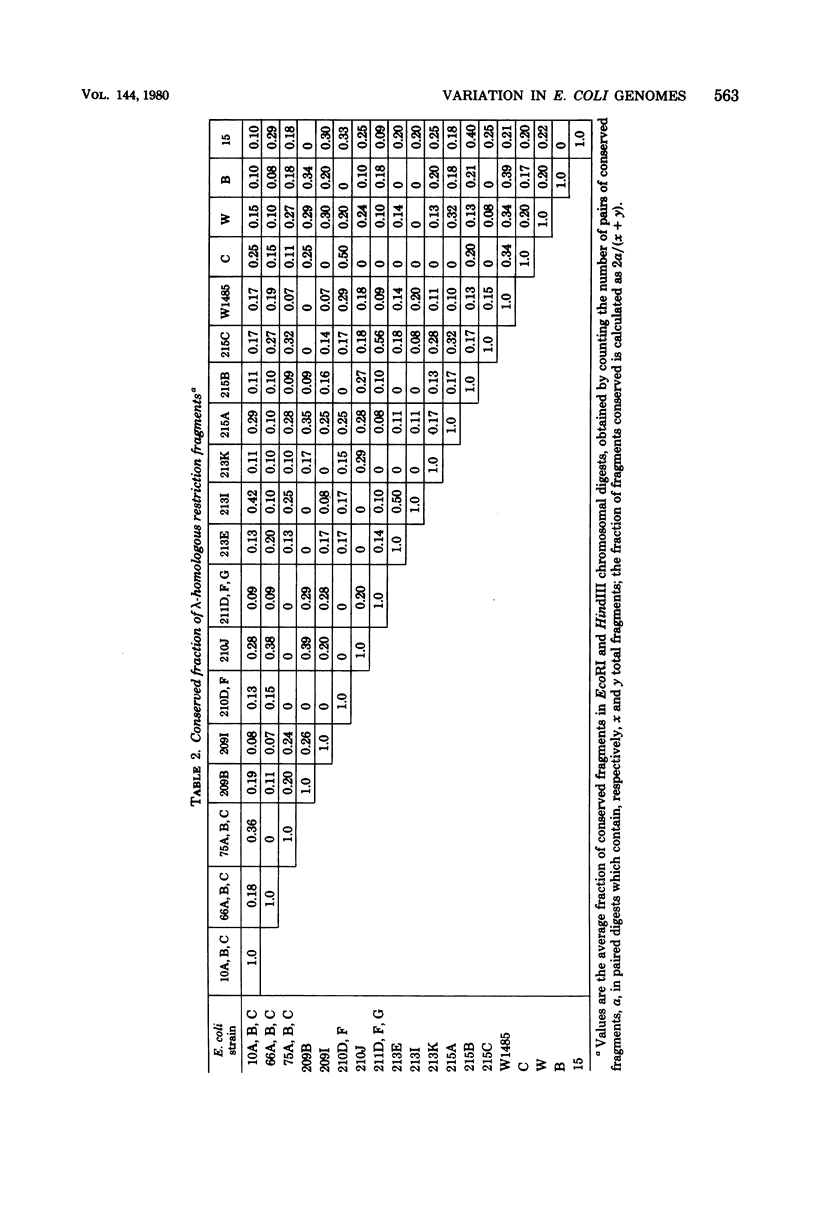
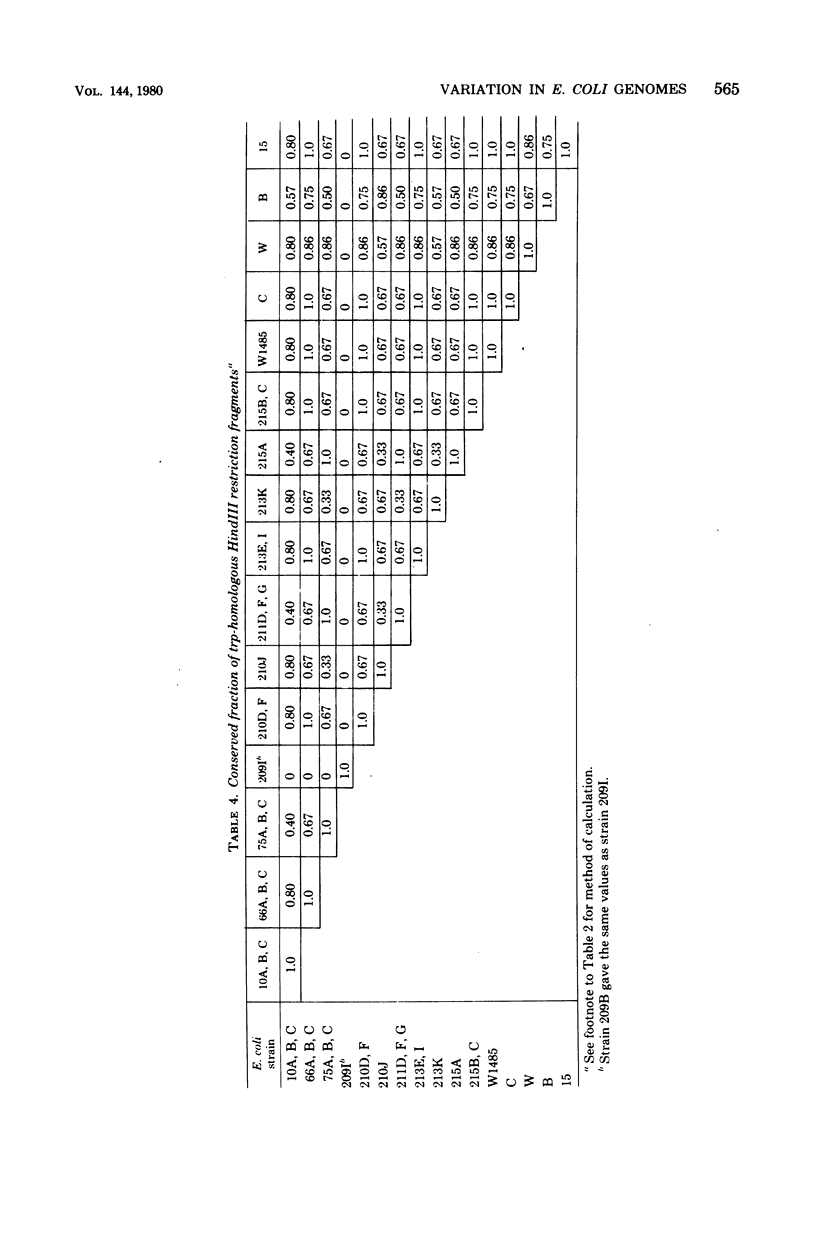
Abstract
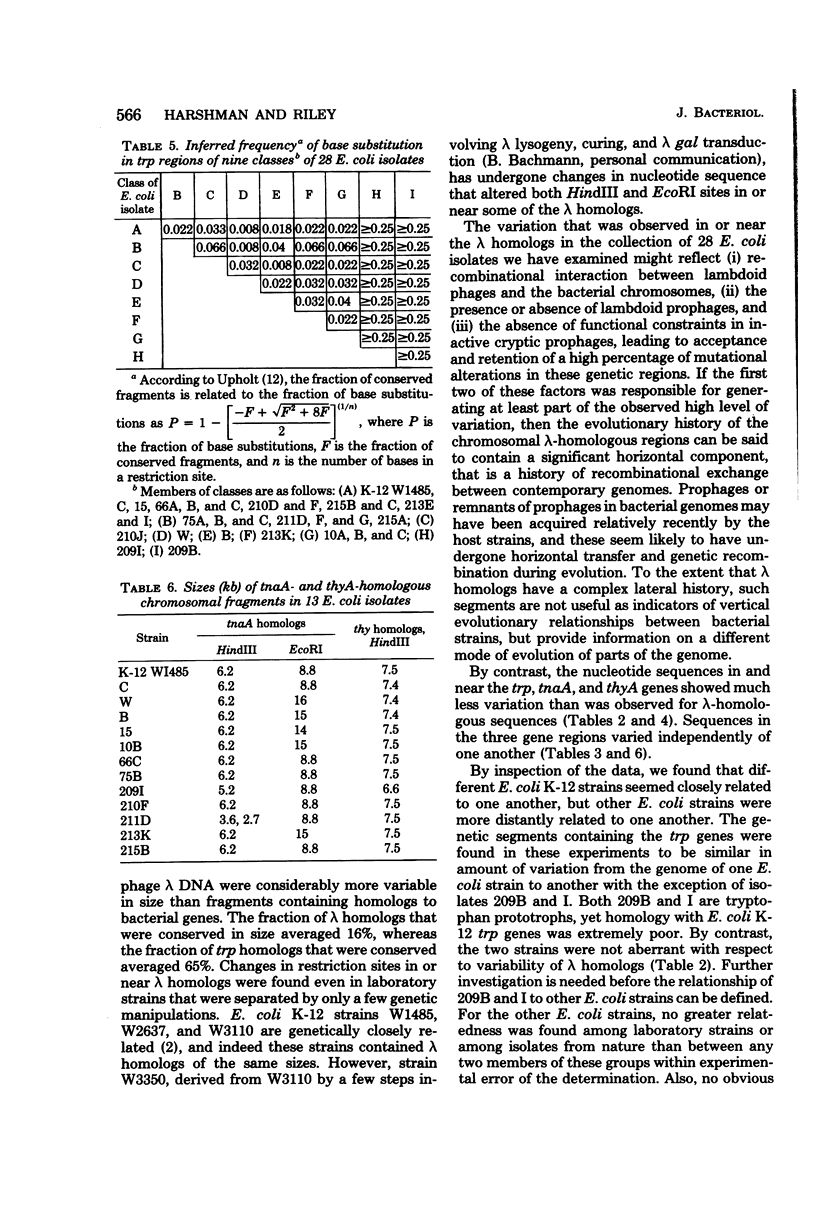
A group of Escherichia coli isolates from nature were compared with one another and with laboratory strains of E. coli with respect to size distribution of chromosomal restriction endonuclease fragments and differences in nucleotide sequences in selected small portions of the genomes. The estimated frequency of base substitutions in nucleotide sequences in and near the trp operons of 26 of the 28 E. coli strains examined ranged from 0.008 to 0.066. Nucleotide sequences in or near lambda prophage homologs were significantly more variable than the sequences in or near trp, tnaA, and thyA genes. Thus, the lambda-homologous regions may have a significant horizontal component in their evolutionary histories, having undergone genetic exchange, whereas the trp, tnaA, and thyA regions may have solely vertical evolutionary histories. The relatedness of the E. coli strains in the genetic regions studied indicated that laboratory strains are not more closely related to one other than they are to isolates from nature. The isolates from natural populations did not form groups related either by host taxa or by geographical region of isolation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Riley M. Conservation and variation of nucleotide sequences within related bacterial genomes: Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):355–365. doi: 10.1128/jb.143.1.355-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. S., Murray N. E., Brammar W. J. Characterization of lambdatrp-transducing bacteriophages made in vitro. J Mol Biol. 1976 Nov 15;107(4):549–569. doi: 10.1016/s0022-2836(76)80082-4. [DOI] [PubMed] [Google Scholar]
- Kaiser K., Murray N. E. Physical characterisation of the "Rac prophage" in E. coli K12. Mol Gen Genet. 1979 Sep;175(2):159–174. doi: 10.1007/BF00425532. [DOI] [PubMed] [Google Scholar]
- Milkman R. Electrophoretic variation in Escherichia coli from natural sources. Science. 1973 Dec 7;182(4116):1024–1026. doi: 10.1126/science.182.4116.1024. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Yanofsky C. Nucleotide sequences of trpA of Salmonella typhimurium and Escherichia coli: an evolutionary comparison. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5244–5248. doi: 10.1073/pnas.76.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Conservation and variation of nucleotide sequences within related bacterial genomes: enterobacteria. J Bacteriol. 1980 Jul;143(1):366–376. doi: 10.1128/jb.143.1.366-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]