Abstract
The immune response plays an important role in staving off cancer; however, mechanisms of immunosuppression hinder productive anti-tumor immunity. T cell dysfunction or exhaustion in tumor-bearing hosts is one such mechanism. PD-1 has been identified as a marker of exhausted T cells in chronic disease states, and blockade of PD-1–PD-1L interactions has been shown to partially restore T cell function. We have found that T cell immunoglobulin mucin (Tim) 3 is expressed on CD8+ tumor-infiltrating lymphocytes (TILs) in mice bearing solid tumors. All Tim-3+ TILs coexpress PD-1, and Tim-3+PD-1+ TILs represent the predominant fraction of T cells infiltrating tumors. Tim-3+PD-1+ TILs exhibit the most severe exhausted phenotype as defined by failure to proliferate and produce IL-2, TNF, and IFN-γ. We further find that combined targeting of the Tim-3 and PD-1 pathways is more effective in controlling tumor growth than targeting either pathway alone.
The importance of the immune system in protection against cancer was originally proposed in the theory of cancer immunosurveillance. This theory holds that the immune system can recognize cancerous cells as they arise and can mount both innate and adaptive immune responses to eliminate them. In support of cancer immunosurveillance is the fact that both immunodeficient or immunosuppressed patients and experimental animals are more susceptible to tumor development (for reviews see Dunn et al., 2004; Zitvogel et al., 2006; Swann and Smyth, 2007). Counter to the role of the immune system in staving off cancer is the ability of tumors to escape the immune system by engendering a state of immunosuppression (for review see Zitvogel et al., 2006). One example of a mechanism of immunosuppression present in tumor-bearing hosts is the promotion of T cell dysfunction or exhaustion.
T cell exhaustion describes a state of T cell dysfunction that was initially observed during chronic lymphocytic choriomeningitis virus (LCMV) infection in mice (Zajac et al., 1998). Exhausted T cells fail to proliferate and exert effector functions such as cytotoxicity and cytokine secretion in response to antigen stimulation. Further studies identified that exhausted T cells are characterized by sustained expression of the inhibitory molecule PD-1 (programmed cell death 1) and that blockade of PD-1 and PD-L1 (PD-1 ligand) interactions can reverse T cell exhaustion and restore antigen-specific T cell responses in LCMV-infected mice (Barber et al., 2006). T cell exhaustion also occurs during chronic infections in humans (for review see Klenerman and Hill, 2005). CD8+ T cells in humans chronically infected with HIV (Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006), hepatitis B virus (Boettler et al., 2006), and hepatitis C virus (HCV; Urbani et al., 2006) express high levels of PD-1, and blocking of PD-1–PD-L interactions can restore T cell function in vitro.
Several lines of evidence also implicate the PD-1–PD-L pathway in T cell exhaustion in cancer. First, PD-1 expression is found on tumor-infiltrating CD8+ T cells in multiple solid tumors (Blank et al., 2006; Ahmadzadeh et al., 2009; Gehring et al., 2009) and on antigen-specific CD8+ T cells in hosts with nonsolid tumors (Yamamoto et al., 2008; Mumprecht et al., 2009). Second, these PD-1+ T cells are dysfunctional. Third, PD-L1 is expressed at high levels in several different cancers (Latchman et al., 2001; Dong et al., 2002; Brown et al., 2003), and high expression of PD-L1 on tumors is strongly associated with poor prognosis (Thompson et al., 2006). Fourth, interference with PD-1–PD-L signaling, either through antibody blockade or PD-1 deficiency, has been shown to improve clinical outcome and restore functional T cell responses in several cancers (Blank et al., 2006; Yamamoto et al., 2008; Mumprecht et al., 2009; Zhang et al., 2009). However, targeting the PD-1–PD-L1 pathway does not always result in reversal of T cell exhaustion (Blackburn et al., 2008; Gehring et al., 2009) and PD-1 expression is not always associated with exhausted phenotype (Petrovas et al., 2006; Fourcade et al., 2009), indicating that other molecules are likely involved in T cell exhaustion.
A recent study in patients with HIV has shown that the immune regulator T cell immunoglobulin mucin (TIM) 3 is up-regulated on exhausted CD8+ T cells (Jones et al., 2008). Tim-3 is a molecule originally identified as being selectively expressed on IFN-γ–secreting Th1 and Tc1 cells (Monney et al., 2002). Interaction of Tim-3 with its ligand, galectin-9, triggers cell death in Tim-3+ T cells. Thus, both Tim-3 and PD-1 can function as negative regulators of T cell responses. In HIV patients, TIM-3 and PD-1 mark distinct populations of exhausted cells, with cells positive for both PD-1 and TIM-3 comprising the smallest fraction (Jones et al., 2008) of CD8+ T cells. Similarly, another group has shown that TIM-3 is up-regulated on exhausted T cells in patients with HCV (Golden-Mason et al., 2009). In this case, cells that coexpress TIM-3 and PD-1 are the most abundant fraction among HCV-specific CD8+ T cells. In both studies, blocking TIM-3 restored T cell proliferation and enhanced cytokine production.
Because targeting the PD-1–PD-L pathway alone does not result in complete restoration of T cell function (Blackburn et al., 2008), and in some cancers targeting the PD-1–PD-L pathway does not restore T cell function at all (Gehring et al., 2009), there is a need to identify other molecules and inhibitory pathways that are involved in T cell exhaustion. Indeed, one study has identified LAG-3 as being expressed on exhausted T cells, and although treatment with anti–LAG-3 alone did not restore T cell function in LCMV-infected mice, it synergized with PD-1 blockade to improve T cell responses and reduce viral load (Blackburn et al., 2009). Unfortunately, this study did not identify whether LAG-3 and PD-1 are expressed on distinct or overlapping populations of exhausted T cells. Given these observations, it appears that targeting multiple pathways may prove most effective in reversing T cell exhaustion.
We report in this paper the coexpression of Tim-3 and PD-1 on a large fraction of tumor-infiltrating lymphocytes (TILs) in mice bearing solid tumors. TILs that coexpress Tim-3 and PD-1 predominate among CD8+ TILs and exhibit the most profound defects in T cell effector function. We further show that combined targeting of the Tim-3 and PD-1 pathways is highly effective in controlling tumor growth.
RESULTS
Tim-3 and PD-1 co expression on T cells in cancer
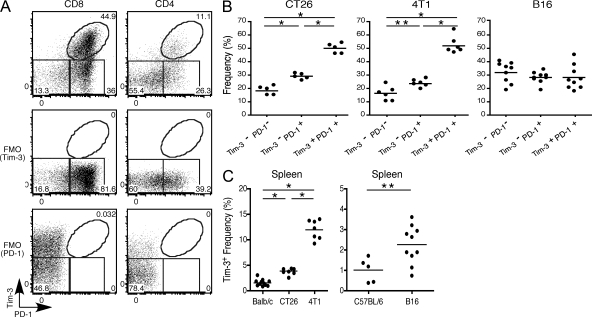
To examine a potential role for Tim-3 in T cell exhaustion in cancer, we first examined the expression of Tim-3 as well as PD-1 in T cells from mice bearing the solid tumor CT26 colon carcinoma. We observed that among CD8+ TILs, cells that coexpress Tim-3 and PD-1 comprise the major population (∼50%) with cells expressing PD-1 alone or neither Tim-3 nor PD-1 comprising smaller populations (∼30% and ∼20%, respectively; Fig. 1, A and B). To extend these observations to other cancers, we examined the CD8+ TILs in mice bearing other solid tumors: 4T1 mammary adenocarcinoma and B16F10 melanoma. Consistent with our observations in mice bearing CT26, cells that coexpress Tim-3 and PD-1 also comprise ∼50% of the CD8+ TILs in mice bearing 4T1 tumor, with cells expressing PD-1 alone or neither Tim-3 nor PD-1 also comprising smaller populations (∼25 and 15%, respectively; Fig. 1 B). In mice bearing B16F10 melanoma, all three populations of CD8+ TILs (Tim-3−PD-1−, Tim-3−PD-1+, and Tim-3+PD-1+) are present at roughly equal frequency. Interestingly, in all three of the tumor models examined we did not observe any Tim-3+PD-1− TILs (Fig. 1 A and not depicted). We also examined CD4+ TILs; however, these are less abundant, and among these we found that the majority were Tim-3-PD-1− with the Tim-3+PD-1+ and Tim-3−PD-1+ populations being roughly equivalent (Fig. 1 A and not depicted). Collectively, these data indicate that Tim-3 and PD-1 coexpressing CD8+ TILs comprise a major population of T cell present in TILs infiltrating different solid tumors.
Figure 1.
PD-1 and Tim-3 expression in TILs. BALB/c mice were implanted with CT26 colon adenocarcinoma or 4T1 mammary adenocarcinoma. C57BL/6 mice were implanted with B16F10 melanoma. TILs were harvested and stained with 7AAD to exclude dead cells and antibodies against CD8, CD4, Tim-3, and PD-1. (A) Expression of Tim-3 and PD-1 on gated CD4+ and CD8+ TILs from a BALB/c mouse bearing CT26 tumor. FMO, fluorescence minus one controls for Tim-3 and PD-1 staining. Data shown are representative of more than five independent analyses. (B) Frequency of CD8+ cells in TILs expressing Tim-3 and PD-1 from tumor-bearing mice. The horizontal bars indicate means. *, P < 0.001; **, P < 0.05, one-way ANOVA followed by Tukey’s multiple comparison test. CT26 (n = 5), 4T1 (n = 6), and B16 (n = 9). (C) Frequency of CD8+Tim-3+ cells in spleens of tumor-bearing mice compared with spleens of naive tumor-free mice. The horizontal bars indicate means. *, P < 0.001, one-way ANOVA, Tukey’s multiple comparison test; **, P = 0.0188, unpaired Student’s t test. BALB/c, n = 11; CT26, n = 8; 4T1, n = 7; C57BL/6, n = 5; B16, n = 10.
We also examined Tim-3 and PD-1 expression in the spleens of tumor-bearing mice. Here, we observed a trend toward increased frequency of CD8+Tim-3+ cells compared with naive mice; however, the extent of this increase was variable among mice bearing different solid tumors (Fig. 1 C). In contrast to the CD8+ TILs, we found little if any evidence for coexpression of PD-1 with Tim-3 among splenic CD8+ T cells in tumor-bearing mice (Fig. S1), suggesting that up-regulation of PD-1 on CD8+ Tim-3+ cells may happen in the tumor environment in response to environmental cues. However, we could distinguish two distinct populations of Tim-3+ cells, Tim-3high and Tim-3low, in the peripheral lymphoid tissue of tumor-bearing mice. Similarly, among splenic CD4+ T cells in tumor-bearing mice, we observed a Tim-3high and Tim-3low population. Interestingly, the Tim-3low population was characterized by coexpression of PD-1, suggesting that these cells may be the precursors of Tim-3+PD-1+ TILs and that they may represent T cells that are in a different functional state from Tim-3high cells (Fig. S1).
T cell dysfunction in TILs expressing Tim-3 and PD-1
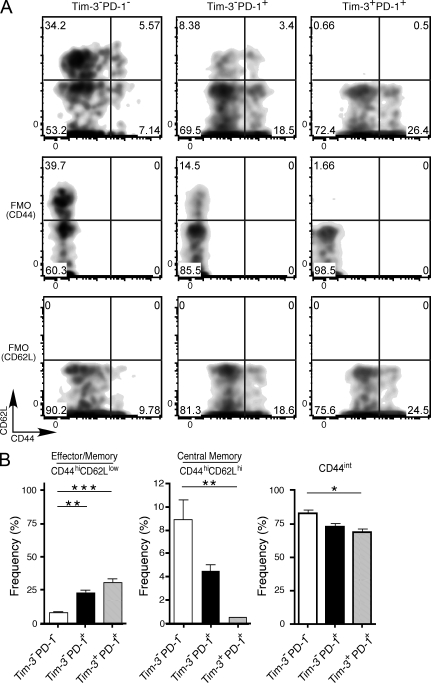
To further characterize the different subsets of CD8+ TILs, we first examined their expression of CD44 and CD62L. We found that the pattern of CD62L and CD44 expression was quite different among the Tim-3−PD-1−, Tim-3−PD-1+, and Tim-3+PD-1+ TILs (Fig. 2, A and B). Among the three populations, the Tim-3+PD-1+ population contained the largest fraction of effector/memory (CD44hiCD62Llow) cells but the lowest fraction of central memory (CD44hiCD62Lhi) cells. Indeed, the majority of Tim-3+PD-1+ TILs were CD62Llow. The majority of TILs in all three populations expressed low to intermediate levels of CD44. Although this CD44int population may comprise some naive cells, it is more likely that this population comprises cells that are transitioning from naive to effector status. The CD44int population was lowest among Tim-3+PD-1+ cells. These data gave the first indication that the three populations of TILs characterized by differential expression of Tim-3 and PD-1 contain cells in different functional states.
Figure 2.
CD44 and CD62L expression in Tim-3– and PD-1–expressing TILs. TILs were harvested from CT26 tumor-bearing mice and stained with 7AAD to exclude dead cells and antibodies against CD8, CD44, CD62L, Tim-3, and PD-1. (A) Representative staining on CD8+ Tim-3−PD-1−, Tim-3−PD-1+, and Tim-3+PD-1+ TILs is shown. FMO, controls for CD44 and CD62L staining are shown. Data are representative of three independent analyses. (B) Summary data showing the frequency of effector/memory (CD44hiCD62Llow) and central memory (CD44hiCD62Lhi) and CD44int cells within the CD8+ Tim-3−PD-1−, Tim-3−PD-1+, and Tim-3+PD-1+ TILs. *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA and Tukey’s multiple comparison test. n = 3. Error bars represent SEM.
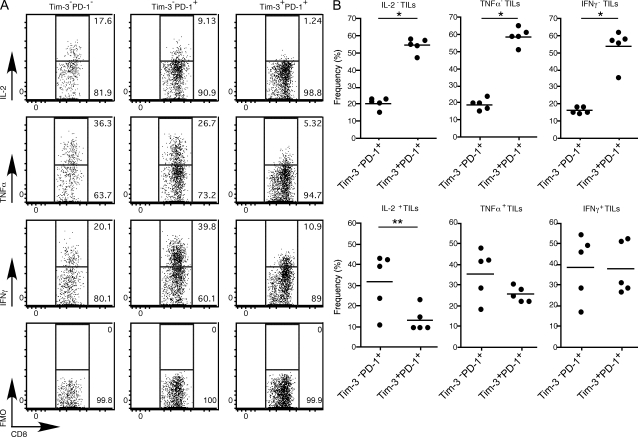
In chronic viral infection, PD-1 has been identified as a marker of dysfunctional or exhausted CD8+ T cells (Barber et al., 2006). Furthermore, it has been observed that there is a hierarchy of T cell exhaustion with CTL function and production of IL-2 being compromised first, followed by loss of TNF and then IFN-γ (Wherry et al., 2003). Therefore, to determine whether any of the Tim-3– and PD-1–expressing TILs exhibited exhausted phenotype, we isolated CD8+ TILs and examined their production of IL-2, TNF, and IFN-γ directly ex vivo. We found that the Tim-3+PD-1+ TILs exhibited the most profound impairment in production of IL-2, TNF, and IFN-γ when compared with Tim-3−PD-1+ TILs and Tim-3−PD-1− TILs (Fig. 3 A). Surprisingly, the Tim-3−PD-1+ TILs produced the most IFN-γ among the three populations of TILs and showed significantly less impairment in the production of IL-2 and TNF than the Tim-3+PD-1+ TILs. These data suggest that the Tim-3+PD-1+ TILs represent the most exhausted TILs and that Tim-3−PD-1+ TILs may contain a mixture of exhausted T cells and effector T cells. To further confirm these observations, we determined the abundance of Tim-3+PD-1+ cells and Tim-3−PD-1+ cells within the cytokine-producing and -nonproducing TILs (Fig. 3 B). We found that Tim-3+PD-1+ cells are the most abundant (55–60%) population among cytokine-nonproducing TILs, outnumbering Tim-3−PD-1+ cells by three- to fourfold. Examination of cytokine-producing TILs revealed that Tim-3+PD-1+ cells are less abundant than Tim-3−PD-1+ among IL-2–producing TILs. A similar trend was observed with TNF, although this did not reach statistical significance. Both populations were equally represented among IFN-γ–producing TILs. Interestingly, this stepwise loss in abundance of Tim-3+PD-1+ cells among cytokine-producing TILs seems to follow the hierarchy of T cell exhaustion, suggesting that exhaustion is likely a dynamic process in vivo and that Tim-3+PD-1+ cells may be the first to develop exhausted phenotype.
Figure 3.
Cytokine production in TILs from CT26 tumor-bearing mice. TILs were harvested from CT26 tumor-bearing mice and stimulated with PMA and ionomycin before intracytoplasmic cytokine staining. (A) Expression of cytokine in Tim-3−PD-1−, Tim-3−PD-1+, and Tim-3+PD-1+ CD8+ TILs. Data shown are representative of five independent analyses. FMO, fluorescence minus one (anti-cytokine antibody). (B) Frequency of Tim-3−PD-1+ and Tim-3+PD-1+ cells among CD8+ cytokine-producing and -nonproducing TILs (n = 5). The horizontal bars indicate means. *, P < 0.0001; **, P = 0.0261, unpaired Student’s t test.
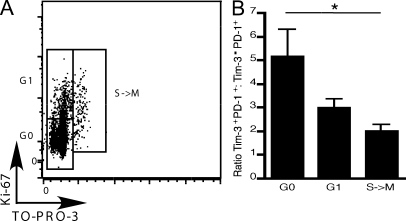
As stated in the previous paragraph, loss of the ability to proliferate in response to TCR stimulation is among the first effector functions lost in exhausted T cells. We therefore examined the ability of TILs to proliferate directly ex vivo by determining expression of Ki-67, a nuclear protein expressed by cells which have entered into cell cycle. However, it has been noted that in individuals chronically infected with HIV, cells that are arrested in G1 can express Ki-67+ (Combadière et al., 2000). We therefore also examined DNA content by simultaneously staining with TO-PRO-3 iodide. By doing so, we can discern cells arrested in G1 from cells that have progressed to S, G2, and M phase. We isolated TILs and stimulated them directly ex vivo before examination of Ki-67 expression and DNA content. We then determined the abundance of Tim-3+PD-1+ and Tim-3−PD-1+ cells in G0, G1, and S-M phases of cell cycle (Fig. 4 A). We found that Tim-3+PD-1+ cells are the most abundant population that is stuck in G0, outnumbering Tim-3−PD-1+ cells by 5 to 1 (Fig. 4 B). Interestingly, when we examined cells that have progressed to the G1 and S-M phases, we found that Tim-3+PD-1+ cells steadily decrease in number, whereas Tim-3−PD-1+ cells steadily increase with progression through cell cycle. Collectively, our data strongly support that coexpression of Tim-3 and PD-1 marks the most exhausted population of TILs, which fail to proliferate and produce IL-2, TNF, and IFN-γ.
Figure 4.
Proliferation and cell cycle entry in TILs from CT26 tumor-bearing mice. TILs were harvested from CT26 tumor-bearing mice and stimulated with 1 µg/ml of anti-CD3 before staining with antibodies against CD8, Tim-3, PD-1, and Ki-67 and TO-PRO-3–iodide. (A) Expression of Ki-67 and TO-PRO-3 staining in CD8+ TILs showing the different phases of the cell cycle: G0, G1, and S→M. Data shown are representative of six independent analyses. (B) Ratio of Tim-3+PD-1+ to Tim-3−PD-1+ TILs (n = 6) in different phases of cell cycle. *, P < 0.05, one-way ANOVA and Tukey’s multiple comparison test. n = 6. Error bars represent SEM.
Effect of targeting the Tim-3 and PD-1 signaling pathways in cancer
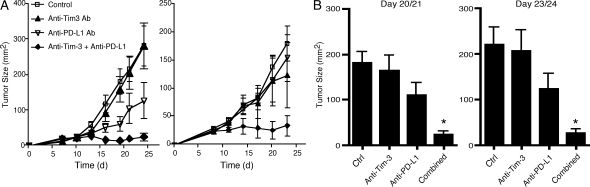
Our observations, along with the previous demonstrations that blockade of either the PD-1 or Tim-3 (Jones et al., 2008; Golden-Mason et al., 2009) signaling pathways can improve T cell function in the context of chronic infections, raised the possibility that combined targeting of these two pathways may prove to be the most efficacious means to restore anti-tumor immunity in vivo. Before commencing in vivo treatments, we first confirmed the expression of the PD-1 and Tim-3 ligands (PD-L1 and galectin-9, respectively) on CT26 tumor (Fig. S2). We then treated CT26 tumor-bearing mice with an anti–Tim-3 antibody, which was previously described to have blocking function in vivo (Monney et al., 2002), anti-PD-L1 antibody, anti–Tim-3 plus anti–PD-L1 antibodies, or control immunoglobulins. We found that treatment with anti–Tim-3 alone had little or no effect and treatment with anti–PD-L1 alone showed a trend toward delayed tumor growth, but this varied between experiments and did not reach statistical significance (Fig. 5). However, combined treatment with anti–Tim-3 and anti–PD-L1 resulted in a dramatic reduction in tumor growth, with 50% of the mice exhibiting complete tumor regression. Indeed, the mice from the combined anti–Tim-3 plus anti–PD-L1 group that exhibited complete regression remained tumor free even after rechallenge (unpublished data). Because CT26 tumor expresses PD-L1 but not Tim-3 (Fig. S2), we controlled for the possibility that anti–PD-L1 antibody could have direct inhibitory effects on tumor growth. We cultured CT26 tumor in the presence of anti–PD-L1 or control immunoglobulin and found that tumor proliferation was not affected (Fig. S3). We have also tested the effect of anti–Tim-3 plus anti–PD-L1 treatment in mice bearing B16 melanoma and found that mice receiving the combined treatment exhibit enhanced survival relative to control immunoglobulin, anti–Tim-3, or anti–PD-L1–treated mice (unpublished data).
Figure 5.
Effect of targeting the Tim-3 and PD-1 signaling pathways on tumor growth. (A) 5 × 105 CT26 cells were implanted into wild-type BALB/c mice. Mice were then treated with anti-Tim-3, anti-PD-L1, anti-Tim-3 + anti-PD-L1, or control immunoglobulins (RatIgG1 + RatIgG2b). Error bars represent SEM. Two independent experiments are shown. Left, control (n = 5), anti–Tim-3 (n = 5), anti–PD-L1 (n = 6), and anti–Tim-3 + anti–PD-L1 (n = 5). Right, control (n = 4), anti–Tim-3 (n = 5), anti–PD-L1 (n = 4), and anti–Tim-3 + anti–PD-L1 (n = 3). (B) Pooled data from the experiments shown in A. Error bars represent SEM. Left, *, P < 0.01 compared with control or anti–Tim-3 group. Right, *, P < 0.01 compared with control group and P < 0.05 compared with anti–Tim-3 group, one-way ANOVA and Tukey’s multiple comparison test.
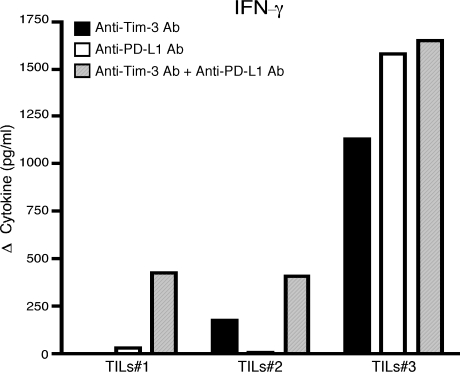
To address directly whether treatment with anti–Tim-3 plus anti–PD-L1 indeed restores TILs function, we isolated TILs from mice bearing CT26 tumor and cultured them in the presence of anti–Tim-3, anti–PD-L1, anti–Tim-3 plus anti–PD-L1 antibodies, or control immunoglobulins (Fig. 6). We found that although both anti–Tim-3 and anti–PD-L1 alone were able to augment IFN-γ production from TILs, this effect was variable and often weaker when compared with the increase in IFN-γ production observed in TILs treated with both anti–Tim-3 and anti–PD-L1 antibodies. Indeed, these data parallel closely what we have seen in our in vivo treatment experiments, where anti–Tim-3 or anti–PD-L1 alone has a limited and/or variable effect on tumor growth (Fig. 5). We have also examined the effect of anti–Tim-3 plus anti–PD-L1 treatment on peripheral T cell responses from tumor-bearing mice and found that, similar to the effects observed on TILs, both anti–Tim-3 and anti–PD-L1 alone had a variable and often weaker effect on IFN-γ production relative to the effect of anti–Tim-3 plus anti–PD-L1 (Fig. S4). Collectively, our data support that combined targeting of the Tim-3 and PD-1 signaling pathways is highly effective in restoring anti-tumor immunity.
Figure 6.
Blockade of the Tim-3 and PD-1 signaling pathways restores IFN-γ production. TILs were harvested from CT26 tumor-bearing mice and cultured in vitro in the presence of soluble anti-CD3 and anti–Tim-3, anti–PD-L1, anti–Tim-3 plus anti–PD-L1, or control immunoglobulins. After 96 h, culture supernatant was collected and IFN-γ measured by cytometric bead array. Data are expressed as the difference in cytokine production over that observed in cultures with control immunoglobulins. Data shown are from three independent TILs samples from two independent experiments.
DISCUSSION
In this paper, we have examined the expression of the inhibitory receptors Tim-3 and PD-1 on TILs in mice bearing solid tumors and found that CD8+ TILs that coexpress Tim-3 and PD-1 not only represent the most abundant TIL population in multiple solid tumors but also represent the most dysfunctional or exhausted population of TILs. We further show that single targeting of the Tim-3 and PD-1 pathways has variable effects on tumor growth, whereas combined targeting of these pathways is highly effective in controlling tumor growth and restoring T cell production of IFN-γ. Similarly, a recent study has found that simultaneous targeting of the Tim-3 and PD-1 pathways also rescues CD8+ T cells from exhaustion in a model of chronic infection (Takamura et al., 2010). Together, these findings support combined targeting of the Tim-3 and PD-1 pathways as an effective treatment not only for cancer but also for other chronic immune conditions where T cell exhaustion is known to occur.
Until recently, PD-1 has been the primary marker for exhausted T cells. However, our data show that PD-1 single-positive TILs likely include bona fide effector T cells that produce IFN-γ, as this population contains the highest frequency of IFN-γ–producing cells, even higher than the PD-1−Tim-3− TILs (Fig. 3 A). Thus, our data suggest that PD-1 is an imperfect marker of exhaustion and that coexpression of Tim-3 clearly marks the T cells with the most exhausted phenotype. However, several questions remain. It is known that triggering of Tim-3 can transmit a death signal into T cells. How then do Tim-3+PD-1+ exhausted T cells persist in chronic conditions? One possibility is that differential levels of Tim-3 expression drive different functional outcomes; i.e., high levels of Tim-3 promote T cell death whereas low levels of Tim-3 transmit an inhibitory signal that allows for cells to escape death and persist in a dysfunctional state. In this regard, we have observed the presence of Tim-3low cells in both the CD4 and CD8 compartments in the periphery of tumor-bearing mice (Fig. S1). It will be intriguing to determine if these T cells are in a different state of effector function compared with Tim-3high cells. A second possibility is that coexpression of PD-1 and/or other inhibitory molecules, such as LAG-3, is responsible for preserving cells with exhausted phenotype. Lastly, the decision between exhaustion and death could be regulated at the level of availability of the Tim-3 ligand galectin-9. In this regard, it still remains to be demonstrated whether development of exhaustion in TILs is dependent on galectin-9 expression on the tumor itself or whether it starts in the periphery and the exhausted phenotype is further amplified by an interaction of Tim-3–Galectin-9 in the tumor.
Aside from the chronicity of disease, little is known about the factors involved in inducing and/or maintaining exhaustion in T cells with the exception of the recent implication of the transcription factor Blimp-1 in promoting exhausted phenotype in CD8+ T cells during chronic LCMV infection (Shin et al., 2009). Our identification of Tim-3+PD-1+ cells as the truly exhausted T cells in chronic conditions will facilitate the examination of the gene programs that drive/maintain exhausted phenotype. A thorough understanding of the signaling pathways downstream of Tim-3 and PD-1 will be an important first step toward identifying molecular mediators of exhaustion.
We have also observed Tim-3 and PD-1 coexpression on CD4+ TILs; however, whether these cells are effector cells, regulatory cells, or exhibit an exhausted phenotype is not known. Whether these cells have an impact on the development of exhaustion in the CD8+ T cells is also not known. In spite of all these considerations, one thing is clear: the Tim-3–Tim-3L pathway and PD-1–PD-L pathways, two pathways which likely evolved to limit tissue pathology after infection, have been co-opted in chronic disease states to promote a state of functional impairment in T cells.
MATERIALS AND METHODS
Animals.
6–8-wk-old female BALB/c or C57BL/6 (The Jackson Laboratory) mice were used in all experiments. All experiments were performed under animal experimentation protocol #04555, which was approved by the Harvard Medical Area Standing Committee on animals.
Isolation of TILs.
TILs were isolated by dissociating tumor tissue in the presence of 2.5 mg/ml collagenase D for 20 min before centrifugation on a discontinuous Percoll gradient (GE Healthcare). Isolated cells were then used in various assays of T cell function.
Flow cytometry.
Single cell suspensions were stained with antibodies against CD4 (RM4-5), CD8 (53–6.7), PD-1 (RMP1-30), CD44 (IM7), CD62L (MEL-14; BioLegend), and Tim-3 (8B.2C12; eBioscience). 7AAD was used to exclude dead cells. For intracytoplasmic cytokine staining, cells were stimulated in vitro with 50 ng/ml PMA and 1 µg/ml ionomycin for 3 h in the presence of Golgi plug (BD). Cells were then harvested and stained with CD8, Tim-3, and PD-1 before fixation and permeabilization. Permeabilized cells were then stained for IL-2 (JES6-5H4), TNF (MP6-XT22), and IFN-γ (XMG1.2). All data were collected on an LSRII (BD) and analyzed with FlowJo software (Tree Star, Inc.).
Ki67 and TO-PRO-3 staining.
TILs were harvested and cultured in vitro in the presence of 1 µg/ml of anti-CD3 for 48 h. Cells were then stained with antibodies against CD8, PD-1, and Tim-3 (8B.2C12) before permeabilization and staining with antibody against Ki-67 (BioLegend) and with TO-PRO-3 iodide (Invitrogen). All data were collected on an LSRII and analyzed with FlowJo software.
Tumor experiments.
5 × 105 CT26 were implanted into the right flank of wild-type BALB/c mice. Mice were treated with 100 µg of anti–Tim-3 (clone 8B.2C12) i.p. on days 0, 2, and 4, 200 µg of anti–PD-L1 (clone 10F.9G2) on days 0, 3, 6, 9, and 12, or isotype control immunoglobulins (Rat IgG1 and RatIgG2b). Tumor surface was measured in two dimensions using a caliper.
In vitro experiments.
TILs were harvested as described and cultured (1–3 × 105/well) in the presence of 5 µg/ml of soluble anti-CD3 and 10 µg/ml of anti–Tim-3 (clone 8B.2C12), anti–PD-L1 (clone 10F.9G2), anti–Tim-3 plus anti–PD-L1, or control immunoglobulins (rat IgG1 and rat IgG2b). After 96 h, culture supernatant was collected and IFN-γ measured by cytometric bead array (BD).
Online supplemental material.
Fig. S1 shows the Tim-3 and PD-1 expression on CD8 and CD4 cells in the spleen of tumor-bearing mice. Fig. S2 shows the expression of PD-L1, Tim-3, and Galectin-9 on CT26 tumor cells. Fig. S3 shows the effects of anti–PD-L1 antibody on the growth of CT26 tumor in vitro. Fig. S4 shows the effect of in vivo targeting of the Tim-3 and PD-1 signaling pathways in tumor-bearing mice on peripheral T cell responses. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100637/DC1.
Acknowledgments
The authors would like to acknowledge Rucha Chandwaskar for technical assistance and Dr. G. Freeman for the provision of anti–PD-L1 antibody.
This work was supported by grants from the National Institutes of Health (V.K. Kuchroo: AI73748, AI056299, NS038037, NS045937, NS030843; A.C. Anderson: NS054096; B.R. Blazer: CA72669, HL56067, AI056299), the National Multiple Sclerosis Society (V.K. Kuchroo), the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard (V.K. Kuchroo), the European Molecular Biology Organization (L. Apetoh), an Innovation Award from the Ragon Institute of the Massachusetts Institute of Technology, Massachussetts General Hospital and Harvard (V.K. Kuchroo), and an award form the Sankyo Foundation of Life Science (K. Sakuishi). V.K. Kuchroo is a recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- HCV
- hepatitis C virus
- LCMV
- lymphocytic choriomeningitis virus
- TIL
- tumor-infiltrating lymphocyte
- TIM
- T cell immunoglobulin mucin
References
- Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., Rosenberg S.A. 2009. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 114:1537–1544 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Freeman G.J., Wherry E.J. 2008. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl. Acad. Sci. USA. 105:15016–15021 10.1073/pnas.0801497105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A., Betts M.R., Freeman G.J., Vignali D.A., Wherry E.J. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C., Kuball J., Voelkl S., Wiendl H., Becker B., Walter B., Majdic O., Gajewski T.F., Theobald M., Andreesen R., Mackensen A. 2006. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int. J. Cancer. 119:317–327 10.1002/ijc.21775 [DOI] [PubMed] [Google Scholar]
- Boettler T., Panther E., Bengsch B., Nazarova N., Spangenberg H.C., Blum H.E., Thimme R. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80:3532–3540 10.1128/JVI.80.7.3532-3540.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Dorfman D.M., Ma F.R., Sullivan E.L., Munoz O., Wood C.R., Greenfield E.A., Freeman G.J. 2003. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 170:1257–1266 [DOI] [PubMed] [Google Scholar]
- Combadière B., Blanc C., Li T., Carcelain G., Delaugerre C., Calvez V., Tubiana R., Debré P., Katlama C., Autran B. 2000. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of the cell cycle. Eur. J. Immunol. 30:3598–3603 [DOI] [PubMed] [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800 [DOI] [PubMed] [Google Scholar]
- Dunn G.P., Old L.J., Schreiber R.D. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329–360 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- Fourcade J., Kudela P., Sun Z., Shen H., Land S.R., Lenzner D., Guillaume P., Luescher I.F., Sander C., Ferrone S., et al. 2009. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J. Immunol. 182:5240–5249 10.4049/jimmunol.0803245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring A.J., Ho Z.Z., Tan A.T., Aung M.O., Lee K.H., Tan K.C., Lim S.G., Bertoletti A. 2009. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 137:682–690 10.1053/j.gastro.2009.04.045 [DOI] [PubMed] [Google Scholar]
- Golden-Mason L., Palmer B.E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B.J., Castelblanco N., Kuchroo V., Gretch D.R., Rosen H.R. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83:9122–9130 10.1128/JVI.00639-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.B., Ndhlovu L.C., Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., et al. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763–2779 10.1084/jem.20081398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P., Hill A. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873–879 10.1038/ni1241 [DOI] [PubMed] [Google Scholar]
- Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- Monney L., Sabatos C.A., Gaglia J.L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E.A., Coyle A.J., Sobel R.A., et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415:536–541 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- Mumprecht S., Schürch C., Schwaller J., Solenthaler M., Ochsenbein A.F. 2009. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 114:1528–1536 10.1182/blood-2008-09-179697 [DOI] [PubMed] [Google Scholar]
- Petrovas C., Casazza J.P., Brenchley J.M., Price D.A., Gostick E., Adams W.C., Precopio M.L., Schacker T., Roederer M., Douek D.C., Koup R.A. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292 10.1084/jem.20061496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Blackburn S.D., Intlekofer A.M., Kao C., Angelosanto J.M., Reiner S.L., Wherry E.J. 2009. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 31:309–320 10.1016/j.immuni.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann J.B., Smyth M.J. 2007. Immune surveillance of tumors. J. Clin. Invest. 117:1137–1146 10.1172/JCI31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura S., Tsuji-Kawahara S., Yagita H., Akiba H., Sakamoto M., Chikaishi T., Kato M., Miyazawa M. 2010. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J. Immunol. 184:4696–4707 10.4049/jimmunol.0903478 [DOI] [PubMed] [Google Scholar]
- Thompson R.H., Kuntz S.M., Leibovich B.C., Dong H., Lohse C.M., Webster W.S., Sengupta S., Frank I., Parker A.S., Zincke H., et al. 2006. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66:3381–3385 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S., et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
- Urbani S., Amadei B., Tola D., Massari M., Schivazappa S., Missale G., Ferrari C. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403 10.1128/JVI.01177-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R., Nishikori M., Kitawaki T., Sakai T., Hishizawa M., Tashima M., Kondo T., Ohmori K., Kurata M., Hayashi T., Uchiyama T. 2008. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 111:3220–3224 10.1182/blood-2007-05-085159 [DOI] [PubMed] [Google Scholar]
- Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., Ahmed R. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gajewski T.F., Kline J. 2009. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 114:1545–1552 10.1182/blood-2009-03-206672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Tesniere A., Kroemer G. 2006. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6:715–727 10.1038/nri1936 [DOI] [PubMed] [Google Scholar]