Abstract
The development and function of natural killer (NK) cells is dictated by signals received through activating and inhibitory receptors expressed on the cell surface. During their maturation in the bone marrow, NK cells undergo an education process that ensures they are tolerant to healthy peripheral tissues. Several recent studies advance our understanding of self-tolerance mechanisms at work in NK cells. These studies demonstrate that the developmental programming in NK cells is not fixed, and that perturbations to the peripheral environment (via transplantation or viral infection, for example) greatly influence the ability of mature NK cells to mount an effector response. This newfound ability of mature NK cells to be “re-educated” may be clinically applicable in the immunotherapeutic use of NK cells against infection and cancer.
The immune system employs intricate mechanisms to maintain self-tolerance; these regulatory mechanisms ensure that immune cells distinguish foreign invaders from healthy tissues. To ensure self-tolerance in T cells, an entire organ (the thymus) is devoted to selecting specific thymocytes that are able to recognize major histocompatability complex (MHC)–self-peptide antigens while deleting thymocytes that recognize these complexes too strongly. Analogous regulatory mechanisms are in place to assure self-tolerance during the development of B cells in the bone marrow. Natural killer (NK) cells are no exception and also undergo an education process during development whereby cells that are potentially self-reactive are rendered anergic.
NK cells, which were first described by several groups in the early 1970s (Greenberg et al., 1973; Herberman et al., 1975; Kiessling et al., 1975; Sendo et al., 1975; Zarling et al., 1975), circulate through the blood and lymphatics and reside in virtually all organs. In these tissues, NK cells are poised to eliminate stressed, virally infected, or transformed cells without prior sensitization while minimizing injury to normal healthy cells. In humans and mice, NK cells survey their environment by using a sophisticated repertoire of evolutionarily selected activating and inhibitory receptors that bind both host- and pathogen-encoded ligands (Lanier, 2005). Because NK cells are powerful effector lymphocytes, their activation must be tightly regulated to ensure that NK cells protect the host from pathogen invasion while avoiding deleterious autoimmune responses. Thus, during their development in the bone marrow, NK cells are trained, or “educated,” to distinguish healthy from abnormal tissues.
Education and self-tolerance of NK cells
In 1986, Kärre et al. (1986) observed that unlike T cells, which respond to foreign protein components bound to MHC molecules, NK cells attack cells that are “missing self,” i.e., lacking MHC molecules. The missing self hypothesis inferred that an NK cell had to possess inhibitory receptors that could bind MHC class I (expressed on virtually all healthy cells), thereby preventing the NK cell from becoming activated during normal healthy conditions (Ljunggren and Kärre, 1990). A couple of years later, Karlhofer et al. (1992) provided the first molecular evidence for the missing self hypothesis by identifying an inhibitory receptor (Ly49A on mouse NK cells) that specifically recognizes MHC class I and suppresses NK cell function. At the same time, amino acid residues in human MHC class I that specifically rendered target cells resistant to NK cell–mediated cytotoxicity were identified (Storkus et al., 1991). Soon thereafter, several groups identified and cloned the genes encoding human inhibitory NK cell receptors that recognize different HLA class I family members (Colonna and Samaridis, 1995; D’Andrea et al., 1995; Gumperz et al., 1995; Wagtmann et al., 1995). The responsible inhibitory receptors were designated the killer cell immunoglobulin-like receptor (KIR) family (Long et al., 1996). Later studies demonstrated that in addition to sensing a loss of MHC class I in target cells, full effector function of NK cells requires triggering of their activating receptors via stress-induced or virus-encoded ligands on target cells (Cerwenka et al., 2001; Diefenbach et al., 2001; Arase et al., 2002; Smith et al., 2002). These studies explain the inability of NK cells to attack healthy cells that display either no MHC class I (e.g., erythrocytes) or low levels of MHC class I (e.g., neurons) on their surface. Although NK cells have been studied for several decades, the activating NK cell receptors and ligands responsible for mediating “missing self” rejection of MHC class I–deficient cells remains elusive.
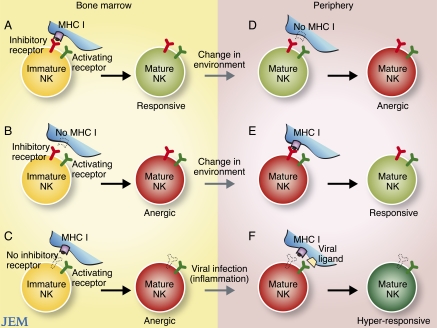
During development, NK cells have been shown to transit through several distinct stages, which are defined by acquisition of function and expression of surface receptors (Yokoyama et al., 2004; Di Santo, 2006). Immature mouse NK cells begin to express inhibitory Ly49 receptors early in development; this initiates an education process whereby inhibitory Ly49 receptor engagement of autologous MHC class I results in the generation of functional effector NK cells in the periphery (Fig. 1 A; Fernandez et al., 2005; Kim et al., 2005). This selection process has been termed “licensing” or “arming” of NK cells. Failure to engage inhibitory receptors during development, due to lack of inhibitory receptor expression on the NK cell or lack of interaction with MHC class I, results in the generation of a subset of anergic or hyporesponsive peripheral NK cells (Fig. 1, B and C; Fernandez et al., 2005; Kim et al., 2005). Similarly, human NK cells that express certain inhibitory KIRs that engage cognate HLA during development gain effector function; in the absence of inhibitory KIR–HLA interactions, human NK cells are rendered hyporesponsive (Anfossi et al., 2006; Yu et al., 2007; Kim et al., 2008). The education of NK cells is also influenced by signals received through activating receptors. In a process analogous to negative selection of developing T cells, ligation of activating receptors on developing NK cells by ubiquitiously expressed cognate viral or self-ligands leads to both a repression of cellular function through that particular receptor and a partial deletion of the subset repertoire (Ogasawara et al., 2005; Oppenheim et al., 2005; Sun and Lanier, 2008b; Tripathy et al., 2008). Altogether, these mechanisms are thought to exist to ensure that mature NK cells do not attack healthy self-tissues.
Figure 1.
Education and re-education of NK cells. The figure depicts the “education” of developing NK cells in different bone marrow environments (left) and the experimental “re-education” of mature NK cells in different peripheral environments (right). (A) Immature NK cells expressing inhibitory receptors that engage MHC class I become responsive mature effector cells. (B) Immature NK cells expressing inhibitory receptors that do not engage MHC class I become anergic cells. (C) Immature NK cells lacking inhibitory receptors that can engage MHC class I also become anergic cells. (D) Mature responsive NK cells that are adoptively transferred into a MHC class I–deficient environment become anergic. (E) Mature anergic NK cells (expressing inhibitory receptors for MHC class I) that are adoptively transferred into a MHC class I–sufficient setting become responsive. (F) During viral infection and inflammation, mature anergic NK cells (lacking inhibitory receptors for MHC class I) become activated and hyperresponsive effector cells.
Re-education of NK cells
Although NK cell precursors are primarily found in the bone marrow, there is evidence that NK cells can also develop in peripheral organs, including the thymus (Vosshenrich et al., 2006), and possibly in human lymph nodes (Freud et al., 2005). There is also evidence that NK cells continue to mature after egress from the bone marrow, as splenic NK cell populations consist of cells displaying varying degrees of maturation (which correlate with their expression of markers such as CD27, Mac-1, and KLRG1; Hayakawa and Smyth, 2006; Huntington et al., 2007a; Chiossone et al., 2009). In studies published in this issue, Joncker et al. and Elliot et al. find that the ability of mature NK cells to produce IFN-γ and kill target cells can be reprogrammed after the cells are exposed to MHC class I environments different than the ones in which they developed. In these studies, splenic NK cells that matured in a MHC class I–sufficient environment and acquired full effector function were adoptively transferred into a new host that was devoid of MHC class I. Surprisingly, these mature NK cells became anergic to receptor stimulation within several days after adoptive transfer into the MHC class I–deficient recipient (Fig. 1 D). In the reciprocal experiment, unresponsive NK cells from MHC class I–deficient mice were adoptively transferred into MHC class I–sufficient hosts (either in high numbers, or into an NK cell–deficient host strain to avoid rejection); these transferred cells gained effector function (Fig. 1 E). Like wild-type NK cells, these previously anergic NK cells now produced high levels of IFN-γ and degranulated robustly when various activating receptors were triggered ex vivo.
Previous data suggested that the education of NK cells is restricted to the bone marrow (Yokoyama et al., 2004; Huntington et al., 2007b). However, these new studies indicate that developmental programming in NK cells is not entirely fixed, and that mature NK cells can be “re-educated,” gaining or losing functional capacity as their new environment dictates. It will be interesting to learn where the reprogramming is occurring. It remains to be determined whether adoptively transferred mature NK cells re-enter the bone marrow, or are reprogrammed in peripheral tissues. In the clinical setting, the functional plasticity of NK cells could potentially be harnessed for NK cell immunotherapy against certain tumors or viruses, where a robust NK cell response against cells that have down-modulated HLA expression would be efficacious.
Unleashing NK cells
In the steady state, both responsive and anergic NK cells reside in peripheral organs. Why is it that NK cells lacking an inhibitory receptor for autologous MHC class I (“unlicensed” or “disarmed” NK cells) are allowed to seed the periphery? Why are these cells not subject to apoptosis like developing T cells bearing TCRs that cannot properly engage MHC during positive selection? In fact, recent studies suggest that these anergic or unlicensed cells play an important role during viral infection. During mouse cytomegalovirus (MCMV) infection, NK cells in both wild-type and MHC class I–deficient mice mount robust effector responses, and NK cells that were previously tolerant to MHC class I–deficient cells in mixed bone marrow chimeric mice rapidly reject their MHC class I–deficient neighbors (Sun and Lanier, 2008a). In this setting, inflammation was able to break self-tolerance in vivo. These findings are consistent with in vitro experiments demonstrating that anergic NK cells can secrete large amounts of IFN-γ when bathed in proinflammatory cytokines such as interleukin (IL) 12 and IL-18 (Yokoyama and Kim, 2006). Likewise, proinflammatory cytokines can activate anergic T cells (Schwartz, 2003).
A recent study surprisingly showed that these unlicensed, anergic NK cells are actually better effectors than licensed NK cells during MCMV infection (Orr et al., 2010). Depletion of unlicensed, but not licensed, NK cells from MCMV-infected wild-type B6 mice resulted in elevated viral titers. Interestingly, adoptively transferred unlicensed NK cells provided robust protection against MCMV challenge in neonate mice, whereas an equal number of licensed NK cells was unable to promote survival any better than the negative control receiving no NK cells. Together, these findings suggest that although inhibitory receptors are required for self-tolerance, they hinder productive immune responses during infection. Thus, an overall lack of inhibitory receptors against autologous MHC class I permits NK cells to respond more robustly against viral infection (Fig. 1 F). Perhaps infection and inflammation stimulate responsive (licensed) and anergic (unlicensed) NK cells similarly, but once the initial threshold for activation is attained, those NK cells without inhibiting receptors intuitively respond faster and more robustly. Although the potential for autoimmunity and greater collateral damage resides in the unlicensed NK cells, it is possible that evolutionary pressures have allowed for the selection and maintenance of this normally anergic subset specifically to deal with infectious pathogens.
In addition, these features may prove useful in the setting of allogeneic hematopoietic stem cell transplantation for the treatment of leukemia patients. In several independent studies, patients receiving inhibitory KIR–HLA mismatched transplants had a lower incidence of leukemia relapse and a higher frequency of survival compared with patients receiving NK cell populations that could be inhibited by host HLA molecules (Hsu et al., 2005; Clausen et al., 2007; Miller et al., 2007; Sobecks et al., 2007; Yu et al., 2009). A greater understanding of how NK cells develop and function may advance the prevention and treatment of certain infectious diseases and cancers.
Acknowledgments
The author thanks Carrie Sun for preparing the figure, and Lewis Lanier and Katharine Hsu for critical reading of the manuscript.
J.C.S. is supported by National Institutes of Health grant AI085034.
References
- Anfossi N., André P., Guia S., Falk C.S., Roetynck S., Stewart C.A., Breso V., Frassati C., Reviron D., Middleton D., et al. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 25:331–342 10.1016/j.immuni.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Arase H., Mocarski E.S., Campbell A.E., Hill A.B., Lanier L.L. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 296:1323–1326 10.1126/science.1070884 [DOI] [PubMed] [Google Scholar]
- Cerwenka A., Baron J.L., Lanier L.L. 2001. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA. 98:11521–11526 10.1073/pnas.201238598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 113:5488–5496 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- Clausen J., Wolf D., Petzer A.L., Gunsilius E., Schumacher P., Kircher B., Gastl G., Nachbaur D. 2007. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clin. Exp. Immunol. 148:520–528 10.1111/j.1365-2249.2007.03360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Samaridis J. 1995. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 268:405–408 10.1126/science.7716543 [DOI] [PubMed] [Google Scholar]
- D’Andrea A., Chang C., Franz-Bacon K., McClanahan T., Phillips J.H., Lanier L.L. 1995. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. J. Immunol. 155:2306–2310 [PubMed] [Google Scholar]
- Di Santo J.P. 2006. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 24:257–286 10.1146/annurev.immunol.24.021605.090700 [DOI] [PubMed] [Google Scholar]
- Diefenbach A., Jensen E.R., Jamieson A.M., Raulet D.H. 2001. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 413:165–171 10.1038/35093109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J.M., Wahle J.A., Yokoyama W.M. 2010. MHC class I–deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I–sufficient environment. J. Exp. Med. 207:2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N.C., Treiner E., Vance R.E., Jamieson A.M., Lemieux S., Raulet D.H. 2005. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 105:4416–4423 10.1182/blood-2004-08-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud A.G., Becknell B., Roychowdhury S., Mao H.C., Ferketich A.K., Nuovo G.J., Hughes T.L., Marburger T.B., Sung J., Baiocchi R.A., et al. 2005. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 22:295–304 10.1016/j.immuni.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Greenberg A.H., Hudson L., Shen L., Roitt I.M. 1973. Antibody-dependent cell-mediated cytotoxicity due to a “null” lymphoid cell. Nat. New Biol. 242:111–113 10.1038/242111a0 [DOI] [PubMed] [Google Scholar]
- Gumperz J.E., Litwin V., Phillips J.H., Lanier L.L., Parham P. 1995. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J. Exp. Med. 181:1133–1144 10.1084/jem.181.3.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y., Smyth M.J. 2006. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176:1517–1524 [DOI] [PubMed] [Google Scholar]
- Herberman R.B., Nunn M.E., Holden H.T., Lavrin D.H. 1975. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer. 16:230–239 10.1002/ijc.2910160205 [DOI] [PubMed] [Google Scholar]
- Hsu K.C., Keever-Taylor C.A., Wilton A., Pinto C., Heller G., Arkun K., O’Reilly R.J., Horowitz M.M., Dupont B. 2005. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 105:4878–4884 10.1182/blood-2004-12-4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington N.D., Tabarias H., Fairfax K., Brady J., Hayakawa Y., Degli-Esposti M.A., Smyth M.J., Tarlinton D.M., Nutt S.L. 2007a. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J. Immunol. 178:4764–4770 [DOI] [PubMed] [Google Scholar]
- Huntington N.D., Vosshenrich C.A., Di Santo J.P. 2007b. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7:703–714 10.1038/nri2154 [DOI] [PubMed] [Google Scholar]
- Joncker N.T., Shifrin N., Delebecque F., Raulet D.H. 2010. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 207:2065–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlhofer F.M., Ribaudo R.K., Yokoyama W.M. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 358:66–70 10.1038/358066a0 [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H.G., Piontek G., Kiessling R. 1986. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 319:675–678 10.1038/319675a0 [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. 1975. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 5:112–117 10.1002/eji.1830050208 [DOI] [PubMed] [Google Scholar]
- Kim S., Poursine-Laurent J., Truscott S.M., Lybarger L., Song Y.J., Yang L., French A.R., Sunwoo J.B., Lemieux S., Hansen T.H., Yokoyama W.M. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 436:709–713 10.1038/nature03847 [DOI] [PubMed] [Google Scholar]
- Kim S., Sunwoo J.B., Yang L., Choi T., Song Y.J., French A.R., Vlahiotis A., Piccirillo J.F., Cella M., Colonna M., et al. 2008. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl. Acad. Sci. USA. 105:3053–3058 10.1073/pnas.0712229105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274 10.1146/annurev.immunol.23.021704.115526 [DOI] [PubMed] [Google Scholar]
- Ljunggren H.G., Kärre K. 1990. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 11:237–244 10.1016/0167-5699(90)90097-S [DOI] [PubMed] [Google Scholar]
- Long E.O., Colonna M., Lanier L.L. 1996. Inhibitory MHC class I receptors on NK and T cells: a standard nomenclature. Immunol. Today. 17:100 10.1016/0167-5699(96)80590-1 [DOI] [PubMed] [Google Scholar]
- Miller J.S., Cooley S., Parham P., Farag S.S., Verneris M.R., McQueen K.L., Guethlein L.A., Trachtenberg E.A., Haagenson M., Horowitz M.M., et al. 2007. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 109:5058–5061 10.1182/blood-2007-01-065383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K., Benjamin J., Takaki R., Phillips J.H., Lanier L.L. 2005. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat. Immunol. 6:938–945 10.1038/ni1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D.E., Roberts S.J., Clarke S.L., Filler R., Lewis J.M., Tigelaar R.E., Girardi M., Hayday A.C. 2005. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 6:928–937 10.1038/ni1239 [DOI] [PubMed] [Google Scholar]
- Orr M.T., Murphy W.J., Lanier L.L. 2010. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 11:321–327 10.1038/ni.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R.H. 2003. T cell anergy. Annu. Rev. Immunol. 21:305–334 10.1146/annurev.immunol.21.120601.141110 [DOI] [PubMed] [Google Scholar]
- Sendo F., Aoki T., Boyse E.A., Buafo C.K. 1975. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J. Natl. Cancer Inst. 55:603–609 [DOI] [PubMed] [Google Scholar]
- Smith H.R., Heusel J.W., Mehta I.K., Kim S., Dorner B.G., Naidenko O.V., Iizuka K., Furukawa H., Beckman D.L., Pingel J.T., et al. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA. 99:8826–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobecks R.M., Ball E.J., Maciejewski J.P., Rybicki L.A., Brown S., Kalaycio M., Pohlman B., Andresen S., Theil K.S., Dean R., Bolwell B.J. 2007. Survival of AML patients receiving HLA-matched sibling donor allogeneic bone marrow transplantation correlates with HLA-Cw ligand groups for killer immunoglobulin-like receptors. Bone Marrow Transplant. 39:417–424 10.1038/sj.bmt.1705609 [DOI] [PubMed] [Google Scholar]
- Storkus W.J., Salter R.D., Alexander J., Ward F.E., Ruiz R.E., Cresswell P., Dawson J.R. 1991. Class I-induced resistance to natural killing: identification of nonpermissive residues in HLA-A2. Proc. Natl. Acad. Sci. USA. 88:5989–5992 10.1073/pnas.88.14.5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Lanier L.L. 2008a. Cutting edge: viral infection breaks NK cell tolerance to “missing self.” J. Immunol. 181:7453–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Lanier L.L. 2008b. Tolerance of NK cells encountering their viral ligand during development. J. Exp. Med. 205:1819–1828 10.1084/jem.20072448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S.K., Keyel P.A., Yang L., Pingel J.T., Cheng T.P., Schneeberger A., Yokoyama W.M. 2008. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 205:1829–1841 10.1084/jem.20072446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich C.A., García-Ojeda M.E., Samson-Villéger S.I., Pasqualetto V., Enault L., Richard-Le Goff O., Corcuff E., Guy-Grand D., Rocha B., Cumano A., et al. 2006. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7:1217–1224 10.1038/ni1395 [DOI] [PubMed] [Google Scholar]
- Wagtmann N., Rajagopalan S., Winter C.C., Peruzzi M., Long E.O. 1995. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 3:801–809 10.1016/1074-7613(95)90069-1 [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M., Kim S. 2006. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol. Rev. 214:143–154 10.1111/j.1600-065X.2006.00458.x [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M., Kim S., French A.R. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405–429 10.1146/annurev.immunol.22.012703.104711 [DOI] [PubMed] [Google Scholar]
- Yu J., Heller G., Chewning J., Kim S., Yokoyama W.M., Hsu K.C. 2007. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J. Immunol. 179:5977–5989 [DOI] [PubMed] [Google Scholar]
- Yu J., Venstrom J.M., Liu X.R., Pring J., Hasan R.S., O’Reilly R.J., Hsu K.C. 2009. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 113:3875–3884 10.1182/blood-2008-09-177055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J.M., Nowinski R.C., Bach F.H. 1975. Lysis of leukemia cells by spleen cells of normal mice. Proc. Natl. Acad. Sci. USA. 72:2780–2784 10.1073/pnas.72.7.2780 [DOI] [PMC free article] [PubMed] [Google Scholar]