Abstract
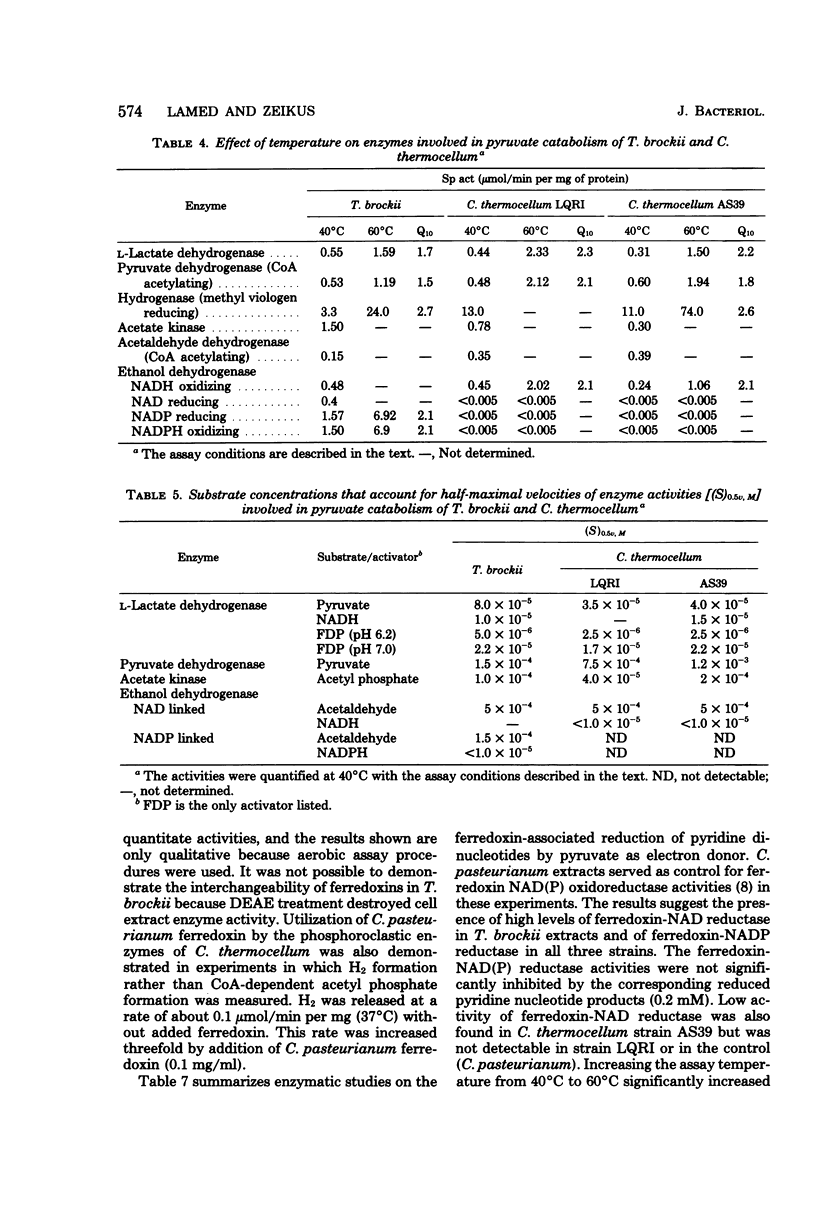
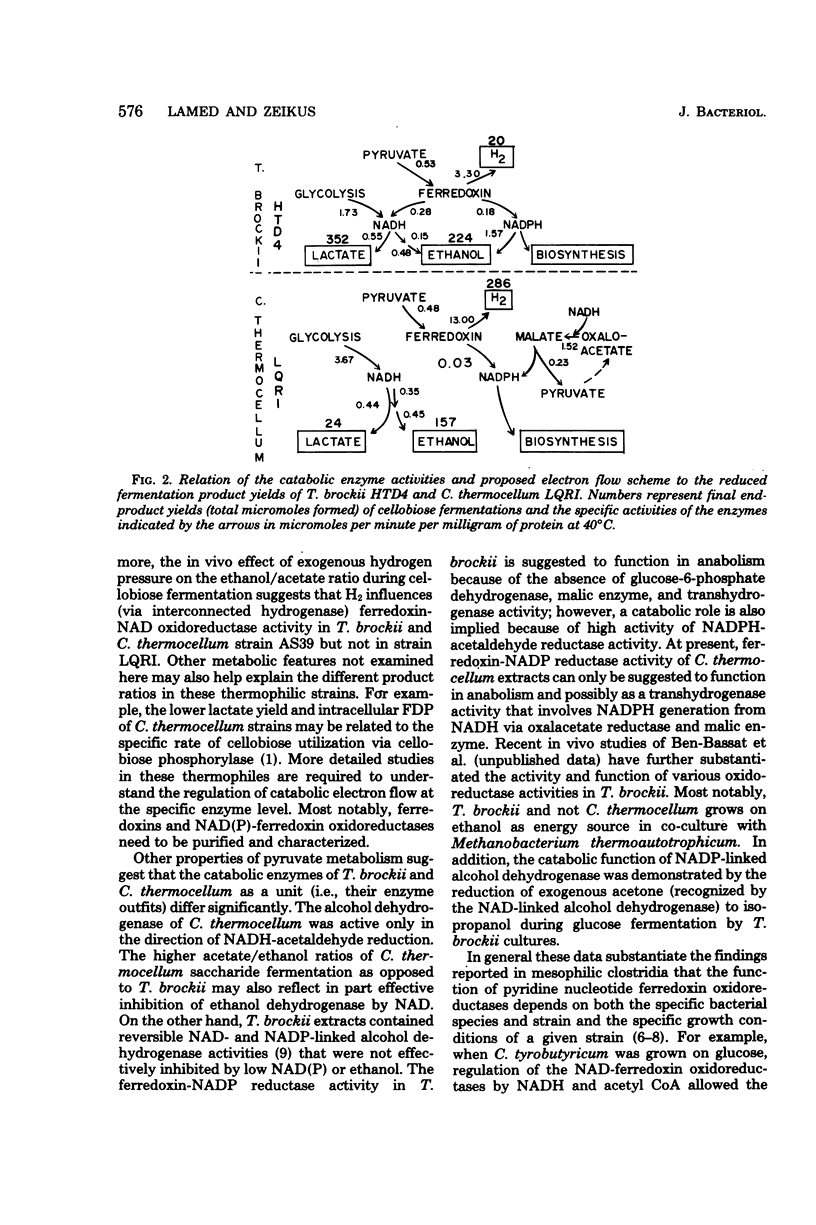
Significant quantitative differences in end-product yields by two strains of Clostridium thermocellum and one strain of Thermoanaerobium brockii were observed during cellobiose fermentation. Most notably, the ethanol/H2 and lactate/acetate ratios were drastically higher for T. brockii as compared with C. thermocellum strains LQRI and AS39. Exogenous H2 addition (0.4 to 1.0 atm) during culture growth increased the ethanol/acetate ratio of both T. brockii and AS39 but had no effect on LQRI. All strains had an operative Embden-Meyerhof glycolytic pathway and displayed catabolic activities of fructose-1,6-diphosphate–activated lactate dehydrogenase, coenzyme A acetylating pyruvate and acetaldehyde dehydrogenase, hydrogenase, ethanol dehydrogenase, and acetate kinase. Enzyme kinetic properties (apparent Km, Vmax, and Q10 values) and the specificity of electron donors/acceptors for different oxidoreductases involved in pyruvate conversion to fermentation products were compared in the three strains. Both species contained ferredoxin-linked pyruvate dehydrogenase and pyridine nucleotide oxidoreductases. Ferredoxin-nicotinamide adenine dinucleotide (NAD) reductase activity was significantly higher in T. brockii than in AS39 and was not detectable in LQRI. H2 production and hydrogenase activity were inversely related to ferredoxin-NAD reductase activity in the three strains. Ferredoxin-NAD phosphate reductase activity was present in cell extracts of both species. Alcohol dehydrogenase activity in C. thermocellum was NAD dependent, unidirectional, and inhibited by low concentrations of NAD and ethanol. Ethanol dehydrogenase activity of T. brockii was both NAD and NADP linked, reversible, and not inhibited by low levels of reaction products. The high lactate yield of T. brockii correlated with increased fructose-1,6-diphosphate. The relation of catabolic enzyme activity and quantitative differences in intracellular electron flow and fermentation product yields of these thermophilic bacteria is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Glass T. L., Bryant M. P., Wolin M. J. Partial purification of ferredoxin from Ruminococcus albus and its role in pyruvate metabolism and reduction of nicotinamide adenine dinucleotide by H2. J Bacteriol. 1977 Aug;131(2):463–472. doi: 10.1128/jb.131.2.463-472.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Glucose fermentation pathway of Thermoanaerobium brockii. J Bacteriol. 1980 Mar;141(3):1251–1257. doi: 10.1128/jb.141.3.1251-1257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- McBEE R. H. The anaerobic thermophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):51–63. doi: 10.1128/br.14.1.51-63.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L. Ethanol fermentation and potential. Biotechnol Bioeng Symp. 1975;(5):345–352. [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Weimer T. K., Zeikus J. G. Cellulolytic and physiological properties of Clostridium thermocellum. Arch Microbiol. 1977 Jul 26;114(1):1–7. doi: 10.1007/BF00429622. [DOI] [PubMed] [Google Scholar]
- Patni N. J., Alexander J. K. Catabolism of fructose and mannitol in Clostridium thermocellum: presence of phosphoenolpyruvate: fructose phosphotransferase, fructose 1-phosphate kinase, phosphoenolpyruvate: mannitol phosphotransferase, and mannitol 1-phosphate dehydrogenase in cell extracts. J Bacteriol. 1971 Jan;105(1):226–231. doi: 10.1128/jb.105.1.226-231.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N. J., Alexander J. K. Utilization of glucose by Clostridium thermocellum: presence of glucokinase and other glycolytic enzymes in cell extracts. J Bacteriol. 1971 Jan;105(1):220–225. doi: 10.1128/jb.105.1.220-225.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange H., Cherrier C., Raval R., Gay R. Regulation of the NADH and NADPH-ferredoxin oxidoreductases in clostridia of the butyric group. Biochim Biophys Acta. 1976 Feb 24;421(2):334–337. doi: 10.1016/0304-4165(76)90300-7. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977 Feb;33(2):289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]



