Abstract
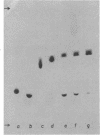
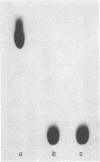
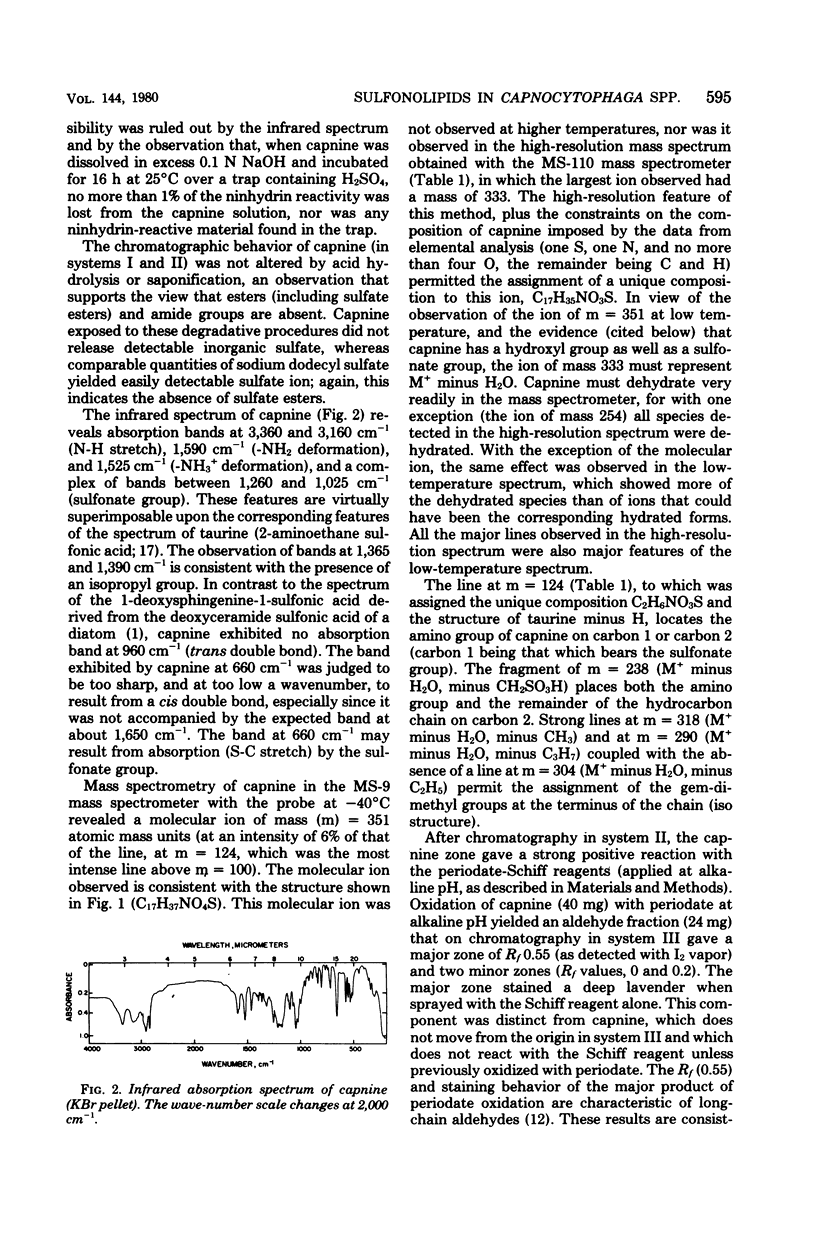
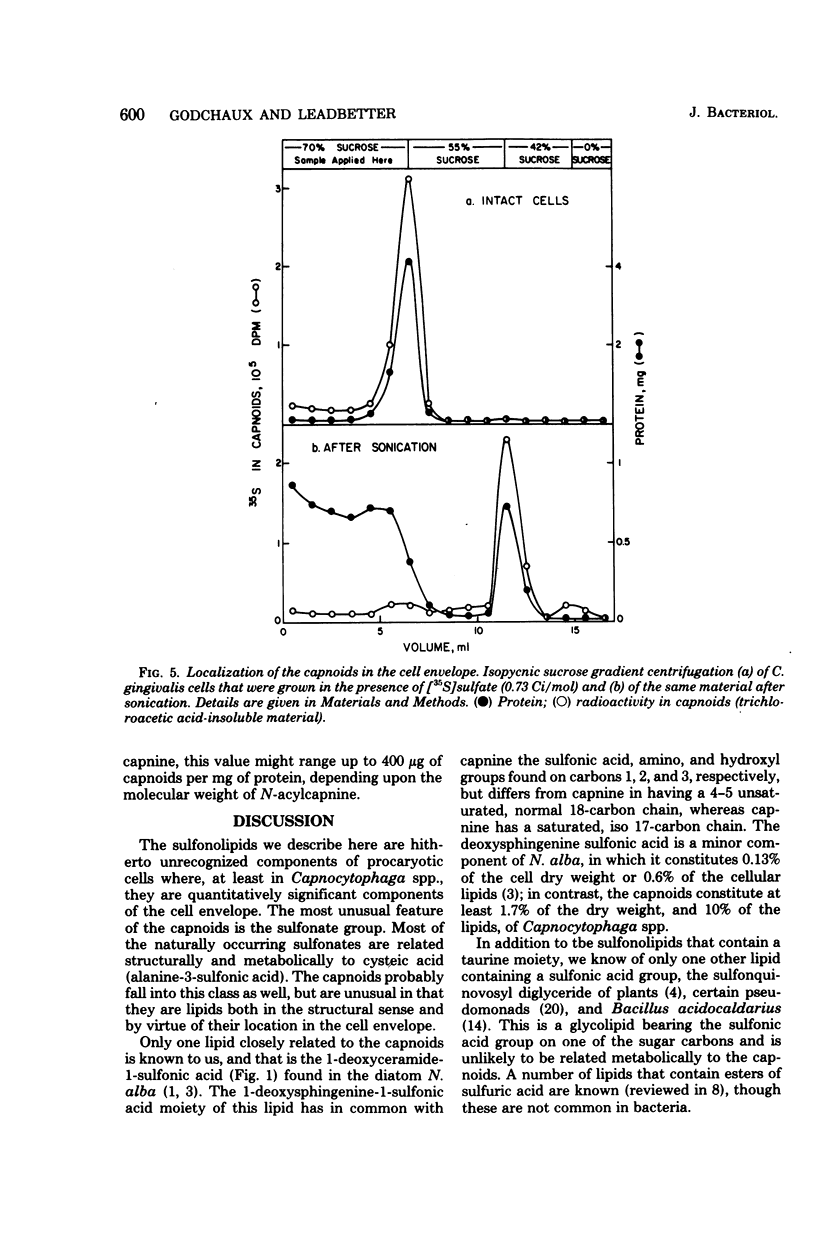
A group of unusual sulfonolipids was found in bacteria of the genus Capnocytophaga. One of these lipids, to which we have assigned the trivial name capnine, was isolated in 98% pure form and was identified, by infrared absorption spectrometry, high-resolution mass spectrometry, and other methods, as 2-amino-3-hydroxy-15-methylhexadecane-1-sulfonic acid. Another lipid appears to be an N-acylated version of capnine; after acid hydrolysis, its sulfur was recovered in a form chromatographically indistinguishable from that of capnine. The new lipids are related structurally to sphingosine and the ceramides, respectively, but differ markedly from those compounds in important respects, notably the presence of the sulfonate group. Some Capnocytophaga strains accumulated mostly capnine, whereas others accumulated mostly N-acylcapnine. All seven strains examined were found to contain the new lipids, in amounts ranging from 7 to 16 mumol/g of cells (wet weight). The lipids were found in isolated cell envelopes, where they were present in amounts ranging up to 400 mg/g of envelope protein; they are, accordingly, major cell components.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Kates M., Volcani B. E. Identification of the sulfolipids in the non-photosynthetic diatom Nitzschia alba. Biochim Biophys Acta. 1978 Jan 27;528(1):89–106. doi: 10.1016/0005-2760(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Anderson R., Kates M., Volcani B. E. Studies on the biosynthesis of sulfolipids in the Diatom Nitzschia alba. Biochim Biophys Acta. 1979 Jun 21;573(3):557–561. doi: 10.1016/0005-2760(79)90230-3. [DOI] [PubMed] [Google Scholar]
- Anderson R., Livermore B. P., Kates M., Volcani B. E. The lipid composition of the non-photosynthetic diatom Nitzschia alba. Biochim Biophys Acta. 1978 Jan 27;528(1):77–88. doi: 10.1016/0005-2760(78)90054-1. [DOI] [PubMed] [Google Scholar]
- BENSON A. A. THE PLANT SULFOLIPID. Adv Lipid Res. 1963;1:387–394. doi: 10.1016/b978-1-4831-9937-5.50016-8. [DOI] [PubMed] [Google Scholar]
- BRADY R. O., KOVAL G. J. The enzymatic synthesis of sphingosine. J Biol Chem. 1958 Jul;233(1):26–31. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fautz E., Rosenfelder G., Grotjahn L. Iso-branched 2- and 3-hydroxy fatty acids as characteristic lipid constituents of some gliding bacteria. J Bacteriol. 1979 Dec;140(3):852–858. doi: 10.1128/jb.140.3.852-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Forcier G., Takacs B. J. Fatty acid composition of gliding bacteria: oral isolates of Capnocytophaga compared with Sporocytophaga. Infect Immun. 1979 Oct;26(1):298–304. doi: 10.1128/iai.26.1.298-304.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J. T., Socransky S. S., Tanner A. C. Histological changes in experimental periodontal disease in rats monoinfected with gram-negative organisms. J Periodontal Res. 1978 Jul;13(4):326–332. doi: 10.1111/j.1600-0765.1978.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Mayberry W. R., Smith P. F. A sulfonolipid and novel glucosamidyl glycolipids from the extreme thermoacidophile Bacillus acidocaldarius. Biochim Biophys Acta. 1976 Jun 22;431(3):550–569. doi: 10.1016/0005-2760(76)90220-4. [DOI] [PubMed] [Google Scholar]
- Leadbetter E. R., Holt S. C., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. I. General characteristics, taxonomic considerations and significance. Arch Microbiol. 1979 Jul;122(1):9–16. doi: 10.1007/BF00408040. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Sutter V. L., Pickett M. J., Blachman U., Greenwood J. R., Grinenko V., Citron D. Detection, identification, and comparison of Capnocytophaga, Bacteroides ochraceus, and DF-1. J Clin Microbiol. 1979 Oct;10(4):557–562. doi: 10.1128/jcm.10.4.557-562.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfro L. J., Dickman K. G. Sulfate transport across the peritubular surface of the marine teleost renal tubule. Am J Physiol. 1980 Aug;239(2):F143–F148. doi: 10.1152/ajprenal.1980.239.2.F143. [DOI] [PubMed] [Google Scholar]
- Sass N. L., Martin W. G. The synthesis of taurine from sulfate. 3. Further evidence for the enzymatic pathway in chick liver. Proc Soc Exp Biol Med. 1972 Mar;139(3):755–761. doi: 10.3181/00379727-139-36232. [DOI] [PubMed] [Google Scholar]
- Williams B. L., Hollis D., Holdeman L. V. Synonymy of strains of Center for Disease Control group DF-1 with species of Capnocytophaga. J Clin Microbiol. 1979 Oct;10(4):550–556. doi: 10.1128/jcm.10.4.550-556.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. J., Nichols B. W., James A. T. The lipids and fatty acid metabolism of photosynthetic bacteria. Biochim Biophys Acta. 1965 Oct 4;106(2):261–273. doi: 10.1016/0005-2760(65)90034-2. [DOI] [PubMed] [Google Scholar]