Abstract
The tumor suppressor gene p53 prevents the initiation of tumor formation by inducing cell cycle arrest, senescence, DNA repair, and apoptosis. Recently, the absence or mutation of p53 was described to facilitate nuclear reprogramming. These findings suggest an influence of p53 on the de-differentiation process, and highlight the similarities between induction of pluripotency and tumor formation.
Recently, Takahashi and Yamanaka (2006) reported that differentiated mouse cells can acquire de novo pluripotency upon the overexpression of four transcription factors, the so-called Yamanaka cocktail (Oct4, Sox2, Klf4, and c-Myc); only 1 yr later, the experiment was successfully reproduced in human somatic cells (Takahashi et al., 2007). This finding offers new opportunities for regenerative medicine, as induced pluripotent stem cells (iPSCs) may represent a rejection-free tissue source, and thus could be generated and considered a patient-specific therapy. In addition, iPSCs obtained from diseased human tissues offer a new tool to study disease modeling and drug screening.
Reprogramming highlights the ability of somatic cells to revert their fate toward a state of pluripotency, a de-differentiation process resembling tumor formation. In this line of thinking, several groups (Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marión et al., 2009; Utikal et al., 2009) reported that p53, a tumor suppressor transcription factor, also acts as a barrier to somatic cell reprogramming. In addition, in this issue Sarig et al. suggest that a mutant form of p53 actually increases the efficiency of the reprogramming process beyond that facilitated by the absence of p53 alone.
Role of p53 during induction of pluripotency
Although the plasticity of differentiated cells has been demonstrated by different approaches (Yamanaka and Blau, 2010), several mechanisms prevent committed cells from de-differentiating into cells of an embryonic state, thus preventing tumor formation. Surprisingly, germline stem cells (GSCs) derived from neonatal, but not adult, testes have been described to spontaneously revert to multipotent GSCs at a very low efficiency, which could be increased by depleting p53 (Kanatsu-Shinohara et al., 2004). These results correlate with a 100-fold increase in the incidence of testicular teratoma reported in p53 knockout mice (Lam and Nadeau, 2003) and establish a relationship between pluripotency, tumorigenesis, and p53. However, further studies demonstrated that pluripotent stem cells could be derived from GSCs obtained from adult testis without manipulating the p53 pathway (Ko et al., 2009).
As the inefficiency of the reprogramming process is a major limitation to the clinical use of iPSCs, many efforts have been directed at improving the efficiency of reprogramming. An initial hypothesis postulated that the limiting step in the reprogramming process was achieveming the appropriate, or correct, level of each exogenous transcription factor in each cell during retroviral infection. To investigate this issue, Wernig et al. (2008) generated chimeric mice from iPSCs obtained with inducible lentiviral vectors encoding Oct4, Sox2, Klf4, and c-Myc. However, only 1–3% of mouse embryonic fibroblasts (MEFs) isolated from these mice were able to give rise to secondary iPSCs after lentivirus induction, even when all the cells contained exactly the same number of viral integrations (Wernig et al., 2008). These findings suggest that other mechanisms, rather than the right stoichiometry among transcription factors, were responsible for the low rate of reprogramming. The suggestion that only a rare population of MEFs could be reprogrammed was then offered, but was subsequently discounted because of the successful reprogramming of different cell types during different stages of differentiation (Yamanaka and Blau, 2010).
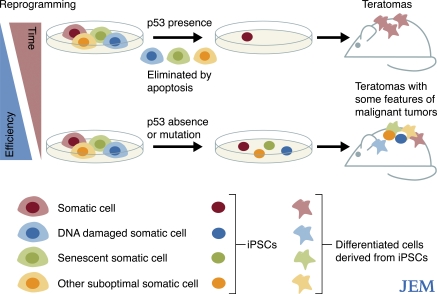
Yamanaka (2007) suggested that c-Myc might induce p53-dependent apoptosis in fibroblasts, which in turn would lead to a reduced rate of reprogramming. The next year, Zhao et al. showed that silencing of p53 combined with overexpression of Utf1 produced a 100-fold increase in iPSC formation (Zhao et al., 2008). Those authors proposed that inhibition of p53 could block apoptosis and senescence, thus facilitating reprogramming. Recently, these suggestions have been demonstrated experimentally, and several reports have described the role played by p53 during different stages of the reprogramming process (Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marión et al., 2009; Utikal et al., 2009; Sarig et al., 2010). After retroviral infection, the overexpression of exogenous transcription factors is thought to activate p53, leading to cell cycle arrest and apoptosis. In addition, at the time of pluripotency induction, a second round of p53-dependent apoptosis is assumed to take place, with cells presenting with DNA damage and/or chromosomal abnormalities excluded from becoming iPSCs. Thus, it is thought that inhibiting p53 prevents apoptosis, senescence, and cell cycle arrest, thereby increasing the efficiency of reprogramming (Fig. 1).
Figure 1.
The p53 pathway decreases the efficiency of reprogramming to iPSCs. In the presence of p53, senescent cells or cells with DNA damage undergo apoptosis, thereby preventing the generation of iPSCs. When the p53 pathway is silenced or mutated, these suboptimal cells can become iPSCs, thus increasing the efficiency of reprogramming. In addition, in the absence of p53-dependent cell cycle arrest, the reprogramming process is accelerated. After transplantation of wild-type iPSCs into immunodeficient mice, these cells give rise to teratomas characterized by differentiated tissues of all three germ layers. In contrast, teratomas generated from p53-deficient iPSCs also contain undifferentiated tissues, thus resembling tumor growths.
However, Hanna et al. (2009) reported that most cells were capable of becoming iPSCs without depleting p53 or immortalizing the cells. For this purpose, those authors used a homogeneous population of clonal B cells obtained from secondary iPSC-generated mice. After induction, the majority of cells were reprogrammed. In the absence of p53, the process was accelerated, correlating with an increase in the proliferation rate. Thus, in this model, lack of p53 only increased the kinetics of reprogramming, without affecting the overall efficiency of the reprogramming process (Hanna et al., 2009). As Hanna et al. (2009) only detected background levels of apoptosis in the B cells, the effect of p53 on reprogramming appears to be more pronounced in cell types with high tolerance to DNA damage. This finding may account for the different reprogramming rates reported for different cell types (Yamanaka and Blau, 2010). In contrast, another group compared the reprogramming efficiency of wild-type and p53-depleted MEFs cultured in 0.5 and 15% fetal bovine serum, respectively. The p53 knockout MEFs exhibited higher reprogramming efficiency, but lower proliferation rate, compared with wild-type MEFs, thus arguing against an effect of p53 on the cell cycle as the cause for the increased reprogramming efficiency (Utikal et al., 2009).
As Klf4 has been observed to repress p53 (Rowland et al., 2005), it follows that Klf4 is dispensable for reprogramming in p53-depleted MEFs. Consistent with this assumption, Kawamura et al. (2009) reported that iPSCs could be generated from p53-depleted human embryonic fibroblasts at a very low efficiency with only OCT4 and SOX2, in the absence of KLF4. However, Zhao et al. (2008) could not generate iPSCs in the absence of KLF4, even after silencing p53. Nevertheless, Melton et al. (Huangfu et al., 2008) successfully reprogrammed human fibroblasts with only OCT4 and SOX2 in the presence of valproic acid (VPA), without inhibiting p53, also at a very low efficiency. It would be very interesting to examine whether VPA acts either by disturbing the p53 pathway or by increasing the proliferation rate or whether its effect is simply an outcome of chromatin modifications and global transcriptional changes.
p53-deficient or mutant iPSCs present tumor-like features
Half of all human cancers contain missense mutations in p53, causing inactivation of the tumor suppressor factor (Hollstein et al., 1991). Most of these mutations not only inhibit p53 tumor suppressor activity, but also engender the mutated protein with an oncogenic ability, referred as a gain of function (Brosh and Rotter, 2009). The mouse p53-R172H mutation, R175H in human, causes a global conformational distortion of the p53 protein that leads to tumor cells with an additional growth advantage, thereby producing a very aggressive tumor phenotype in vitro (Donehower and Lozano, 2009). One gain of function described for this p53 mutant is its ability to interfere with miRNA biogenesis, decreasing the availability of several mature miRNAs involved in the p53 response to DNA damage (Suzuki et al., 2009).
Sarig et al. (2010) explored the role of the R172H p53 mutant in pluripotency induction. To this end, those authors derived MEFs from p53 R172H–knockin mice (Lang et al., 2004) and showed that p53 mutant MEFs were reprogrammed more efficiently than MEFs derived from either wild-type or p53 knockout mice. They also showed a higher reprogramming efficiency in p53 mutant MEFs using only two factors (Oct4 and Sox2) compared with p53 knockout MEFs using three factors (Oct4, Sox2, and Klf4), suggesting an active role of the p53 mutant in reprogramming as in tumorigenesis.
As adult stem cells are capable of self-renewing, it is more likely that a spontaneous p53 mutation in an adult stem cell, rather than in a differentiated cell, gives rise to a tumor. Consistent with this notion, neural stem cells (NSCs) lacking p53 exhibit impaired differentiation, up-regulation of c-Myc, and a tendency for glioma formation. Thus, inhibition of p53 can cause normal stem cells to turn into tumor cells (Zheng et al., 2008). In agreement with this, Hong et al. (2009) reported that teratomas from p53-deficient iPSCs generated with the four factors (Oct4, Sox2, Klf4, and c-Myc) were mostly formed of undifferentiated tissue; this was not the case when c-Myc was excluded from the reprogramming cocktail. Similarly, chimeric mice generated from four-factor iPSCs derived from p53 knockout cells died within 7 wk of birth because of tumor formation (Hong et al., 2009). These findings suggest that c-Myc, in the absence of p53, blocks differentiation and induces tumor formation (Hong et al., 2009; Sarig et al., 2010). Tumorigenesis caused by c-Myc retroviral reactivation has already been described in iPSC chimeric mice, even in the presence of p53 (Okita et al., 2007). This result is consistent with the ability of c-Myc alone to initiate tumor formation in epithelial cells (Wong et al., 2008) and to prevent the differentiation of p53-deficient NSCs (Zheng et al., 2008). Thus, four-factor–generated, p53-deficient iPSCs, with their ability to self-renew and aberrantly differentiate, resemble malignant tumors.
However, Sarig et al. (2010) report that even three-factor (Oct4, Sox2, and Klf4) p53 knockout iPSCs or two-factor (Oct4 and Sox2) p53-R172H iPSCs can give rise to undifferentiated tissues in teratomas. Although the gain of function of p53-R172H may contribute to this aberrant differentiation process, these findings may also be explained by the fact that, even in the absence of c-Myc, p53 deficiency should lead to genomic instability and, consequently, to impaired differentiation. p53-deficient iPSCs did in fact become unstable with passaging (Hong et al., 2009; Marión et al., 2009; Sarig et al., 2010). In addition, the presence of double-stranded DNA breaks and activation of the DNA damage response was observed in teratomas generated from p53 knockout iPSCs (Marión et al., 2009). Marión et al. (2009) proposed that the DNA damage response observed in the p53-null teratomas was similar to that observed in human tumors (Bartkova et al., 2005). However, tumor formation was not described in chimeras generated from two- and three-factor p53-depleted iPSCs (Kawamura et al., 2009; Marión et al., 2009), although p53 knockout mice develop tumors at early stages of life (Donehower and Lozano, 2009). Therefore, although iPSCs generated in the absence of wild-type p53 exhibit features characteristic of pluripotency, in terms of self-renewal, gene expression, and morphology, they also present with features related to tumorigenesis, such as impaired differentiation (Sarig et al., 2010).
Conclusions
Several studies have described the involvement of p53 in the mechanism underlying the inefficient process of iPSC generation. p53 leads to a reduction in the efficiency of reprogramming, inducing apoptosis, and cell cycle arrest, particularly in those cells presenting with DNA damage, thereby preventing the reprogramming of suboptimal cells. Although an approach involving the depletion of p53 would result in an increased rate of iPSC generation, it would not be feasible for therapeutic use. The transient inhibition of p53 using chemicals has been proposed in efforts to increase the reprogramming efficiency, particularly when using plasmids rather than retroviruses in the reprogramming procedure. However, the absence of an effect on the genome integrity of the generated iPSCs remains to be shown and is a prerequisite for therapeutic consideration. In addition, although p53 mutations in patient cells would be advantageous for reprogramming over nonmutated cells, iPSCs should be analyzed to exclude the possibility of any p53 mutations, as these have the potential to give rise to tumors after transplantation of the differentiated cells into patients. In summary, future efforts to increase the efficiency of somatic cell reprogramming should preferably be aimed at selecting for primary cells with lower levels of p53 and/or higher proliferative ability, rather than silencing the p53 pathway.
Acknowledgments
We thank Dong Wook Han for critical comments on the manuscript.
References
- Bartkova J., Horejsí Z., Koed K., Krämer A., Tort F., Zieger K., Guldberg P., Sehested M., Nesland J.M., Lukas C., et al. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 434:864–870 10.1038/nature03482 [DOI] [PubMed] [Google Scholar]
- Brosh R., Rotter V. 2009. When mutants gain new powers: news from the mutant p53 field. Nat. Rev. Cancer. 9:701–713 [DOI] [PubMed] [Google Scholar]
- Donehower L.A., Lozano G. 2009. 20 years studying p53 functions in genetically engineered mice. Nat. Rev. Cancer. 9:831–841 [DOI] [PubMed] [Google Scholar]
- Hanna J., Saha K., Pando B., van Zon J., Lengner C.J., Creyghton M.P., van Oudenaarden A., Jaenisch R. 2009. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 462:595–601 10.1038/nature08592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C.C. 1991. p53 mutations in human cancers. Science. 253:49–53 10.1126/science.1905840 [DOI] [PubMed] [Google Scholar]
- Hong H., Takahashi K., Ichisaka T., Aoi T., Kanagawa O., Nakagawa M., Okita K., Yamanaka S. 2009. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 460:1132–1135 10.1038/nature08235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Osafune K., Maehr R., Guo W., Eijkelenboom A., Chen S., Muhlestein W., Melton D.A. 2008. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 26:1269–1275 10.1038/nbt.1502 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Inoue K., Lee J., Yoshimoto M., Ogonuki N., Miki H., Baba S., Kato T., Kazuki Y., Toyokuni S., et al. 2004. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 119:1001–1012 10.1016/j.cell.2004.11.011 [DOI] [PubMed] [Google Scholar]
- Kawamura T., Suzuki J., Wang Y.V., Menendez S., Morera L.B., Raya A., Wahl G.M., Belmonte J.C. 2009. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 460:1140–1144 10.1038/nature08311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Tapia N., Wu G., Kim J.B., Bravo M.J., Sasse P., Glaser T., Ruau D., Han D.W., Greber B., et al. 2009. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 5:87–96 10.1016/j.stem.2009.05.025 [DOI] [PubMed] [Google Scholar]
- Lam M.Y., Nadeau J.H. 2003. Genetic control of susceptibility to spontaneous testicular germ cell tumors in mice. APMIS. 111:184–190, discussion :191 10.1034/j.1600-0463.2003.11101221.x [DOI] [PubMed] [Google Scholar]
- Lang G.A., Iwakuma T., Suh Y.A., Liu G., Rao V.A., Parant J.M., Valentin-Vega Y.A., Terzian T., Caldwell L.C., Strong L.C., et al. 2004. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 119:861–872 10.1016/j.cell.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Li H., Collado M., Villasante A., Strati K., Ortega S., Cañamero M., Blasco M.A., Serrano M. 2009. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 460:1136–1139 10.1038/nature08290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marión R.M., Strati K., Li H., Murga M., Blanco R., Ortega S., Fernandez-Capetillo O., Serrano M., Blasco M.A. 2009. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 460:1149–1153 10.1038/nature08287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. 2007. Generation of germline-competent induced pluripotent stem cells. Nature. 448:313–317 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- Rowland B.D., Bernards R., Peeper D.S. 2005. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell Biol. 7:1074–1082 10.1038/ncb1314 [DOI] [PubMed] [Google Scholar]
- Sarig R., Rivlin N., Brosh R., Bornstein C., Kamer I., Ezra O., Molchadsky A., Goldfinger N., Brenner O., Rotter V. 2010. Mutant p53 facilitates somatic cell reprogramming and augments the malignant potential of reprogrammed cells. J. Exp. Med. 207:2127–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H.I., Yamagata K., Sugimoto K., Iwamoto T., Kato S., Miyazono K. 2009. Modulation of microRNA processing by p53. Nature. 460:529–533 10.1038/nature08199 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Utikal J., Polo J.M., Stadtfeld M., Maherali N., Kulalert W., Walsh R.M., Khalil A., Rheinwald J.G., Hochedlinger K. 2009. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 460:1145–1148 10.1038/nature08285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M., Lengner C.J., Hanna J., Lodato M.A., Steine E., Foreman R., Staerk J., Markoulaki S., Jaenisch R. 2008. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 26:916–924 10.1038/nbt1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.J., Liu H., Ridky T.W., Cassarino D., Segal E., Chang H.Y. 2008. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2:333–344 10.1016/j.stem.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. 2007. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 1:39–49 10.1016/j.stem.2007.05.012 [DOI] [PubMed] [Google Scholar]
- Yamanaka S., Blau H.M. 2010. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 465:704–712 10.1038/nature09229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yin X., Qin H., Zhu F., Liu H., Yang W., Zhang Q., Xiang C., Hou P., Song Z., et al. 2008. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 3:475–479 10.1016/j.stem.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Zheng H., Ying H., Yan H., Kimmelman A.C., Hiller D.J., Chen A.J., Perry S.R., Tonon G., Chu G.C., Ding Z., et al. 2008. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 455:1129–1133 10.1038/nature07443 [DOI] [PMC free article] [PubMed] [Google Scholar]