Abstract
Some mature natural killer (NK) cells cannot be inhibited by major histocompatibility complex (MHC) I molecules, either because they lack corresponding inhibitory receptors or because the host lacks the corresponding MHC I ligands for the receptors. Such NK cells nevertheless remain self-tolerant and exhibit a generalized hyporesponsiveness to stimulation through activating receptors. To address whether NK cell responsiveness is set only during the NK cell differentiation process, we transferred mature NK cells from wild-type (WT) to MHC I–deficient hosts or vice versa. Remarkably, mature responsive NK cells from WT mice became hyporesponsive after transfer to MHC I–deficient mice, whereas mature hyporesponsive NK cells from MHC I–deficient mice became responsive after transfer to WT mice. Altered responsiveness was evident among mature NK cells that had not divided in the recipient animals, indicating that the cells were mature before transfer and that alterations in activity did not require cell division. Furthermore, the percentages of NK cells expressing KLRG1, CD11b, CD27, and Ly49 receptors specific for H-2b were not markedly altered after transfer. Thus, the functional activity of mature NK cells can be reset when the cells are exposed to a changed MHC environment. These findings have important implications for how NK cell functions may be curtailed or enhanced in the context of disease.
An important role of NK cells is to eliminate cells that extinguish or diminish expression of self-MHC class I molecules, which commonly occurs as a result of viral infection or cellular transformation (Herberman et al., 1975; Kiessling et al., 1975; Biron et al., 1999; Diefenbach and Raulet, 2002). This capacity arises because NK cells express stimulatory and inhibitory receptors that engage ligands on normal cells. The majority of inhibitory receptors belong to the KIR (in human), Ly49 (in mouse), and CD94/NKG2A (both in human and mouse) families and are specific for MHC I molecules (Raulet et al., 1997; Moretta et al., 2001; Lanier, 2005). When an NK cell encounters a normal cell, engagement of the inhibitory receptors conveys signals that counteract stimulatory signaling. Lysis occurs when inhibition is lost because the target cell lacks one or more self-MHC molecules or when target cells express high levels of stimulatory ligands that override inhibition (Raulet and Vance, 2006).
NK cells vary in the number and specificity of MHC-specific inhibitory receptors that they express (Raulet et al., 1997). Recent studies demonstrate that NK cells vary in basal responsiveness to stimulatory receptor engagement depending on the number of expressed inhibitory receptors specific for self-MHC molecules (Yu et al., 2007; Brodin et al., 2009; Joncker et al., 2009). Cells with several self-MHC–specific receptors exhibit the greatest basal responsiveness and thus mediate the greatest activity against target cells that lose MHC. Cells with no self-MHC–specific receptors are the most hyporesponsive, to the extent that they fail to attack otherwise normal cells lacking MHC molecules. These data suggest that the responsiveness set point of individual NK cells is tuned depending on the balance of inhibitory and stimulatory ligands that each NK cell encounters on neighboring cells in the normal environment (Joncker and Raulet, 2008).
An important unanswered question is whether the basal responsiveness of NK cells is set only once during NK cell development or, alternatively, can be readjusted when the fully mature NK cell is exposed to a changing MHC environment. Readjustments of NK cell responsiveness, if they occur, may account for instances under which NK cells fail to eliminate tumors or pathogen-infected cells and will be important to address when testing therapies designed to augment or suppress NK cell activity in the context of disease.
RESULTS AND DISCUSSION
Splenic NK cells reset their functional potential downward when transferred to MHC I–deficient mice
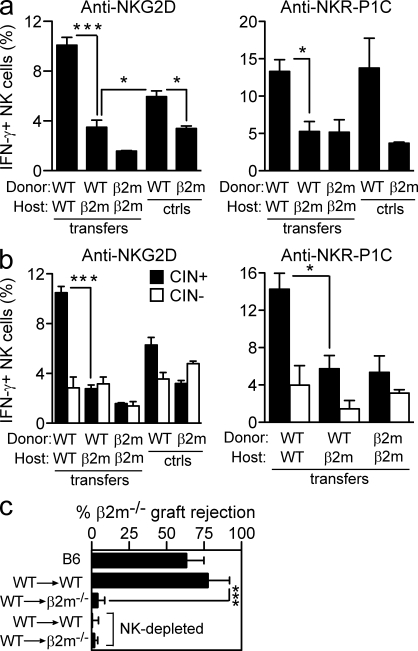
We used an adoptive transfer system to test whether the responsiveness of NK cells can be reset after the NK cells reach maturity. Splenocytes from C57BL/6 mice, containing a majority of responsive NK cells (Fernandez et al., 2005; Joncker et al., 2009), were transferred to irradiated congenic MHC I–deficient or control WT mice, and responsiveness was tested 10 d later. To test responsiveness, splenocytes from the recipients were stimulated in vitro with plate-bound antibodies specific for the stimulatory receptors NKG2D or NKR-P1C. Intracellular IFN-γ production by donor NK cells was determined by gating on the congenic CD45 marker. The results demonstrated a striking hyporesponsiveness of WT NK cells to NKG2D and NKR-P1C stimulation after transfer to MHC I–deficient hosts, comparable with that of MHC I–deficient NK cells after transfer to MHC I–deficient hosts (Fig. 1 a). Hyporesponsiveness arose from exposure to the MHC-deficient environment because the same NK cells transferred to WT mice exhibited strong responsiveness (Fig. 1 a and not depicted). Time course experiments demonstrated that some resetting occurred as little as 4 d after transfer but required at least 7–10 d to be fully established (unpublished data).
Figure 1.
Responsive NK cells reset their functional potential downward in the absence of MHC I contact. Responses of WT NK cells after transfer to WT or β2m-deficient hosts. (a and b) 10 d after transfer, splenic NK cells were stimulated for 5 h with 5 µg/ml of plate-bound NKG2D or 50 µg/ml NKR-P1C antibody as indicated. The percentage of IFN-γ–producing NK cells among gated donor NK cells (a) or gated donor NK cells expressing one or more of Ly49C, Ly49I, and/or CD94/NKG2A (CIN+) or lacking those three receptors (CIN−; b) was determined by flow cytometry. Responses of NK cells from unmanipulated WT and β2m−/− mice are shown for comparison. Experiments were repeated five to six times with n = 3–5 each for transfer groups. For relevant comparisons, statistical significance was determined with a two-tailed unpaired (a) or paired (b) Student’s t test (*, P < 0.05; ***, P < 0.0005). ctrls, controls. (c) At day 12 after transfer, groups of adoptive transfer recipients (n = 3–4) received grafts of CFSEhigh-labeled β2m−/− spleen cells mixed with an equal number of CFSElow-labeled WT spleen cells. Rejection of β2m−/− target cells was determined by flow cytometry of spleen cells 18 h later. Some groups of transfer recipients were pretreated i.p. twice (on days −2 and −1 relative to the time of engraftment) with 200 µg PK136 (NK1.1) antibody to deplete NK cells, as indicated. The experiment was repeated three times with n = 3–6 mice for each (***, P = 0.0002). Data represent means ± SEM.
In WT B6 mice, the responsive NK cells express one or more of the three receptors that bind appreciably to H-2b class I molecules, Ly49C, Ly49I, and/or CD94/NKG2A (CIN+ NK cells; Fernandez et al., 2005; Joncker et al., 2009). NK cells lacking these three receptors (CIN− NK cells) are largely hyporesponsive in B6 mice. The alterations in NK cell responsiveness after transfer occurred for the most part in the CIN+ NK cell population, as expected (Fig. 1 b). The CIN− population was relatively hyporesponsive after transfer in all combinations tested, and the differences in CIN− cells responsiveness were neither statistically significant nor reproducible (unpublished data).
Resetting of NK cell function after transfer to MHC I–deficient mice was also evident in an in vivo assay of NK cell function. 12 d after transfer of WT splenocytes to irradiated WT or MHC I–deficient mice, we tested the capacity of the mice to reject spleen cell grafts from MHC I–deficient mice (Fig. 1 c). Only minimal rejection occurred in irradiated WT control mice that did not receive a cell transfer (unpublished data). When WT recipients were restored with WT spleen cells, strong rejection occurred. The rejection was mediated by NK cells, as shown by depleting NK cells before engraftment. In contrast, MHC I–deficient recipients that were restored with WT NK cells failed to reject MHC I–deficient grafts. An absence of NK cells could not account for the failure to reject the grafts because these recipients contained as many donor NK cells as did WT recipients of WT NK cells (unpublished data).
Hyporesponsive NK cells reset their functional potential upwards when exposed to MHC I+ cells
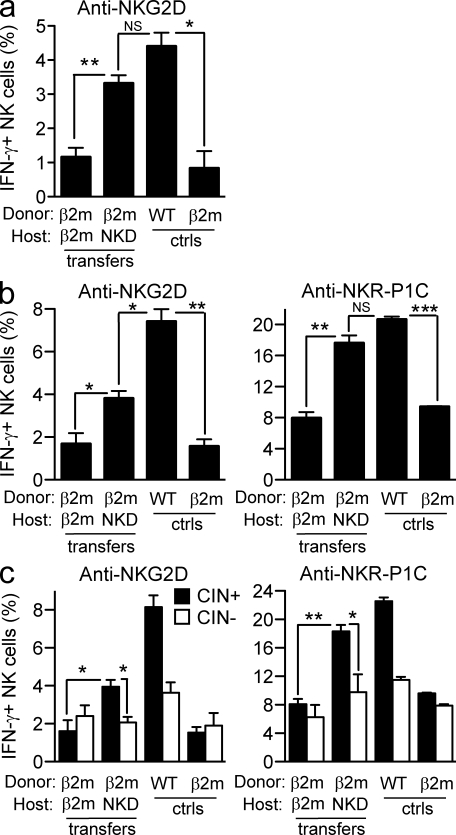
Having found that exposure of WT NK cells to an MHC I–deficient environment renders the NK cell population hyporesponsive, we asked whether the reverse was true, i.e., whether exposure of MHC I–deficient NK cells to an MHC I+ environment increases the responsiveness of the NK cells. MHC I–deficient splenocytes could not be transferred into WT hosts because the host’s NK cells rejected the donor cells. Therefore, we used an NK cell–deficient strain (NKD mice; Kim et al., 2000) as recipients. When MHC I–deficient splenocytes were transferred to irradiated NKD (MHC I+) mice for 10 d, the responsiveness of the NK cells was significantly enhanced compared with the responsiveness of the same NK cells after transfer to MHC I–deficient hosts (Fig. 2). In some repetitions, the response approached the level observed in WT mice (Fig. 2 a), and in others, it was intermediate between WT and MHC I deficient (Fig. 2 b). Similar results were obtained whether the adoptive transfer hosts were pretreated with polyinosinic-polycytidylic acid (Poly I:C) for 36 h to enhance NK activity (Fig. 2, b and c) or were tested without pretreatment (Fig. 2 a). The increase in responsiveness after transfer to NKD mice was most pronounced in the CIN+ population, whereas the CIN− population was by comparison relatively hyporesponsive (Fig. 2 c). Collectively, the data indicate that the responsiveness of peripheral NK cells can be reset both downward and upward depending on the steady-state MHC environment to which the NK cells are exposed.
Figure 2.
Hyporesponsive NK cells reset their functional potential upwards when exposed to MHC I–expressing cells. (a–c) Responses of β2m−/− NK cells after transfer to MHC I–expressing NK cell–deficient (NKD) or β2m-deficient hosts. Stimulations and assays were performed as in Fig. 1. The percentage of IFN-γ–producing NK cells among gated donor NK cells (a and b) or gated donor CIN+ and CIN− NK cells (c) was determined by flow cytometry. Recipients were either left untreated (a) or treated with Poly I:C (b and c) 36 h before in vitro stimulation to augment the responses. Experiments were repeated four times with n = 3–6 mice each for transfer groups. For relevant comparisons, statistical significance was determined with a two-tailed unpaired (a and b) or paired (c) Student’s t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). Data represent means ± SEM. ctrls, controls.
Resetting occurs in mature NK cells
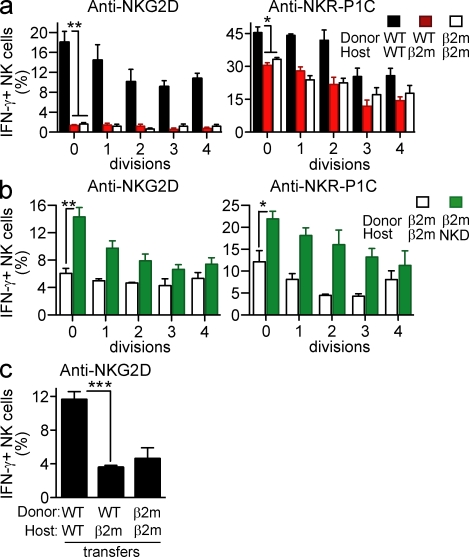
A shorter transfer time of 4 d revealed similar trends in terms of both upward and downward resetting of NK activity, but the differences were not statistically significant, suggesting that >4 d are required for maximal resetting (unpublished data). Although we would not expect extensive NK differentiation from stem cells to occur in ≤10 d, it remained possible that the changes in activity we observed after transfer of spleen cells applied only to new NK cells that differentiated in the adoptive host from donor hematopoietic precursor cells. If this occurred, the new results would not speak to whether the MHC environment influences the activity of mature (as opposed to differentiating) NK cells. To distinguish NK cells that were already mature at the time of transfer from NK cells that differentiated after transfer, we took advantage of the knowledge that NK cell differentiation involves extensive cellular proliferation (Kim et al., 2002). For this analysis, donor spleen cells were labeled with CFSE before transfer, and the extent of CFSE dilution was assessed in NK cells harvested 10 d after transfer. A significant fraction of NK cells (between 10 and 30% in different experiments) had not divided 10 d after transfer (unpublished data). By gating on NK cells that had not divided after transfer, we observed the same patterns of responsiveness that we had observed in the bulk populations: WT (or MHC I deficient) NK cells transferred to MHC I–deficient hosts exhibited relatively low responsiveness (Fig. 3 a), whereas MHC I–deficient (or WT) NK cells transferred to MHC I+ hosts exhibited elevated responsiveness (Fig. 3 b). These data argue that the changes in NK responsiveness after transfer occur in nondividing mature NK cells, as opposed to representing the activity of newly differentiated NK cells that arose after transfer. In general, NK cells in all groups that had undergone several divisions exhibited lower activity, suggesting that many of them corresponded to NK cells that differentiated after transfer from precursor cells but had yet to attain full maturity (Fig. 3).
Figure 3.
Functional resetting occurs even in NK cells that do not divide after transfer. (a) CFSE-labeled WT splenocytes were transferred to irradiated WT or β2m-deficient hosts. (b) CFSE-labeled β2m−/− splenocytes were transferred to irradiated β2m−/− or MHC I–expressing NK cell–deficient (NKD) hosts. 10 d after transfer, NK cells were stimulated and assayed as in Fig. 1, except that the donor cells were gated based on CFSE level, distinguishing cells that had divided 0, 1, 2, 3, or 4 times after initial labeling. Experiments were repeated two to four times with n = 3–6 mice for each. (c) WT splenocytes were transferred to unirradiated, CD8a- and CD4-depleted WT, or β2m-deficient hosts. 9 d after transfer, NK cells were stimulated and assayed as in Fig. 1. The experiment was performed twice with n = 3–5 mice. For some comparisons, statistical significance was determined with a two-tailed unpaired Student’s t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). Data represent means ± SEM.
The experiments to this point were performed in irradiated hosts, so an additional analysis was performed to determine whether downward NK cell resetting occurs after transfer to unirradiated hosts. Preliminary experiments showed that unirradiated MHC I–deficient hosts reject WT hematopoietic cells (unpublished data). The rejection was likely caused in part by the action of rare CD8+ CTLs in such hosts, which were shown, using in vitro experiments, to be amplified after challenge with MHC I+ cells (Glas et al., 1994). However, we determined that it was necessary to deplete the hosts of both CD8+ and CD4+ T cells before transfer to prevent rejection of MHC I+ cells (unpublished data), possibly reflecting the action of MHC I–specific antibodies in the rejection process. Therefore, in the subsequent experiments, the hosts were depleted of both CD8+ and CD4+ T cells before transfer and the cells were parked for a slightly shorter time period before analysis. WT cells transferred to unirradiated MHC I–deficient mice were rendered hyporesponsive to NKG2D stimulation, similar to our findings in irradiated mice (Fig. 3 c). In unirradiated hosts, the donor NK cells underwent little or no homeostatic proliferation, as expected (unpublished data). Thus, downward resetting does not depend on the use of irradiated hosts. We were unable to perform the converse experiment (upward resetting after transfer of MHC I–deficient cells to unirradiated WT mice) because of rejection of donor cells by residual NK cells in unirradiated NKD mice.
NK subset composition after transfer
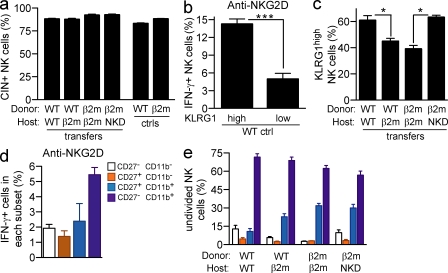
To determine whether functional resetting correlated with changes in subset composition after transfer, we determined the phenotypes of gated CFSEhigh NK cells, which we infer correspond to transferred NK cells rather than NK cells that differentiated from precursor cells after transfer. Because CIN+ and CIN− NK cells correspond to responsive and hyporesponsive NK cells in normal B6 mice (Fernandez et al., 2005), we determined whether the proportions of these subsets changed after transfer. Analysis showed that the proportions of CIN+ and CIN− NK cells among total NK cells or among those that did not divide after transfer did not change significantly in the various transfer combinations (Fig. 4 a). As already emphasized, the changes in responsiveness after transfer can be accounted for by changes in the responsiveness of CIN+ NK cells after transfer (Figs. 1 and 2).
Figure 4.
Phenotypic changes associated with resetting. (a) 10 d after transfer of CFSE-labeled splenocytes, the frequencies of CIN+ and CIN− NK cells were determined among gated donor NK cells that remained undivided after transfer by staining with Ly49C, Ly49I, and NKG2A antibodies. (b) IFN-γ response of KLRG1high and KLRG1low WT NK cells to stimulation with 5 µg/ml plate-bound NKG2D antibody. (c) Percentages of KLRG1high cells among undivided donor NK cells at day 10 after transfer. (d) IFN-γ response of CD27− CD11b−, CD27+ CD11b−, CD27+ CD11b+, and CD27− CD11b+ NK cell subsets from WT mice upon stimulation with 5 µg/ml anti-NKG2D. (e) Distribution of undivided donor NK cells among the CD27/CD11b-defined subsets at day 10 after transfer. Asterisks indicate a statistically significant difference as calculated by two-tailed paired (b) or unpaired (c) Student’s t test (*, P < 0.05; ***, P < 0.0005). Experiments were performed two to four times with n = 3–6 mice, except one repeat of panel d where n = 2 mice. Data represent means ± SEM.
KLRG1 is expressed by more WT than β2-microglobulin−/− (β2m−/−) NK cells and by more CIN+ than CIN− NK cells in WT mice (Corral et al., 2000; Fernandez et al., 2005) and thus correlates with the responsive subset. Direct assays confirmed that in normal B6 mice, KLRG1high NK cells reproducibly responded better to antireceptor antibodies than KLRG1low NK cells (Fig. 4 b). Interestingly, WT NK cells that had been transferred to MHC I–deficient hosts exhibited a modestly reduced percentage of KLRG1high NK cells, compared with WT NK cells that had been transferred to WT hosts (Fig. 4 c). Conversely, MHC I–deficient NK cells that had been transferred to MHC I+ hosts exhibited a modestly increased percentage of KLRG1high NK cells compared with MHC I–deficient NK cells recovered from MHC I–deficient hosts. However, these differences were small compared with the functional differences we observed (Figs. 1 and 2), suggesting that resetting results in changes in both the composition of NK subsets and their functional activity.
Expression of the CD11b and CD27 markers define four NK subsets, labeled R0 (CD27− CD11b−), R1 (CD27+ CD11b−), R2 (CD27+ CD11b+), and R3 (CD27− CD11b+). The R3 population includes somewhat higher percentages of Ly49+ and KLRG1high NK cells and greater expression of genes involved in NK effector functions (Hayakawa and Smyth, 2006; Chiossone et al., 2009; unpublished data). Consistent with these features, but in contrast to the findings of others (Hayakawa and Smyth, 2006), we consistently observed that R3 NK cells were more responsive than R2 NK cells, or in some cases roughly equal in responsiveness (Fig. 4 d and not depicted). R1 and R0 cells showed substantially less functional activity. Although there were modest changes in the percentages of R2 and R3 cells when comparing the various transfer combinations, the changes did not provide an adequate explanation for the functional differences we observed (Fig. 4 e).
The responsiveness of developing NK cells is determined by the MHC environment in which they mature
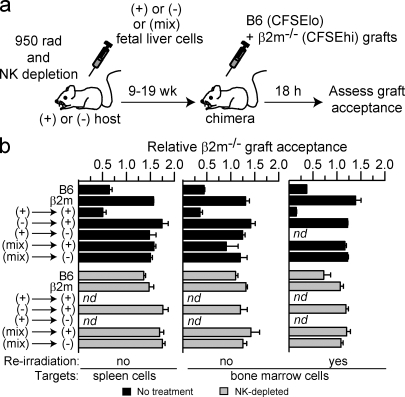
A previous study showed that in stable chimeras in which WT and MHC I–deficient fetal liver precursors codeveloped into NK cells in the same animal, the resulting NK cells were impaired in rejecting MHC I–deficient bone marrow grafts (Wu and Raulet, 1997). Because those experiments were performed with methods that are currently little used, we have repeated these experiments using a modern assay to confirm and extend the results. Fetal liver chimeras were prepared combining MHC I+ and/or MHC I–deficient fetal liver cells and hosts in various combinations (Fig. 5 a). After allowing between 9 and 19 wk for reconstitution, we tested NK function in vivo by determining whether the chimeras could reject spleen cell or bone marrow grafts from MHC I–deficient mice (Fig. 5 b). MHC I+ hosts reconstituted with MHC I+ fetal liver cells ([(+) into (+)] chimeras) rejected the grafts, and the rejection was prevented when NK cells were depleted from the mice before transfer. In contrast, the grafts were accepted by chimeras in which either the host or transferred fetal liver cells contained MHC I–deficient cells, including the [(+) into (−)], [(−) into (+)], [(+) into (−)], [(mix) into (+)], and [(mix) into (−)] chimeras. The outcome was the same whether the MHC I–deficient cells used to test graft rejection were spleen cells or bone marrow cells and whether the hosts were reirradiated or not before applying the test grafts (Fig. 5 b). Analysis showed that the chimeras were generally reconstituted with >95% donor cells and that the mixed chimeras contained between 25 and 50% of donor β2m−/− cells at the time of assay (unpublished data). Collectively, these data indicate that developing NK cells were rendered tolerant to MHC I–deficient cells when the NK cells developed in an environment containing MHC I–deficient cells. Tolerance occurred similarly whether the MHC I–deficient cells were derived from radioresistant host cells or donor fetal liver cells.
Figure 5.
The responsiveness of developing NK cells is set depending on the MHC environment in which they mature. (a) Experimental scheme for producing mixed fetal liver cell chimeras and testing rejection of MHC I–deficient cells. WT (+), β2m−/− (−), or a 1:1 mix of WT and β2m−/− (mix) fetal liver cells was used to reconstitute lethally irradiated and NK-depleted hosts. (b) After 9–17 wk of reconstitution, chimeras were reirradiated or not before testing whether the chimeras rejected β2m−/− spleen cells or bone marrow cells, as indicated. Unmanipulated B6 and β2m−/− mice were tested in parallel as controls for rejection and tolerance, respectively. The rejection assay was performed by injecting CFSEhigh-labeled β2m−/− cells (spleen or bone marrow) mixed with an equal number of CFSElow-labeled WT spleen cells as an internal control. Rejection of β2m−/− spleen cells was determined by flow cytometry of spleen cells 18 h later. Some groups of chimeras were pretreated i.p. twice (on days −2 and −1) with 200 µg PK136 (NK1.1) antibody (NK depleted), as indicated. Data represent means ± SEM (n = 2–5 mice). The experiment was performed three times with spleen cell targets and once with bone marrow cell targets. nd, not done.
Concluding remarks
Our results indicate that the responsiveness of NK cells remains plastic once the cells reach maturity and can be reset when the cells are exposed to an environment in which MHC expression differs. Resetting was not dependent on NK cell proliferation. Of note, the results contrast with the finding that sustained blockade of inhibitory receptors in vivo with mAb failed to render NK cells hyporesponsive (Sola et al., 2009; Vahlne et al., 2010). The discrepancy may reflect difficulties in maintaining complete blockade of the inhibitory receptors under these conditions.
In the chimera experiments, donor MHC I–deficient cells induced tolerance to MHC I–deficient cells (e.g., in [(−) into (+)] or [(mix) into (+)] chimeras), whereas in the (−) into (+) adoptive transfers, the NK cells were converted to high responsiveness. The two results may reflect the different assays used, but another possibility is that in the chimeras, a specific population of donor MHC I–deficient stromal cell precursors eventually seeded and repopulated niches in the bone marrow or elsewhere. The donor stromal cells may be specialized for inducing NK cell hyporesponsiveness and/or responsiveness. Donor cell repopulation of stromal niches may not occur in the adoptive transfers either because of the short duration of the transfers or because the relevant cells are rare or absent in the spleen compared with the fetal liver. A dominant role of stromal cells in resetting was consistent with the observation that MHC expression, or not, by donor lymphohematopoietic cells in the resetting experiments had little or no impact on the ultimate responsiveness of cotransferred NK cells (Figs. 1–3). The relevant stromal cells may be nonhematopoietic in origin (although present in fetal liver) or, alternatively, represent a specialized subset of hematopoietic cells.
In the adoptive transfers, and in some combinations of the chimeras, the responsiveness of donor NK cells was determined by whether the recipient cells expressed MHC I or not, indicating that trans interactions between NK cell inhibitory receptors and MHC molecules on other cells played an important role in determining the responsiveness of NK cells. It remains possible that cis interactions between the inhibitory receptors and MHC molecules in the same NK cell play a role in determining NK responsiveness in other contexts (Chalifour et al., 2009). Indeed, it is possible that two or more independent mechanisms based on different types of interactions, and possibly operative at different stages of the NK cell lifecycle (developing vs. mature cells), work in parallel to ensure NK cell hyporesponsiveness.
Although not addressed in this study, it is plausible that the resetting of NK responsiveness also occurs in cases in which NK cells are exposed to cells with reduced expression of other inhibitory ligands or increased expression of stimulatory ligands. NK cell responsiveness may also be altered by other environmental cues such as those that accompany infections. For example, an early study showed that Listeria monocytogenes infections of mice cause hyporesponsive NK cells to produce similar amounts of IFN-γ to responsive NK cells (Fernandez et al., 2005). Furthermore, hyporesponsive Ly49C/I− NK cells respond even better than Ly49C/I+ NK cells in cytomegalovirus-infected mice (Orr et al., 2010). Finally, Sun and Lanier (2008) showed that mouse cytomegalovirus infection caused a rapid rejection of MHC I–deficient donor cells in [(mix) into (+)] chimeras.
We propose that the capacity of NK cells to reset their responsiveness represents a mechanism to set the triggering threshold of NK cells to an appropriate level under normal conditions, to prevent autoreactivity, while preserving optimal reactivity to MHC I–deficient or other stimulatory target cells. The results have important implications for understanding NK cell functions in the context of disease. First, the downward resetting of NK cell activity that occurs after transfer to MHC I–deficient mice provides one plausible explanation for how NK cells become inactivated in certain tumors. Multiple studies have identified NK cells in tumor-bearing patients that are anergic or incapable of attacking the tumor cells (Costello et al., 2002; Epling-Burnette et al., 2007). NK cells that are exposed to tumor cells, which frequently extinguish MHC I molecules, may initially lyse the tumor cells, but persistent exposure may cause the NK cells to adopt a hyporesponsive state. Conversely, although NK cells may protect against autoimmunity in some cases (Lünemann et al., 2009), they reportedly contribute to the development of autoimmunity in others (Poirot et al., 2004; Feuerer et al., 2009; Lünemann et al., 2009); the potential for NK cells to reset their responsiveness upwards in a changing environment could play a role in promoting autoimmunity in these instances. A deeper understanding of the signals that mediate these different outcomes will aid in determining the role of resetting in the genesis and prevention of inflammatory disease, malignancy, and infectious disease.
MATERIALS AND METHODS
Mice and adoptive transfers.
Mice were bred at the University of California, Berkeley, from breeders obtained from The Jackson Laboratory (C57BL/6J, designated B6 or WT), Charles River (B6-Ly5.2/Cr, which express Ly5.1), or W. Yokoyama (Washington University in St. Louis, St. Louis, MO; NKD mice). β2m−/− mice on the B6 background were described previously (Zijlstra et al., 1989). B6 β2m−/−–Ly5.1 mice were bred in our facilities. WT or β2m−/− splenocytes were injected i.v. in hosts that had received 6 Gy of irradiation from a 137Cs source 4–5 h earlier. For adoptive transfers in unirradiated mice, recipients were treated i.p. with 200 µg of depleting CD8a and CD4 antibodies the day before transfer and at days 1, 4, and 7 after transfer. Each host received 108 donor splenocytes corresponding to 2.5–3.5 × 106 NK cells. Where indicated, donor NK cells were labeled with CFSE (Invitrogen) before transfer. Animal protocols were approved by the Animal Care and Use Committee at the University of California, Berkeley, and animals were maintained in accordance with the guidelines of the National Institutes of Health.
Radiation chimeras.
Fetal liver chimeras were prepared as described in Wu and Raulet (1997). The recipients received 9.5 Gy for 5 h before injection with 107 liver cells from embryonic day 14–17 donors or 107 of each type for mixed chimeras. WT recipients of MHC I–deficient fetal liver cells were pretreated with 200 µg PK136 antibody 1 and 2 d before irradiation of the mice.
Antibodies and flow cytometry analysis.
Antibodies against NK1.1 (PK136), CD3-ε (145-2C11), NKG2A/C/E (20d5), Ly49I (YLI-90), CD45.1 (A20), CD45.2 (104), IFN-γ (XMG1.2), NKG2D (MI6; Jamieson et al., 2002), NKp46 (29A1.4), KLRG1 (2F1), CD27 (LG.3A10), and CD11b (M1/70) were purchased from eBioscience and BD. Ly49C antibody 4LO3311 (a gift from S. Lemieux, l’Institut national de la recherche scientifique–Institut Armand-Frappier, Laval, Québec, Canada; Gosselin et al., 1997) was purified and conjugated to biotin according to standard methods. Biotin-conjugated mAbs were detected with streptavidin Pacific blue (Invitrogen). Depleting NK1.1 antibody (PK136) was purified from CELLine classical 1000 supernatants (Integra Biosciences). Depleting CD8a (2.43) and CD4 (GK1.5) antibodies were purchased from Bio X Cell. Before staining, cells were preincubated for 20 min with 2.4G2 hybridoma supernatant to block FcγRII/III receptors. Flow cytometry was performed on a cytometer (LSR II; BD), and data were analyzed with the FlowJo software (Tree Star, Inc.).
In vitro NK cell stimulation and IFN-γ assay.
As previously described (Joncker et al., 2009), flat-bottomed, high protein-binding plates (Thermo Fisher Scientific) were coated with NKG2D or NKR-P1C antibodies, and NK cells (106 spleen cells per well) were stimulated therein for 5 h in the presence of 1 µg/ml GolgiPlug (BD) and 1,000 U/ml recombinant human IL-2 (Roche) before staining the cells for intracellular IFN-γ accumulation. In some experiments, as indicated, mice were injected i.p. with 200 µg Poly I:C (Sigma-Aldrich) 1.5 d before harvesting spleens.
CFSE-labeled spleen and bone marrow cell engraftment assay.
Spleen and bone marrow graft rejection assays were performed as previously described (Joncker et al., 2009). Spleen or bone marrow cells from Ly5 congenic β2m−/− were labeled with 10 µM CFSE (CFSEhigh), and corresponding B6 cells were labeled with 1 µM CFSE (CFSElow) for 10 min at 37°C. The two types of cells were mixed 1:1 and injected (5 × 106 each) i.v. into mice that had been irradiated, or not, as indicated, earlier in the day with 9.5 Gy. Some mice were predepleted of NK cells by two i.p. injections of 200 µg PK136 (anti-NK1.1). Recipient spleens cells, harvested 18 h after transplantation, were analyzed for CFSE+ congenic MHC I–deficient and control B6 cells based on the intensity of CFSE staining. Percent graft rejection (Fig. 1 c) was defined as 100 × (1 − [%β2m−/− cells/%B6 cells]) among CFSE+ recipient spleen cells, after normalizing to the mean corresponding rejection obtained when the same cells were injected into β2m−/− recipients, which cannot reject class I–deficient grafts (Bix et al., 1991). Relative β2m−/− graft acceptance (Fig. 5 b) was calculated as the ratio of β2m−/− to B6 cells among CFSE+ recipient spleen cells.
Acknowledgments
L. Zhang, J. Beck, and A. Griffith provided excellent technical support. We thank Dr. W. Yokoyama for NKD mice, H. Nolla and A. Valeros for assistance with flow cytometry, and Dr. R. Vance for helpful comments on the manuscript.
This work was supported by National Institutes of Health grant RO1AI35021 to D.H. Raulet.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- β2m
- β2-microglobulin
- Poly I:C
- polyinosinic-polycytidylic acid
References
- Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189–220 10.1146/annurev.immunol.17.1.189 [DOI] [PubMed] [Google Scholar]
- Bix M., Liao N.S., Zijlstra M., Loring J., Jaenisch R., Raulet D. 1991. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 349:329–331 10.1038/349329a0 [DOI] [PubMed] [Google Scholar]
- Brodin P., Lakshmikanth T., Johansson S., Kärre K., Höglund P. 2009. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 113:2434–2441 10.1182/blood-2008-05-156836 [DOI] [PubMed] [Google Scholar]
- Chalifour A., Scarpellino L., Back J., Brodin P., Devèvre E., Gros F., Lévy F., Leclercq G., Höglund P., Beermann F., Held W. 2009. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 30:337–347 10.1016/j.immuni.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 113:5488–5496 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- Corral L., Hanke T., Vance R.E., Cado D., Raulet D.H. 2000. NK cell expression of the killer cell lectin-like receptor G1 (KLRG1), the mouse homolog of MAFA, is modulated by MHC class I molecules. Eur. J. Immunol. 30:920–930 [DOI] [PubMed] [Google Scholar]
- Costello R.T., Sivori S., Marcenaro E., Lafage-Pochitaloff M., Mozziconacci M.J., Reviron D., Gastaut J.A., Pende D., Olive D., Moretta A. 2002. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 99:3661–3667 10.1182/blood.V99.10.3661 [DOI] [PubMed] [Google Scholar]
- Diefenbach A., Raulet D.H. 2002. The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol. Rev. 188:9–21 10.1034/j.1600-065X.2002.18802.x [DOI] [PubMed] [Google Scholar]
- Epling-Burnette P.K., Bai F., Painter J.S., Rollison D.E., Salih H.R., Krusch M., Zou J., Ku E., Zhong B., Boulware D., et al. 2007. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 109:4816–4824 10.1182/blood-2006-07-035519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N.C., Treiner E., Vance R.E., Jamieson A.M., Lemieux S., Raulet D.H. 2005. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 105:4416–4423 10.1182/blood-2004-08-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M., Shen Y., Littman D.R., Benoist C., Mathis D. 2009. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 31:654–664 10.1016/j.immuni.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas R., Ohlén C., Höglund P., Kärre K. 1994. The CD8+ T cell repertoire in beta 2-microglobulin-deficient mice is biased towards reactivity against self-major histocompatibility class I. J. Exp. Med. 179:661–672 10.1084/jem.179.2.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin P., Lusignan Y., Brennan J., Takei F., Lemieux S. 1997. The NK2.1 receptor is encoded by Ly-49C and its expression is regulated by MHC class I alleles. Int. Immunol. 9:533–540 10.1093/intimm/9.4.533 [DOI] [PubMed] [Google Scholar]
- Hayakawa Y., Smyth M.J. 2006. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176:1517–1524 [DOI] [PubMed] [Google Scholar]
- Herberman R.B., Nunn M.E., Holden H.T., Lavrin D.H. 1975. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer. 16:230–239 10.1002/ijc.2910160205 [DOI] [PubMed] [Google Scholar]
- Jamieson A.M., Diefenbach A., McMahon C.W., Xiong N., Carlyle J.R., Raulet D.H. 2002. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 17:19–29 10.1016/S1074-7613(02)00333-3 [DOI] [PubMed] [Google Scholar]
- Joncker N.T., Raulet D.H. 2008. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol. Rev. 224:85–97 10.1111/j.1600-065X.2008.00658.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncker N.T., Fernandez N.C., Treiner E., Vivier E., Raulet D.H. 2009. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J. Immunol. 182:4572–4580 10.4049/jimmunol.0803900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. 1975. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 5:117–121 10.1002/eji.1830050209 [DOI] [PubMed] [Google Scholar]
- Kim S., Iizuka K., Aguila H.L., Weissman I.L., Yokoyama W.M. 2000. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA. 97:2731–2736 10.1073/pnas.050588297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Iizuka K., Kang H.S., Dokun A., French A.R., Greco S., Yokoyama W.M. 2002. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 3:523–528 10.1038/ni796 [DOI] [PubMed] [Google Scholar]
- Lanier L.L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274 10.1146/annurev.immunol.23.021704.115526 [DOI] [PubMed] [Google Scholar]
- Lünemann A., Lünemann J.D., Münz C. 2009. Regulatory NK-cell functions in inflammation and autoimmunity. Mol. Med. 15:352–358 10.2119/molmed.2009.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Bottino C., Vitale M., Pende D., Cantoni C., Mingari M.C., Biassoni R., Moretta L. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197–223 10.1146/annurev.immunol.19.1.197 [DOI] [PubMed] [Google Scholar]
- Orr M.T., Murphy W.J., Lanier L.L. 2010. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 11:321–327 10.1038/ni.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot L., Benoist C., Mathis D. 2004. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc. Natl. Acad. Sci. USA. 101:8102–8107 10.1073/pnas.0402065101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D.H., Vance R.E. 2006. Self-tolerance of natural killer cells. Nat. Rev. Immunol. 6:520–531 10.1038/nri1863 [DOI] [PubMed] [Google Scholar]
- Raulet D.H., Held W., Correa I., Dorfman J.R., Wu M.-F., Corral L. 1997. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol. Rev. 155:41–52 10.1111/j.1600-065X.1997.tb00938.x [DOI] [PubMed] [Google Scholar]
- Sola C., André P., Lemmers C., Fuseri N., Bonnafous C., Bléry M., Wagtmann N.R., Romagné F., Vivier E., Ugolini S. 2009. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc. Natl. Acad. Sci. USA. 106:12879–12884 10.1073/pnas.0901653106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Lanier L.L. 2008. Cutting edge: viral infection breaks NK cell tolerance to “missing self”. J. Immunol. 181:7453–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlne G., Lindholm K., Meier A., Wickström S., Lakshmikanth T., Brennan F., Wilken M., Nielsen R., Romagné F., Wagtmann N.R., et al. 2010. In vivo tumor cell rejection induced by NK cell inhibitory receptor blockade: maintained tolerance to normal cells even in the presence of IL-2. Eur. J. Immunol. 40:813–823 10.1002/eji.200939755 [DOI] [PubMed] [Google Scholar]
- Wu M.-F., Raulet D.H. 1997. Class I-deficient hemopoietic cells and nonhemopoietic cells dominantly induce unresponsiveness of natural killer cells to class I-deficient bone marrow cell grafts. J. Immunol. 158:1628–1633 [PubMed] [Google Scholar]
- Yu J., Heller G., Chewning J., Kim S., Yokoyama W.M., Hsu K.C. 2007. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J. Immunol. 179:5977–5989 [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. 1989. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 342:435–438 10.1038/342435a0 [DOI] [PubMed] [Google Scholar]