Abstract
Generation of reactive oxygen species (ROS) is a common denominator in many conditions leading to cell death in the cochlea, yet little is known of the cochlea’s endogenous mechanisms involved in preventing oxidative stress and its consequences in the cochlea. We have recently described a corticotropin-releasing factor (CRF) signaling system in the inner ear involved in susceptibility to noise-induced hearing loss. We use biochemical and proteomics assays to define further the role of CRF signaling in the response of cochlear cells to aminoglycoside exposure. We demonstrate that activity via the CRF2 class of receptors protects against aminoglycoside-induced ROS production and activation of cell death pathways. This study suggests for the first time a role for CRF signaling in protecting the cochlea against oxidative stress, and our proteomics data suggest novel mechanisms beyond induction of free radical scavengers that are involved in its protective mechanisms.
Keywords: corticotropin-releasing factor receptors, oxidative stress, mass spectrometry, iTRAQ, cochlea, OC-K3 cells
Numerous external agents, including high-intensity noise and various drugs, damage hair cells and/or support cells of the inner ear (Santi and Duvall, 1978; Rybak, 1986; Huizing and de Groot, 1987; Fredelius et al., 1990). Because mammalian hair cells do not regenerate, cytotoxic stress-induced damage can lead to significant hearing loss. Despite differences in the initial targeted compartments of the inner ear by the interactions of ototoxic drugs and damaging noise, these agents ultimately seem to produce similar cellular pathologies and likely have overlapping mechanisms of action, perhaps including activation of the same cellular signaling pathways, that lead to cell death (Kopke et al., 1999).
Metabolically induced and noise-activated oxidative stress and production of reactive oxygen species (ROS) play a significant role in cochlear injury (Yamane et al., 1995). Given the natural activity state of the inner ear, highly active endogenous defenses against the initiation of oxidative stress/damage must exist, yet little is known of these mechanisms. Indeed, most research to date has focused on mitigating ROS-associated damage, despite the fact that the presence of elevated ROS levels indicates that damage has already taken place. That endogenous mechanisms exist to protect against the earliest phases of cochlear damage is exemplified by the efficacy of sound-conditioning paradigms in attenuating both noise-induced and drug-induced hearing loss (Canlon et al., 1988; Campo et al., 1991; Subramaniam et al., 1992; Suryadevara et al., 2009). Two effects induced by sound conditioning suggested as underlying mechanisms by which conditioning protects against cochlear injury are an increase in cochlear antioxidant enzymes (Jacono et al., 1998; Harris et al., 2006; Henderson et al., 2006) and inhibition of apoptosis (Niu et al., 2003). Protective effects of sound conditioning have been reported to last for as long as 60 days (McFadden et al., 1997), suggesting involvement of intracellular signaling pathways. Because exposure to initially nondamaging noise has been shown to result nonetheless in significant functional defects with aging (Kujawa and Liberman, 2006) and because sound conditioning has also been shown to be effective against temporary hearing threshold shifts in humans (Miyakita et al., 1992), a clearer and more detailed definition of the endogenous mechanisms involved in cochlear protection is fundamental not only to our understanding of basic cochlear function, but also for designing future interventional strategies designed to lessen the deleterious effects following exposure to intense stimuli.
We recognized that the inner ear is constantly exposed to stress-inducing stimuli, similar to the extent that skin also must cope with such impacts. Therefore, we have modeled our hypotheses concerning the identity of components making up the endogenous stress response system of the inner ear on that of skin. One signaling system expressed in skin that plays a major role in stress-response involves corticotropin-releasing factor (CRF) and its receptors (Slominski et al., 1999, 2000, 2001). We have previously shown that cells of the inner ear also express CRF, urocortin (a CRF-related peptide), and CRF receptors (Vetter et al., 2002). We have also demonstrated a role for one of the CRF receptors, CRF2, in controlling auditory brainstem response (ABR) thresholds and establishing susceptibility to noise-induced permanent threshold shifts of the ABR (Graham et al., 2010). Here we define a role for CRF2 in protection against aminoglycoside-induced ROS generation and its associated activation of cell death pathways. We go on to use a global proteomics approach to begin defining mechanisms that may explain the ability of CRF2 to provide such protection. These preliminary protein data may hold information on potential novel therapeutic targets for future rational drug design useful in combating damage induced by exposure to ototoxic compounds.
MATERIALS AND METHODS
Reagents
All laboratory-grade chemicals were purchased from Sigma (St. Louis, MO) unless otherwise indicated. Reactive oxygen species-detecting dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; DCF) was obtained from Invitrogen (Carlsbad, CA), the micro-BCA protein estimation assay kit from Pierce Inc. (Rockford, IL), fetal bovine serum (FBS) from Hyclone-Thermo-Scientific (Logan UT), and Dulbecco’s modified Eagle’s medium (DMEM) cell culture medium from Gibco/Invitrogen. The caspase-3 assay kit and SOD assay kit were obtained from BioVision.
Cell Culture
The immortalized organ of Corti cell line (OC-K3) used throughout the experiments was a kind gift from Dr. F. Kalinec (Kalinec et al., 1999, 2003). The cells were initially grown in 250-ml T-neck Falcon flasks. Media consisted of 10 ml DMEM supplemented with 10% fetal bovine serum (FBS) and 50 U/ml recombinant interferon-γ (Sigma) per flask. Cultures were maintained at 33°C, 10% CO2 for proliferative (permissive) conditions. When 80–90% confluent, cells were transferred to differentiation conditions. The proliferative culture medium was replaced with DMEM supplemented with 10% FBS without interferon-γ and moved to 39°C, 5% CO2. Cells were maintained under differentiation conditions for 10 days prior to performing all experiments. No antibiotics were used during cell culturing to avoid potential elevation of antibiotic signaling prior to the evaluation stages of the experiments.
Cellular Signaling Assays
Measurement of ROS levels
To measure ROS levels, OC-K3 cells were grown in six-well plates and allowed to differentiate for 10 days. To perform the experiment, differentiation medium was replaced with serum-free DMEM and stimulated with H2O2 (100 µM), urocortin II (UcnII) peptide (100 nM), gentamicin (200 µM), or vehicle control for 1 hr. To determine whether UcnII could protect against ROS-activating insults, the cells were first preincubated with UcnII (100 nM) for 1 hr, followed by either H2O2 (100 µM) or gentamicin (200 µM) for 1 hr. The cells were washed twice with ice-cold phosphate-buffered saline (PBS) and incubated at 39°C for 30 min in the presence of 10 µM DCF, prepared in PBS. Cells were then washed twice with PBS and scraped into 300 µl of 1% Triton X-100 prepared in PBS. The cells were kept on ice for 30 min and homogenized by trituration using a 27-gauge needle coupled to a 1-ml syringe. Homogenates were centrifuged at 12,000g for 15 min at 4°C, the supernatant collected, and total protein estimation was performed by using a micro-BCA kit (Pierce Inc.). To measure ROS accumulation, 50 µl of cell suspension was diluted to 500 µl with PBS, and any resultant fluorescence level was measured in triplicate for each experiment using a Perkin Elmer luminescence spectrometer LS50B (Perkin Elmer, Waltham, MA) at an excitation wavelength of 485 nm and an emission wavelength of 522 nm. As a blank control, 50 µl of 1% Triton X-100 in PBS was used in place of cell extract. DCF fluorescence intensity was obtained and expressed as percentage of control (blank) fluorescence per milligram protein. As a negative control, cells were incubated with PBS or DMSO, and we followed the same procedure to measure the ROS level. The experiments were repeated three times, and the average and SEM were calculated and plotted in Prism 4.0 (GraphPad Software, San Diego, CA).
Superoxide dismutase (SOD) assay
SOD activity was measured by using an SOD Assay Kit according to the manufacturer’s protocol (BioVision). Briefly, OC-K3 cells were grown in six-well plates and differentiated for 10 days. Medium was replaced with serum-free DMEM and stimulated with or without UcnII peptide (100 nM) and gentamicin (200 µM) for 1 hr. After drug treatment, cells were washed twice with ice-cold PBS and scraped into ice-cold 0.5% Triton X-100 prepared in PBS. The cells were kept on ice for 30 min and homogenized by trituration with a 27-gauge needle coupled to a 1-ml syringe. Homogenates were centrifuged at 12,000g for 15 min at 4°C, the supernatant was collected, and total protein estimation was performed by using a micro-BCA kit. (Pierce Inc.). Next, 40 µg protein lysate was loaded into a 96-well microplate and appropriate buffer and enzyme solution added, followed by incubation at 37°C for 30 min. Absorbance was read at 450 nm using a Bio-Rad microplate reader. The experiments were repeated three times, and the average and SEM were calculated. The inhibition rate of SOD on WST-1 dye formation was expressed as SOD activity per milligram protein.
Caspase-3 activity measurement
Activation of caspase-3 was evaluated in OC-K3 cell lysates using a Caspase-3 Fluorometric Assay Kit, following the manufacturer’s recommendations (BioVision). Briefly, OC-K3 cells were maintained under differentiation conditions for 10 days. Prior to the experiment, cultures were serum starved for 2 hr, prestimulated with UcnII for 5 min, and then challenged with 200 µM gentamicin for 1 hr. The cells from each condition were washed twice with ice-cold PBS and scraped into 200 µl ice-cold lysis buffer. The cells were homogenized by trituration through a 27-gauge needle and 1-ml syringe. The homogenates were centrifuged at 12,000g for 15 min at 4°C, the supernatant was collected, and total protein estimation was performed by using a micro-BCA kit. (Pierce Inc.). Caspase-3 activity was performed with 100 µg protein per assay. Fold increase in caspase-3 activity was determined by comparing fluorescence of 7-amino-4-trifluoromethyl coumarin in control and drug-treated cell lysates using a Perkin Elmer luminescence spectrometer LS50B at an excitation wavelength of 400 nm and an emission wavelength of 505 nm. The caspase-3 activity was expressed per milligram protein, and relative activity for treated samples was expressed as percentage relative to blank control. The experiments were repeated three times, and average and SEM of the three experiments were calculated.
Peptide Labeling Using Isobaric Tagging for Relative and Absolute Quantification Techniques and LC-MS/MS Analysis
Cell culture
OC-K3 cells were initially grown in 250-ml T-neck flasks (Falcon) consisting of 10 ml DMEM supplemented with 10% FBS and 50 U/ml recombinant interferon-γ (Sigma). Cultures were maintained at 33°C, 10% CO2 for proliferative (permissive) conditions. When 80–90% confluent, the cells were differentiated. The culture medium was replaced with DMEM supplemented with 10% FBS without interferon-γ and moved to 39°C, 5% CO2. After 10 days, plates were assigned to one of four conditions: baseline, no stimulation; gentamicin (200 µM) stimulation for 1 hr; UcnII (100 nM) stimulation for 1 hr; or prestimulation for 1 hr with 100 nM UcnII, followed by a wash and a sequential challenge with 200 µM gentamicin for 1 hr. Cells were washed twice with ice-cold PBS and scraped into mammalian cell lysis buffer (T-Per; Pierce), without adding any protease inhibitors. The cells were homogenized by triturating through a 27-gauge needle and 1-ml syringe. The homogenates were centrifuged at 12,000g for 15 min at 4°C, supernatant collected, and the protein estimation performed by using a micro-BCA kit (Pierce Inc.).
Protein precipitation for the isobaric tagging for relative and absolute quantification protocol
Isobaric tagging for relative and absolute quantification (iTRAQ) protein labeling and mass spectrometry were performed at the University of Victoria Proteomics Centre (Victoria, British Columbia, Canada). One hundred micrograms of protein was precipitated by adding 9 volumes of ice-cold acetone and incubating overnight at 4°C. After removal of the acetone, the protein pellet was resuspended in 30 µl of 0.5 M triethyl ammonium bicarbonate.
iTRAQ labeling
Numerous methods have evolved to label proteins for quantitative proteomics analysis. One of the methods, iTRAQ, is well suited for analysis of protein expression changes over discrete time intervals. Briefly, peptides are labeled with isobaric reagents carrying reporters of differing molecular weights. In the system used here, four reagents are used that show up in the mass spectrometer as peaks at 114, 115, 116, or 117 Da and hence are simply termed reagents 114, 115, 116, and 117 (for further description see Vetter et al., 2009). Samples undergoing the different treatments (no treatment control, gentamicin treatment, urocortin II treatment, and urocortin pretreatment followed by gentamicin challenge) can then be labeled with one of the reporter tags to differentiate protein expression levels for each condition (see below for assignments). Labeling of protein lysates for all conditions was performed using the iTRAQ labeling kit (Applied Biosystems) and following the manufacturer’s recommendations (see also Vetter et al., 2009). Briefly, the samples were each denatured using a final concentration of 0.2% SDS and cysteines were reduced with 2 µl 50 mM TCEP. Tubes were held at 60°C for 1 hr and spun briefly, and 1 µl iodoacetamide was added to block cysteines. Finally, samples were trypsinized at 37°C overnight.
On the following day, the digested samples were labeled with the iTRAQ reagents, following the manufacturer’s protocol such that the control, unstimulated sample was combined with reagent 114, gentamicin-stimulated sample was combined with reagent 115, UcnII-stimulated sample was combined with reagent 116, and the UcnII-pretreated gentamicin-challenged sample was combined with reagent 117. Labeling proceeded at room temperature for 1 hr. Labeled samples were then combined in a 1:1:1:1 ratio in a fresh Eppendorf tube.
Strong cation exchange (SCX) HPLC
A Vision Workstation (Applied Biosystems, Foster City, CA) was equipped with a polysulfoethyl A (poly-LC) 100 mm × 4.6 mm, 5-µm, 300-Å SCX column. Buffer A was 10 mM KPO4 (pH 2.7), 25%ACN. Buffer B was 10 mM KH2PO4, 25%ACN, 0.5 M KCl. The flow rate was set to 0.5 ml/min.
Samples were brought up to 2 ml with buffer A and injected onto the column. The column was allowed to equilibrate for 20 min in buffer A before a gradient was applied, 0–35% B in 30 min. Fractions were collected every minute after injection. The collected fractions were then reduced in volume in a Speed-Vac (Savant Instruments) and transferred to autosampler vials (LC Packings)
LC-MS/MS materials and methods
LC-MS/MS analysis was performed with an integrated Famos autosampler, Switchos-II switching pump, and UltiMate micropump (LC Packings) system with a hybrid quadrupole-time of flight (TOF) LC/MS/MS mass spectrometer (QStar Pulsar i) equipped with a nanoelectrospray ionization source (Proxeon) and fitted with a 10-lm fused-silica emitter tip (New Objective). Chromatographic separation was achieved on a 75 µm × 15 cm C18 PepMap Nano LC column (3 µm, 100 Å; LC Packings), and a 300 µm × 5 mm C18 PepMap guard column (5 µm, 100 Å; LC Packings) was in place before switching inline with the analytical column and the mass spectrometer (MS). The mobile phase (solvent A) consisted of water/acetonitrile (98:2 v/v) with 0.05% formic acid (FA) for sample injection and equilibration on the guard column at a flow rate of 100 µl/min. A linear gradient was created upon switching the trapping column inline by mixing with solvent B, which consisted of acetonitrile/water (98:2 v/v) with 0.05% formic acid, and the flow rate was reduced to 200 nl/min for high-resolution chromatography and introduction into the mass spectrometer.
LC-MS/MS procedure
Samples were brought up to 20 µl with 5% ACN and 3% FA and transferred to autosampler vials (LC Packings). Ten microliters of sample was injected in 95% solvent A and allowed to equilibrate on the trapping column for 10 min to wash away any contaminants. Upon switching inline with the MS, a linear gradient from 95% to 40% solvent A was developed for 40 min, and in the following 5 min the composition of the mobile phase was increased to 20% A, before decreasing to 95% A for a 15 min equilibration before the next sample injection. MS data were acquired automatically in Analyst QS 1.0 software (Service Pack 8; ABI MDS SCIEX). An information-dependent acquisition method consisted of a 1-sec TOF-MS survey scan of mass range 400–1,200 amu and two 2.5-sec product ion scans of mass range 100–1,500 amu. The two most intense peaks over 20 counts with charge states 2–5 were selected for fragmentation, and a 6-amu window was used to prevent the peaks from the same isotopic cluster from being fragmented again. Once an ion was selected for MS/MS fragmentation, it was put on an exclude list for 180 sec. Curtain gas was set at 23; nitrogen was used as the collision gas, and the ionization tip voltage used was 2,700 V. If the observed A215 was greater than 0.1 for any fraction collected during the SCX, a 2.5-hr gradient (95–50% solvent A) was used to compensate for the higher peptide concentration in that fraction.
Data Analysis
Data files were processed offline at Tufts University School of Medicine in ProteinPilot software (v 2.0.1, revision 67476; Applied Biosystems MSD SciEx). Identification of significant expression changes was accomplished by first establishing an Unused ProtScore (confidence level) cutoff of 1.3 (this metric establishes the number of peptides of any spectrum that have not already been used by a higher scoring, “winning” protein; the greater number of unused peptides, the greater the confidence in the protein identification), thereby filtering the data set for detected proteins with a 95% or greater confidence ID call. This first filter ensured that all proteins examined further were identified with a high level of confidence. Next, this filtered data set was again filtered by ratio change of any reaction ratio, with proteins that attained a 1.2 ratio or higher or a 0.8 ratio or lower arbitrarily considered to be proteins of potential interest. Finally, the data set was again filtered by P value of the reaction ratios (relative to the mean ratio change of the entire data set), with a cutoff first of 0.05 to establish the protein ID list and next with a cutoff of 0.01 to establish proteins that were examined further for functional classification. After these steps, only the most stringently identified proteins of highest statistical significance in terms of expression level changes were considered in this analysis. A Venn diagram was drawn using the Venny software (Oliveros, 2007) with the statistically significant proteins as input for each condition. R statistical framework was used to cluster differentially expressed proteins that met the highest stringency conditions to generate a heat map of functional clusters, color coded according to their row z-scores. Heat maps were created using the heatmap.2 function in the gplots package.
RESULTS
CRF2 Activation Protects Against Cytotoxic Effects of Aminoglycoside Exposure
ROS formation is decreased by activation of CRF2
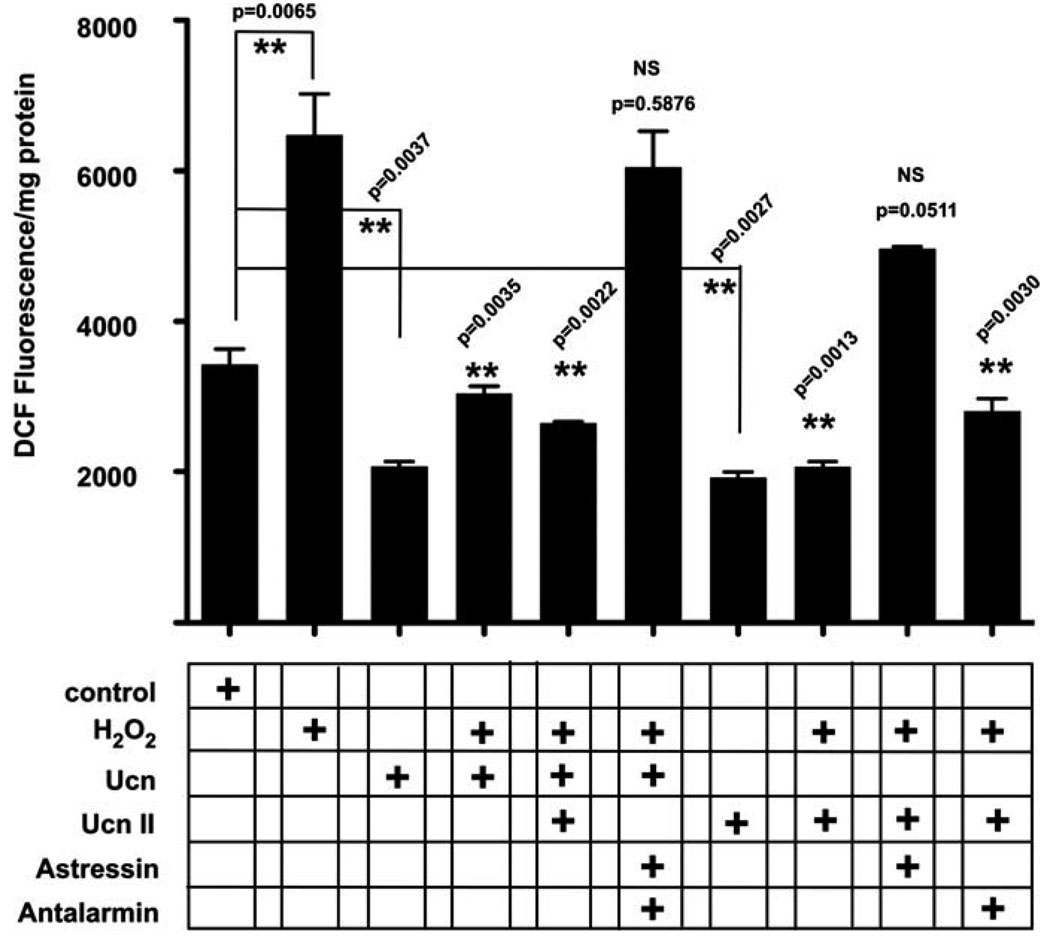
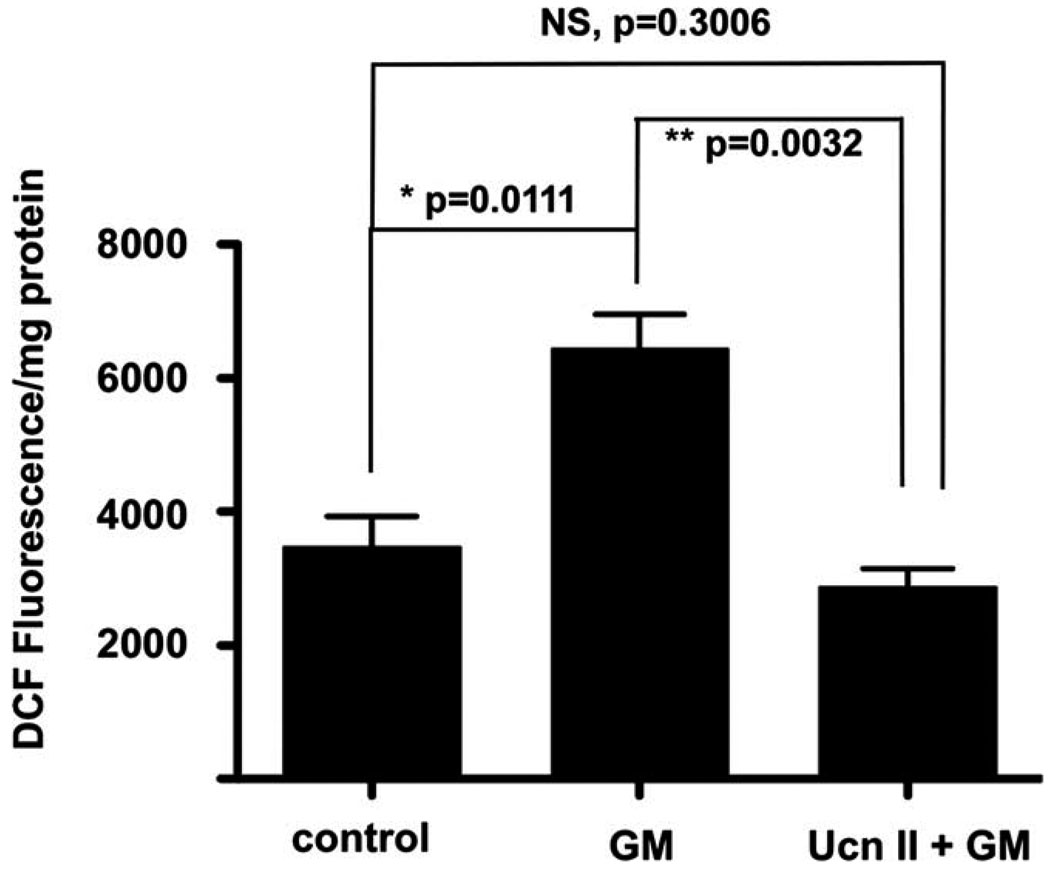
ROS formation was initiated in OC-K3 cells by a 1-hr exposure to either 100 µM H2O2 (Fig. 1) or 200 µM of the aminoglycoside gentamicin (Fig. 2). ROS detection and quantification were performed using H2DCFDA dye (DCF) by monitoring fluorescence intensity. Baseline measures of unstimulated samples served as control. Both H2O2 (Fig. 1) and gentamicin (Fig. 2) increased DCF intensity approximately two-fold over baseline measures. Ucn (100 nM), which is maximally active at CRF2 but has some efficacy also at CRF1, and UcnII (100 nM), which is active only at CRF2, significantly decreased basal DCF signal (P = 0.037, P = 0.0027, respectively; Fig. 1). Pretreatment for 1 hr with either Ucn or UcnII prior to challenge with H2O2 (Fig. 1) produced a significant block of H2O2-induced DCF signal (Ucn, P = 0.0035; UcnII, P = 0.0013). Simultaneous application of the CRF1 and CRF2 antagonists antalarmin and astressin2B, respectively, prior to H2O2 application blocked the protective effect of Ucn, whereas application of astressin2B alone was sufficient to block the protective effects of UcnII application on H2O2-induced DCF fluorescence increase. Antalarmin did not block the protection offered by UcnII against H2O2-induced ROS formation (DCF fluorescence; Fig. 1). In a manner similar to protection against H2O2, UcnII preapplication 1 hr prior to gentamicin challenge protected the cells from gentamicin-induced DCF increase (P = 0.0032; Fig. 2).
Fig. 1.
CRF-related peptides protect OC-K3 cells from H2O2-induced ROS formation. Reactive oxygen species formation was monitored by using DCF fluorescence and expressed as fluorescent signal per milligram protein. H2O2 induced a significant increase in fluorescence, indicating a spike in ROS formation. Urocortin (Ucn) pretreatment protected against H2O2-induced ROS formation (fourth bar), as did UcnII (eighth bar). Antalarmin, a CRF1 antagonist, was ineffective in blocking the effect of UcnII on its own (last bar). However, astressin2B, a CRF2 antagonist, completely blocked the protective effects of UcnII, even when Ucn was added to the CRF2 activation cocktail. Interestingly, both Ucn and UcnII also demonstrated a significant decrease in basal ROS levels (third and seventh bars, respectively). **P < 0.01.
Fig. 2.
UcnII protects against gentamicin-induced ROS formation. Gentamicin, an aminoglycoside and well-known ototoxic agent, induces significant ROS generation over background (second bar). Pretreat-ment for 1 hr with UcnII, which specifically activates CRF2, protects cells against gentamicin-induced ROS formation (third bar), by normalizing the ROS levels to control values. *P < 0.05, **P < 0.01.
SOD activity is increased by stimulation of CRF2
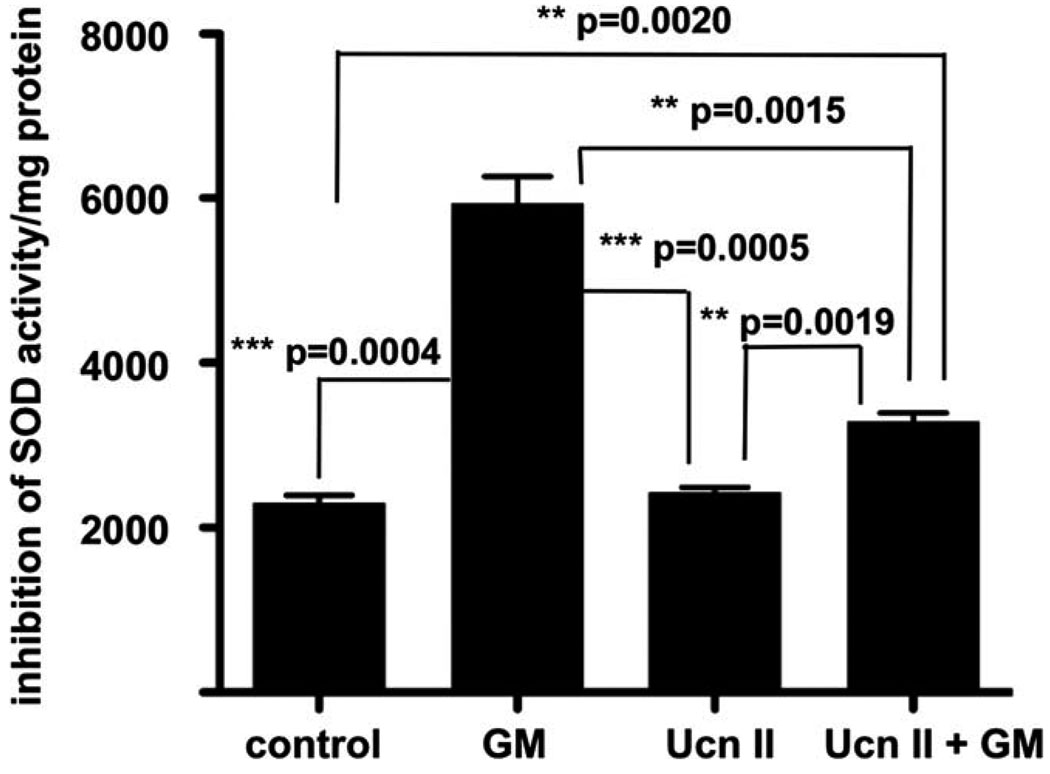
The SOD activity assay performed measures SOD-induced inhibition of soluble dye formation as a result of WST-1 reduction following superoxide anion formation. Dye formation is linearly related to the ability of SOD to catalyze dismutation of superoxide anions to O2 and H2O2 and therefore is indicative of the amount of SOD present. Thus, rising levels of SOD will inhibit dye formation, whereas lower SOD levels result in enhanced dye formation. Exposure to 200 µM gentamicin inhibited SOD activity approximately three times over basal activity (P = 0.0004; Fig. 3). Similar to the effects observed for H2O2-induced ROS formation, UcnII pretreatment significantly blocked the gentamicin inhibition of SOD activity (P = 0.0015), although it was not able to return the measure completely to baseline (difference between UcnII + gentamicin and baseline, P = 0.0020). However, the protective effect of pre-exposure to UcnII decreased the SOD inhibition by approximately half (Fig. 3).
Fig. 3.
UcnII protects against gentamicin-induced inhibition of superoxide dismutase (SOD) activity. SOD activity is significantly inhibited upon challenge with gentamicin (second bar). Although UcnII application does not alter baseline SOD activity (third bar), pretreatment with UcnII significantly blocked gentamicin inhibition of SOD activity (thus lessening the formation of WST1 formazan dye), suggesting that the cellular ROS response induced by gentamicin is lessened by CRF2 activity. *P < 0.05, **P < 0.01, ***P < 0.001.
Caspase-3 activity is inhibited by stimulation of CRF2
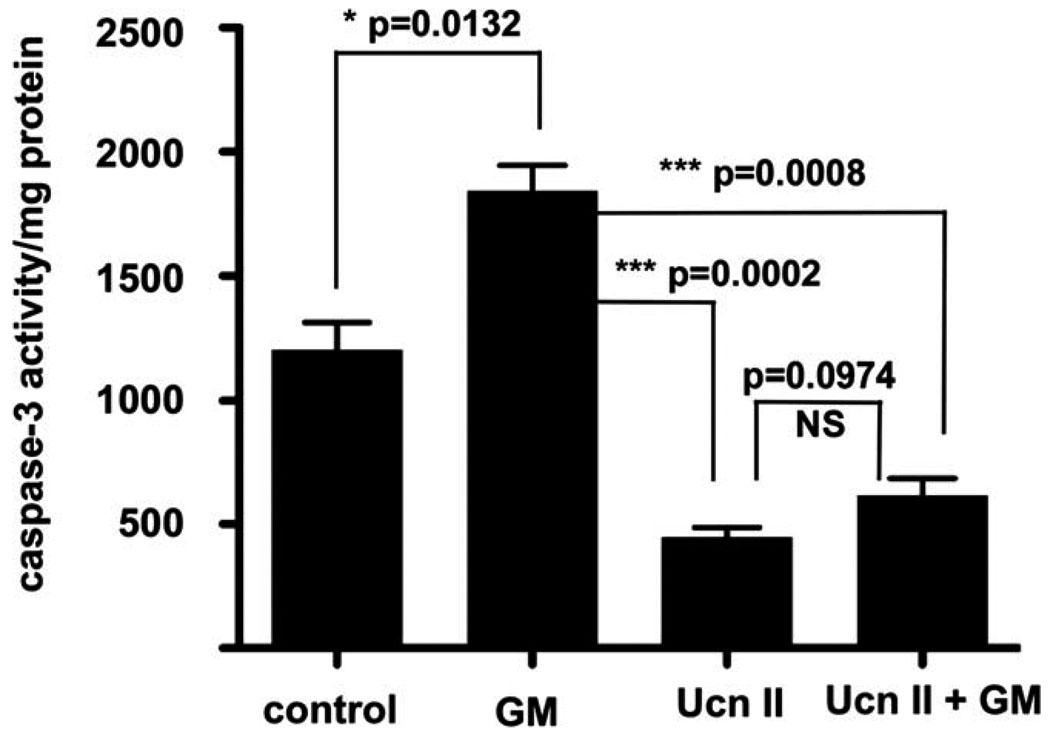
Caspases are intimately involved in apoptotic responses to both extrinsic and intrinsic cell death signals. In the inner ear, caspase-3 has been shown to play a major role in hair cell apoptosis following numerous insults (Van De Water et al., 2004). We therefore examined the ability of CRF2 activity to inhibit caspase-3 activity in a caspase-3 assay. Application of gentamicin increased caspase-3 activity by approximately 53% (P = 0.0132; Fig. 4). However, application of UcnII alone was able to decrease the baseline caspase-3 activity by 63% (P = 0.0028) over untreated cells. UcnII pretreatment similarly protected the cells from a gentamicin-mediated increase in caspase-3 activity (67% decrease; P = 0.0008 vs. gentamicin treatment alone). Thus, UcnII treatment was able to control one of the most important effectors of apoptosis induced in the inner ear significantly.
Fig. 4.
UcnII induces lower basal caspase-3 activity and protects against gentamicin-induced caspase-3 activation. Control, nonstimulated caspase-3 activity is significantly elevated by application of gentamicin (bar 2). Application of UcnII not only significantly reduces basal level caspase-3 activity (bar 3) but pretreatment also protects against gentamicin-induced caspase-3 activity. *P < 0.05, ***P < 0.001.
Differential Protein Expression Assayed by iTRAQ Labeling and LC-MS/MS
To ascertain more precisely the potential mechanisms underlying the ability of UcnII, and therefore of CRF2 activation, to protect against oxidative stress and its accompanying signaling cascades, we used a shotgun proteomics procedure to perform a differential proteomic expression analysis on the OC-K3 cells under various conditions. The iTRAQ technique uses a tagging procedure that allows multiplexed samples to be compared quantitatively (Ross et al., 2004; Choe et al., 2007; Vetter et al., 2009) against each other or against a baseline condition. OC-K3 cells were grown under differentiation conditions for 10 days, and separate plates were then treated with 200 µM gentamicin for 1 hr; 100 nM UcnII for 1 hr; pretreated first for 1 hr with UcnII, followed by gentamicin exposure for 1 hr; or left untreated. Lysates were generated and samples were labeled with iTRAQ reagents, following the manufacturer’s instructions. Control, untreated samples were labeled with reagent 114, gentamicin treated samples with reagent 115, UcnII treated samples with reagent 116, and the double treatment, UcnII + gentamicin samples with reagent 117. Samples were mixed and subjected to LC-MS/MS analysis.
Global analysis of alterations to baseline protein expression induced by gentamicin, UcnII, or combined exposure
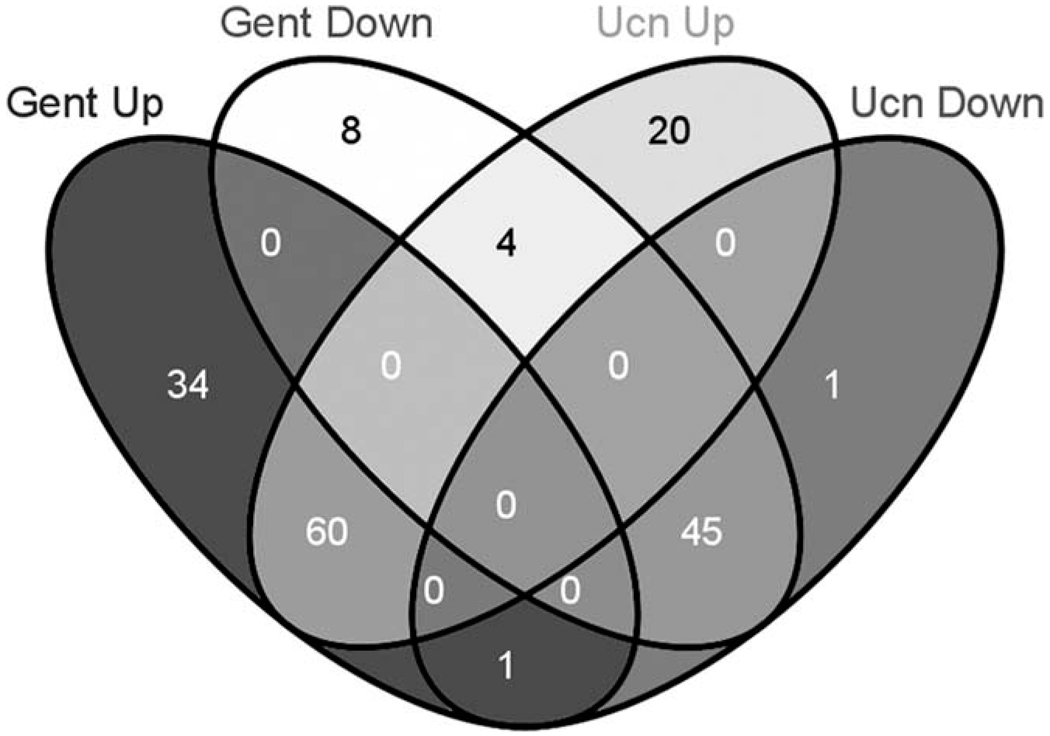
iTRAQ results generate sequence and quantitative expression information for each peptide detected. Criteria used for acceptable protein identification and differential expression assessment are described in Materials and Methods. Seventeen thousand one hundred seventy-seven spectra were obtained, among which 81% (13,956) met the 95% confidence level cutoff criterion for protein identification [Unused ProtScore cutoff 1.3 or higher; represented by a −log10 (P value) ≤ 0.05 established under the Paragon algorithm (Shilov et al., 2007) run within ProteinPilot for confidence in protein identification], resulting in identification of 5,667 peptides. These peptides were mapped to 942 total proteins identified by the mass spectrometer (see Supp. Info. Table I). Further refinement of the initial protein list was performed so that proteins from the initial list were then filtered to meet requirements for a ratio change relative to baseline (i.e., ratio with reaction 114) of 1.2 or greater or 0.8 or less (i.e., over- or under-expressed, respectively) and at least one ratio associated with the different conditions for each protein demonstrating a P value ≤ 0.05. This second filter produced Table I. A further refinement of the Table I data set was performed to select only for those proteins having an Unused ProtScore of 2.0 or greater [i.e., a log10 (P value) = 0.01], representing a protein identification confidence score of 99% or greater) and P ≤ 0.05. This list was generated to define those proteins that are likely to define future validation targets for therapeutic intervention, because confidence in identity is highest. This filter defined the 173 proteins of Table I that met such a criterion, and these proteins were used to investigate further the occurrence of contraregulated proteins between the gentamicin exposure and UcnII exposure conditions. Interestingly, a four-way Venn diagram (Fig. 5) demonstrated that only five proteins were contraregulated between these conditions, four that were down-regulated under gentamicin exposure while being up-regulated upon UcnII exposure and one that was up-regulated under gentamicin exposure while being down regulated with UcnII exposure (listed in Table II). These data seem to indicate that the mechanism by which UcnII exerts its protective effects against gentamicin-induced damage does not include a simple rescue of protein expression levels altered by the actions of gentamicin.
TABLE I.
Unused ProtScore ≥1.3; 0.8 ≥ X:114 ≥ 1.2: Any P Value for Any Protein ≤ 0.05
| Name | Symbol | Unused | 115:114 | P | 116:114 | P | 117:114 | P |
|---|---|---|---|---|---|---|---|---|
| Acetyl-coenzyme A acyltransferase 2 | Acaa2 | 3 | 1.19 | 0.10655 | 1.16 | 0.04989 | 1.25 | 0.02612 |
| Alpha-actinin-1 | Actn1 | 10.07 | 0.72 | 0.00520 | 0.97 | 0.56941 | 0.86 | 0.01405 |
| Splice Isoform 2 of alpha-adducin | Add1 | 2.19 | 1.23 | 0.12389 | 1.24 | 0.02382 | 0.92 | 0.10495 |
| Adenosylhomocysteinase | Ahcy | 7.3 | 1.28 | 0.00857 | 1.21 | 0.01460 | 1.04 | 0.48866 |
| Testis-specific SSeCKS | Akap12 | 14.83 | 1.35 | 0.00047 | 1.13 | 0.08924 | 1.07 | 0.23754 |
| Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial precursor | Aldh4a1 | 4.59 | 1.42 | 0.00405 | 1.02 | 0.74304 | 1.08 | 0.03851 |
| Annexin A3 | Anxa3 | 20.05 | 0.77 | 6.68E-09 | 0.88 | 0.00001 | 0.90 | 0.00055 |
| Annexin A4 | Anxa4 | 22.79 | 0.71 | 8.04E–07 | 0.92 | 0.08543 | 0.87 | 0.00284 |
| Amyloid-like protein 2, isoform 751 | Aplp2 | 3.05 | 0.77 | 0.03011 | 0.91 | 0.02647 | 0.96 | 0.01915 |
| Splice Isoform APP751 of Amyloid beta A4 protein precursor | App | 14.74 | 0.77 | 0.00072 | 0.80 | 0.00025 | 0.95 | 0.27227 |
| ADP-ribosylation factor 5 | Arf5 | 6 | 1.29 | 0.00633 | 1.11 | 0.23170 | 1.03 | 0.72005 |
| ADP-ribosylation factor-like 10B | Arl8a | 2 | 0.94 | 0.18331 | 0.75 | 0.00380 | 0.85 | 0.02373 |
| ARMET protein precursor | Armet | 10.25 | 0.80 | 0.00717 | 0.90 | 0.01279 | 0.97 | 0.49759 |
| Actin-related protein 2/3 complex subunit 3 | Arpc3 | 2.15 | 0.97 | 0.86568 | 1.29 | 0.03126 | 1.15 | 0.12455 |
| ATP synthase alpha chain, mitochondrial precursor | Atp5a1 | 19.49 | 1.30 | 3.56E–07 | 1.18 | 0.02213 | 1.19 | 0.00002 |
| ATP synthase gamma chain, mitochondrial precursor | Atp5c1 | 1.4 | 1.38 | 0.00818 | 1.36 | 0.00843 | 1.34 | 0.01042 |
| ATP synthase B chain, mitochondrial precursor | Atp5f1 | 6.01 | 1.33 | 0.00001 | 1.20 | 0.01632 | 1.25 | 0.00034 |
| ATPase, F0 complex, subunit D, mitochondrial | Atp5h | 4.61 | 1.26 | 0.01414 | 1.14 | 0.19651 | 1.20 | 0.08039 |
| V-type proteon ATPase 16 kDa proteolipid subunit | Atp6v0c | 2 | 0.49 | 0.00166 | 1.30 | 0.04380 | 1.26 | 0.04152 |
| ATPase, H+ transporting, V1 subunit B, isoform 2 | Atp6v1b2 | 4.15 | 0.66 | 0.06271 | 0.62 | 0.01397 | 0.75 | 0.09479 |
| B-cell receptor-associated protein 31 | Bcap31 | 2 | 0.55 | 0.02015 | 0.84 | 0.15366 | 1.01 | 0.87081 |
| Biglycan precursor | Bgn | 5.7 | 0.78 | 0.05639 | 0.78 | 0.03182 | 0.92 | 0.28058 |
| Splice isoform 2C of dihydropyridine-sensitive L-type, calcium channel alpha-2/delta subunits precursor |
Cacna2d1 | 2 | 0.72 | 0.00060 | 0.81 | 0.01788 | 0.91 | 0.06214 |
| Cullin associated and neddylation disassociated 1 | Cand1 | 8.41 | 1.22 | 0.01944 | 1.18 | 0.00333 | 1.08 | 0.10822 |
| Capping protein (actin filament) muscle Z-line, beta isoform a | Capzb | 2 | 1.38 | 0.01499 | 1.31 | 0.02294 | 1.22 | 0.01440 |
| Splice Isoform Short of H-2 class II histocompatibility antigen, gamma chain | Cd74 | 3.15 | 0.59 | 0.02162 | 0.90 | 0.54316 | 1.10 | 0.59026 |
| Cytoskeleton-associated protein 4 | Ckap4 | 8.97 | 1.04 | 0.53204 | 1.22 | 0.01480 | 1.18 | 0.02673 |
| UMP-CMP kinase homolog | Cmpk1 | 4 | 1.37 | 0.07191 | 1.28 | 0.02922 | 1.07 | 0.23327 |
| Calponin-2 | Cnn2 | 2 | 7.10 | 0.00984 | 3.05 | 0.01954 | 2.78 | 0.01604 |
| Calponin-3 | Cnn3 | 6.02 | 1.36 | 0.04934 | 0.97 | 0.71265 | 0.98 | 0.73881 |
| Collagen alpha-1(XII) chain, isoform 4 | Col12a1 | 13.26 | 0.79 | 0.03761 | 0.75 | 0.00963 | 1.13 | 0.15020 |
| Procollagen, type I, alpha 2 | Col1a2 | 42.23 | 0.71 | 4.0E–17 | 0.71 | 2.4E–20 | 0.93 | 0.01803 |
| Collagen alpha-l(III) chain precursor | Col3a1 | 49.83 | 0.53 | 1.2E–13 | 0.59 | 5.0E–15 | 0.85 | 0.00015 |
| Collagen alpha-l(IV) chain precursor | Col4a1 | 2.02 | 0.59 | 0.02488 | 0.77 | 0.10088 | 0.85 | 0.11560 |
| Collagen alpha-l(IV) chain | Col5a1 | 13.66 | 0.68 | 0.00254 | 0.82 | 0.00911 | 0.96 | 0.62754 |
| Coatomer subunit beta | Copb1 | 4.16 | 1.55 | 0.00167 | 1.41 | 0.00067 | 1.19 | 0.03385 |
| Coatomer subunit beta | Copb2 | 3.22 | 1.42 | 0.02954 | 1.33 | 0.00297 | 1.23 | 0.06778 |
| Cytochrome c oxidase subunit VIb isoform 1 | Cox6b1 | 2 | 1.32 | 0.00053 | 1.15 | 0.02654 | 1.17 | 0.00400 |
| Cp protein | Cp | 2 | 1.20 | 0.16834 | 1.45 | 0.02559 | 1.32 | 0.00547 |
| Cystatin C precursor | Cst3 | 6 | 0.78 | 0.10577 | 0.74 | 0.00278 | 0.94 | 0.29031 |
| Doublecortin and calcium/calmodulin-dependent protein kinase-like 1 | Dclk1 | 2.01 | 1.36 | 0.02664 | 1.10 | 0.14404 | 1.07 | 0.22534 |
| 2,4-Dienoyl-CoA reductase, mitochondrial precursor | Decr1 | 2 | 1.42 | 0.05570 | 1.14 | 0.08451 | 1.47 | 0.02531 |
| Cytoplasmic dynein heavy chain | Dync1h1 | 11.32 | 1.33 | 0.00330 | 1.08 | 0.34341 | 0.97 | 0.55667 |
| Dynein, cytoplasmic, intermediate chain 2 | Dync1i2 | 2 | 1.26 | 0.00278 | 1.14 | 0.03052 | 0.82 | 0.09319 |
| EGF-like repeats and discordin I-like domains 3 | Edil3 | 2.59 | 0.66 | 0.00606 | 0.73 | 0.00157 | 0.97 | 0.62209 |
| Elongation factor 1-alpha 1 | Eef1a1 | 16.59 | 1.44 | 4.37E–11 | 1.15 | 0.00033 | 1.03 | 0.60766 |
| Elongation factor 1-beta | Eef1b2 | 2.96 | 1.58 | 0.00405 | 1.54 | 0.00099 | 1.26 | 0.00316 |
| Elongation factor 1B gamma | eEF1bg | 6 | 1.29 | 0.00202 | 1.12 | 0.02556 | 0.95 | 0.03239 |
| Elongation factor 2 | Eef2 | 19.7 | 1.23 | 0.00282 | 1.11 | 0.05674 | 0.95 | 0.27293 |
| Eukaryotic translation initiation factor 3, subunit 8 | Eif3c | 2.02 | 1.14 | 0.25951 | 1.23 | 0.05069 | 1.20 | 0.03923 |
| Eukaryotic translation initiation factor 3, subunit 6 interacting protein | Eif3eip | 2.01 | 1.17 | 0.04418 | 1.39 | 0.00859 | 1.25 | 0.02601 |
| Splice isoform 2 of eukaryotic initiation factor 4A–II | Eif4a2 | 2 | 1.41 | 0.10876 | 1.27 | 0.03575 | 1.11 | 0.14509 |
| Eukaryotic translation initiation factor 5A | Eif5a | 1.7 | 1.30 | 0.00755 | 1.04 | 0.34764 | 0.89 | 0.12779 |
| Alpha-enolase | Eno1 | 18.9 | 1.22 | 0.00002 | 1.08 | 0.09906 | 0.97 | 0.45577 |
| Type 1 tumor necrosis factor receptor shedding aminopeptidase | Erap1 | 9.35 | 0.77 | 0.00440 | 0.94 | 0.20115 | 0.89 | 0.04079 |
| ERO1-like protein alpha precursor | Ero1a | 5.05 | 0.80 | 0.00259 | 0.81 | 0.00479 | 0.97 | 0.34587 |
| Coagulation factor III (fragment) | F3 | 4 | 0.73 | 0.00373 | 0.86 | 0.01499 | 0.86 | 0.05201 |
| Fatty acid synthase | Fasn | 10.01 | 1.32 | 0.03305 | 1.09 | 0.22451 | 0.94 | 0.54579 |
| FK506-binding protein 10 precursor | Fkbp10 | 10.04 | 0.74 | 0.00349 | 0.83 | 0.00003 | 0.92 | 0.00582 |
| Flotillin 2 | Flot2 | 2.2 | 1.43 | 0.05516 | 1.55 | 0.00575 | 1.51 | 0.03622 |
| Fibronectin precursor | Fn1 | 16.79 | 1.08 | 0.16536 | 1.17 | 0.07149 | 1.27 | 0.00253 |
| Fascin | Fscn1 | 4 | 1.32 | 0.00156 | 1.15 | 0.06462 | 0.93 | 0.16715 |
| Mannosyl-oligosaccharide glucosidase | Gcs1 | 2 | 1.41 | 0.00834 | 1.31 | 0.16586 | 1.26 | 0.02932 |
| Hypothetical glycosyl transferase, family 25 | Glt25d1 | 4.64 | 0.77 | 0.04454 | 0.77 | 0.01608 | 0.86 | 0.04285 |
| Predicted: similar to ribosomal protein L27 isoform 1 | Gm6301 | 4 | 1.17 | 0.00009 | 1.27 | 0.00007 | 1.11 | 0.00140 |
| Predicted: similar to 40S ribosomal protein S7 (S8) isoform 1 | Gm6472 | 2.01 | 1.16 | 0.03182 | 1.23 | 0.01406 | 1.15 | 0.06964 |
| Glypican-4 precursor | Gpc4 | 10.91 | 0.69 | 0.00077 | 0.96 | 0.51617 | 1.01 | 0.83763 |
| Gelsolin precursor | Gsn | 10.06 | 1.36 | 0.00008 | 1.36 | 0.00021 | 1.15 | 0.02775 |
| histone 1, H2ab | H2a1 | 4 | 1.44 | 0.00396 | 1.46 | 0.00027 | 1.06 | 0.13184 |
| Histocompatibility 2, class II, locus DMa | H2Dma | 2 | 1.16 | 0.15268 | 1.23 | 0.11280 | 1.27 | 0.02078 |
| Hemoglobin alpha subunit | Hba-a2 | 2.52 | 1.52 | 0.03220 | 1.27 | 0.15750 | 1.08 | 0.54494 |
| Hexosaminidase A | Hexa | 6.01 | 0.75 | 0.00063 | 0.76 | 0.00153 | 0.87 | 0.07372 |
| Beta-hexosaminidase beta chain precursor | Hexb | 2.04 | 1.80 | 0.03116 | 1.33 | 0.02409 | 1.59 | 0.02675 |
| Hypothetical twin-arginine translocation pathway signal containing protein | Hgsnat | 2 | 0.88 | 0.47902 | 0.74 | 0.00251 | 0.92 | 0.36047 |
| Histone H1.2 | Hist1h1c | 4.01 | 1.27 | 0.00946 | 1.50 | 0.00358 | 1.04 | 0.63660 |
| Histone H2B | Hist3h2ba | 4.02 | 1.27 | 0.00333 | 1.40 | 0.01046 | 1.04 | 0.10255 |
| Hematological and neurological expressed 1-like protein | Hn1l | 2 | 1.37 | 0.01644 | 1.30 | 0.02738 | 1.17 | 0.14991 |
| Heterogeneous nuclear ribonucleoprotein C | Hnrnpc | 2.92 | 1.10 | 0.48055 | 1.22 | 0.04580 | 1.18 | 0.18811 |
| Heterogeneous nuclear ribonucleoprotein F | Hnrpf | 5.7 | 1.01 | 0.51723 | 1.20 | 0.02655 | 1.12 | 0.01691 |
| Heterogeneous nuclear ribonucleoprotein M | Hnrpm | 9.16 | 2.77 | 0.00048 | 1.51 | 0.00073 | 1.45 | 0.00077 |
| Haptoglobin precursor | Hp | 2 | 6.51 | 0.02087 | 6.33 | 0.03657 | 3.30 | 0.00731 |
| Heat shock protein HSP 90-alpha | Hsp90aa1 | 8.18 | 1.29 | 0.00031 | 1.19 | 0.00952 | 1.00 | 0.98177 |
| Heat shock 70 kDa protein 4 | Hspa4 | 2.22 | 2.20 | 0.02308 | 1.74 | 0.01329 | 1.54 | 0.11746 |
| Isocitrate dehydrogenase 1 (NADP+), soluble | Idh1 | 4.05 | 1.42 | 0.00091 | 1.48 | 0.00276 | 1.30 | 0.01552 |
| Interferon-Inducible protein homolog | Ifitm3 | 10 | 1.48 | 0.01991 | 1.33 | 0.02416 | 1.43 | 0.04082 |
| Insulin-like growth factor binding protein 7 | Igfbp7 | 4 | 0.64 | 0.00086 | 0.64 | 0.00012 | 0.99 | 0.24127 |
| Predicted similar to RAB6A, member PvAS oncogene family | IPI00458129 | 2 | 2.04 | 0.01115 | 1.35 | 0.20837 | 1.40 | 0.05857 |
| Predicted similar to murinoglobulin 1 | IPI00458628 | 2.22 | 1.18 | 0.24269 | 1.47 | 0.03579 | 1.55 | 0.01363 |
| Predicted similar to 60S ribosomal protein L26-like 1 | IPI00461916 | 2.96 | 1.20 | 0.00986 | 1.23 | 0.01941 | 1.07 | 0.05728 |
| Predicted similar to 40S ribosomal protein S2 isoform 1 | IPI00462315 | 4.12 | 1.13 | 0.11148 | 1.23 | 0.01681 | 1.03 | 0.74056 |
| Predicted similar to cortactin isoform B | IPI00624988 | 2 | 1.23 | 0.00759 | 1.13 | 0.15730 | 1.01 | 0.74524 |
| Predicted similar to syndecan binding protein | IPI00658860 | 1.7 | 0.78 | 0.17799 | 0.84 | 0.01026 | 0.76 | 0.00081 |
| Predicted similar to 60S ribosomal protein L18 | IPI00660168 | 2.01 | 1.36 | 0.00576 | 1.33 | 0.01801 | 1.20 | 0.00237 |
| Predicted similar to peroxiredoxin 6 | IPI00667059 | 2 | 1.31 | 0.01308 | 1.14 | 0.10505 | 1.09 | 0.41735 |
| Predicted: similar to heterogeneous nuclear ribonucleoprotein A3 | IPI00668080 | 12.39 | 0.45 | 0.00413 | 0.82 | 0.02460 | 0.74 | 0.04749 |
| Predicted: similar to 40S ribosomal protein S6 | IPI00671512 | 2.22 | 1.53 | 0.03667 | 1.57 | 0.09780 | 1.36 | 0.11542 |
| Predicted: similar to 60S ribosomal protein L22 | IPI00672745 | 2 | 0.99 | 0.31630 | 1.33 | 0.02302 | 1.11 | 0.03785 |
| Similar to aldo-keto reductase family 1, member B3 (aldose reductase) isoform 3 | IPI00672751 | 6.52 | 1.24 | 0.00038 | 1.04 | 0.33160 | 0.92 | 0.04130 |
| Predicted: similar to nuclear mitotic apparatus protein 1 isoform 8 | IPI00679098 | 4.31 | 1.73 | 0.00666 | 1.71 | 0.00980 | 1.33 | 0.08625 |
| Ras GTPase-activating-like protein IQGAP1 | Iqgap1 | 4.55 | 1.27 | 0.07468 | 1.24 | 0.03558 | 1.17 | 0.07167 |
| Interalpha trypsin inhibitor, heavy chain 2, full insert sequence | Itih2 | 4.8 | 0.81 | 0.15388 | 0.84 | 0.00319 | 0.77 | 0.00269 |
| Kinesin family member 5B | Kif5b | 1.34 | 0.37 | 0.00131 | 0.29 | 0.00033 | 0.40 | 0.00097 |
| Lysosomal-associated transmembrane protein 4A | Laptm4a | 4.16 | 0.74 | 0.01955 | 0.91 | 0.18230 | 0.87 | 0.23043 |
| LIM and SH3 domain protein 1 | Lasp1 | 1.87 | 1.28 | 0.00081 | 1.02 | 0.82691 | 0.89 | 0.07143 |
| Splice Isoform 1 of leukemia inhibitory factor receptor precursor | Lifr | 2 | 0.73 | 0.07785 | 0.79 | 0.02379 | 0.83 | 0.05927 |
| Splice isoform A of lamin-A/C | Lmna | 8.74 | 1.42 | 0.00177 | 1.48 | 0.00429 | 0.90 | 0.02039 |
| Lamin-B1 | Lmnb1 | 2.02 | 2.05 | 0.01306 | 1.82 | 0.00986 | 0.85 | 0.18476 |
| DNA replication licensing factor MCM6 | Mcm6 | 4.14 | 1.76 | 0.01764 | 1.68 | 0.00668 | 1.00 | 0.92590 |
| 72 kDa type IV collagenase precursor | Mmp2 | 3.57 | 0.69 | 0.02808 | 0.71 | 0.02033 | 0.95 | 0.49834 |
| Moesin | Msn | 15.87 | 1.23 | 0.00184 | 1.06 | 0.20770 | 1.06 | 0.21205 |
| Microtubule-associated protein 4, isoform 1 | Mtap4 | 10.01 | 1.46 | 0.04986 | 1.14 | 0.22768 | 1.07 | 0.44384 |
| Nicotinamide phosphoribosyltransferase | Nampt | 2 | 1.35 | 0.02439 | 1.07 | 0.24788 | 0.87 | 0.17672 |
| Nucleosome assembly protein 1-like 4 | Nap1l4 | 4 | 1.18 | 0.06649 | 1.22 | 0.00632 | 0.98 | 0.80709 |
| Beta-soluble NSF attachment protein | Napb | 2 | 1.07 | 0.65191 | 1.55 | 0.04053 | 1.24 | 0.17255 |
| Neural cell adhesion molecule 2 | Ncam2 | 2 | 1.38 | 0.01051 | 0.91 | 0.27592 | 0.91 | 0.31025 |
| Nucleolin | Ncl | 8.03 | 0.64 | 0.02786 | 1.25 | 0.00204 | 0.96 | 0.36012 |
| N-myc downstream regulated 1 | Ndrg1 | 4 | 1.55 | 0.00619 | 1.34 | 0.00082 | 1.34 | 0.00041 |
| Predicted: similar to Nucleophosmin (NPM) isoform 2 | Npm1 | 7.31 | 1.04 | 0.70252 | 1.25 | 0.04476 | 1.02 | 0.83235 |
| Splice Isoform 2 of Prolyl 4-hydroxylase alpha-1 subunit precursor | P4ha1 | 14.27 | 0.82 | 0.00054 | 0.80 | 0.00002 | 0.93 | 0.02886 |
| Splice Isoform 1 of Programmed cell death 6-interacting protein | Pdcd6ip | 5.69 | 1.31 | 0.01627 | 1.24 | 0.05127 | 1.23 | 0.08927 |
| Phosphatidylethanolamine-binding protein | Pebp1 | 3.4 | 2.11 | 0.03143 | 1.77 | 0.03640 | 1.61 | 0.02473 |
| Prohibitin | Phb | 6 | 1.38 | 0.04142 | 1.21 | 0.12291 | 1.08 | 0.38330 |
| Metalloendopeptidase homolog PEX | Phex | 4 | 0.46 | 0.02234 | 0.53 | 0.01021 | 0.80 | 0.01815 |
| Procollagen-lysine,2-oxoglutarate 5-dioxygenase 2 precursor | Plod2 | 19.3 | 0.61 | 3.14E–10 | 0.69 | 3.21E–08 | 0.84 | 0.00002 |
| Peptidyl-prolyl cis-trans isomerase, mitochondrial precursor | Ppif | 2.36 | 1.55 | 0.09794 | 1.35 | 0.02459 | 1.11 | 0.40978 |
| Serine/threonine-protein phosphatase PP1-alpha catalytic subunit | Ppp1ca | 4 | 1.15 | 0.11370 | 1.23 | 0.01697 | 1.22 | 0.19022 |
| Proteasome (prosome, macropain) subunit, alpha type 2 | Psma2 | 6 | 0.79 | 0.00584 | 0.85 | 0.01403 | 0.82 | 0.00292 |
| Proteasome subunit alpha type 6 | Psma6 | 7.3 | 1.04 | 0.63170 | 1.20 | 0.17251 | 1.21 | 0.02916 |
| Proteasome subunit beta type 4 precursor | Psmb4 | 1.7 | 0.67 | 0.00041 | 0.85 | 0.14877 | 0.84 | 0.08104 |
| Proteosome subunit, beta type 9 | Psmb9 | 2 | 0.73 | 0.00017 | 0.81 | 0.00302 | 0.86 | 0.02689 |
| 26S proteasome non-ATPase regulatory subunit 12 | Psmd12 | 2.01 | 1.89 | 0.01565 | 1.91 | 0.01172 | 1.46 | 0.01089 |
| Polypyrimidine tract binding protein 1 | Ptbp1 | 6.8 | 1.29 | 0.01348 | 1.34 | 0.00353 | 1.39 | 0.00342 |
| Prothymosin alpha | Ptma | 2 | 0.78 | 0.10205 | 1.72 | 0.00193 | 1.04 | 0.74595 |
| Parathymosin | Ptms | 2.21 | 0.47 | 0.00653 | 1.21 | 0.02743 | 0.80 | 0.03355 |
| Pituitary tumor-transforming gene 1 protein-interacting protein precursor | Pttg1ip | 2.4 | 0.70 | 0.05267 | 0.79 | 0.00607 | 0.92 | 0.56299 |
| Pentraxin-related protein PTX3 precursor | Ptx3 | 9 | 0.72 | 0.01151 | 0.87 | 0.02842 | 0.77 | 0.00052 |
| Ras-related protein Rap-1b | Rap1b | 6.01 | 0.78 | 0.00019 | 0.80 | 0.00002 | 0.88 | 0.00139 |
| Heterogeneous nuclear ribonucleoprotein G | Rbmx | 2.25 | 1.53 | 0.00002 | 1.30 | 0.03901 | 1.40 | 0.00004 |
| Reticulocalbin-3 precursor | Rcn3 | 8.96 | 0.77 | 0.01407 | 0.83 | 0.00019 | 0.84 | 0.00147 |
| Predicted: similar to 60S ribosomal protein L11 | Rpl11 | 2.01 | 1.41 | 0.00013 | 1.38 | 0.00005 | 1.25 | 0.00527 |
| 60S ribosomal protein L13 | Rpl13 | 9.36 | 1.39 | 0.07805 | 1.45 | 0.02174 | 1.24 | 0.11835 |
| Ribosomal protein L14, cytosolic homolog | Rpl14 | 6 | 1.39 | 0.00465 | 1.45 | 0.00063 | 1.34 | 0.00957 |
| 60S ribosomal protein L15 | Rpl15 | 2.09 | 1.17 | 0.31704 | 1.31 | 0.01177 | 1.19 | 0.00001 |
| 60S ribosomal protein L34 | Rpl34 | 2.51 | 1.93 | 0.00475 | 2.43 | 0.00218 | 1.80 | 0.00601 |
| 60S ribosomal protein L7 | Rpl7 | 8.13 | 1.16 | 0.03492 | 1.30 | 0.00114 | 1.12 | 0.01597 |
| Ribosomal protein, Large P2 | Rplp2 | 6 | 1.21 | 0.04420 | 1.25 | 0.01254 | 1.18 | 0.00833 |
| 40S ribosomal protein S27 | Rps27 | 2 | 1.24 | 0.00371 | 1.24 | 0.01741 | 1.15 | 0.06352 |
| 40S ribosomal protein S4, X isoform | Rps4x | 5.7 | 1.49 | 0.00200 | 1.53 | 0.00107 | 1.31 | 0.01517 |
| 40S ribosomal protein S9 homolog | Rps9 | 7.01 | 1.16 | 0.00244 | 1.27 | 0.00016 | 1.06 | 0.17068 |
| Similar to ribosomal_S3Ae | RS3Ae | 10.78 | 1.08 | 0.21645 | 1.29 | 0.00287 | 1.14 | 0.08070 |
| Reticulon 3 isoform 2 | Rtn3 | 2 | 0.88 | 0.17876 | 0.79 | 0.09679 | 0.74 | 0.04592 |
| S100 calcium-binding protein A11 (Calgizzarin) | S100a11 | 4 | 1.45 | 1.27E–06 | 1.40 | 4.63E–06 | 1.30 | 4.85E–06 |
| S100 calcium-binding protein A4 | S100a4 | 4 | 1.35 | 0.02872 | 1.22 | 0.11344 | 1.04 | 0.72927 |
| Lysosome membrane protein II | Scarb2 | 10.96 | 0.84 | 0.00314 | 0.78 | 0.00055 | 0.90 | 0.00567 |
| Retinoid-inducible serine carboxypeptidase precursor | Scpep1 | 5.7 | 0.69 | 0.01942 | 0.80 | 0.00492 | 0.88 | 0.10695 |
| Septin-2 | Sept2 | 4 | 1.39 | 0.00221 | 1.33 | 0.00322 | 1.10 | 0.01895 |
| Septin 7 | Sept7 | 4.03 | 1.24 | 0.00549 | 0.95 | 0.50655 | 0.98 | 0.59337 |
| Placental thrombin inhibitor | Serpinb6a | 11.41 | 1.25 | 0.00006 | 1.15 | 0.00209 | 1.12 | 0.03375 |
| Glia derived nexin precursor | Serpine2 | 9.71 | 0.68 | 0.00036 | 0.74 | 0.00331 | 0.98 | 0.74391 |
| Splicing factor, proline- and glutamine-rich | Sfpq | 8.23 | 1.47 | 0.00645 | 1.38 | 0.00114 | 1.42 | 0.00045 |
| SH3 domain-binding glutamic acid-rich-like protein | Sh3bgrl | 2 | 0.93 | 0.08474 | 0.82 | 0.07161 | 0.78 | 0.01127 |
| ADP/ATP translocase 2 | Slc25a5 | 4.7 | 0.75 | 0.00768 | 0.81 | 0.02415 | 1.08 | 0.20140 |
| Solute carrier family 29 | Slc29a1 | 4 | 0.70 | 0.03439 | 0.79 | 0.05475 | 0.86 | 0.19471 |
| Splice Isoform 1 of Choline transporter-like protein 2 | Slc44a2 | 4.16 | 0.78 | 0.00775 | 0.78 | 0.00097 | 0.85 | 0.01557 |
| Synaptosomal-associated protein 23 | Snap23 | 2 | 1.35 | 0.01229 | 0.90 | 0.28088 | 1.11 | 0.24989 |
| Small nuclear ribonucleoprotein Sm D3 | Snrpd3 | 2 | 1.08 | 0.08386 | 1.21 | 0.04227 | 1.25 | 0.02455 |
| Small nuclear ribonucleoprotein E | Snrpe | 2 | 0.78 | 0.02898 | 0.95 | 0.20513 | 1.02 | 0.40027 |
| Superoxide dismutase [Mn], mitochondrial precursor | Sod2 | 5 | 0.78 | 0.00325 | 0.81 | 0.03148 | 0.86 | 0.05072 |
| Hsc70-interacting protein | St13 | 5.3 | 1.28 | 0.03667 | 1.31 | 0.08439 | 1.12 | 0.38800 |
| Transgelin | Tagln | 3.1 | 1.24 | 0.03847 | 1.09 | 0.38651 | 0.99 | 0.94747 |
| Transaldolase | Taldo1 | 4.26 | 1.39 | 0.00149 | 1.51 | 0.00264 | 1.19 | 0.09349 |
| Protein-glutamine gamma-glutamyltransferase 2 | Tgm2 | 4 | 0.83 | 0.00727 | 0.74 | 0.00072 | 1.00 | 0.82842 |
| Trans-Golgi network integral membrane protein 2 precursor | Tgoln2 | 2 | 0.80 | 0.16160 | 0.64 | 0.04075 | 0.98 | 0.71384 |
| Thioesterase superfamily member 2 | Them2 | 4 | 0.71 | 0.00205 | 0.78 | 0.00750 | 1.04 | 0.11957 |
| Transketolase | Tkt | 10 | 1.29 | 0.00489 | 1.25 | 0.00691 | 1.05 | 0.40896 |
| Transmembrane emp24 domain trafficking protein 2 | Tmed2 | 2 | 1.37 | 0.06743 | 1.43 | 0.01484 | 1.59 | 0.18361 |
| Hypothetical protein Tmem 106b | Tmem 106b | 3.05 | 0.80 | 0.00724 | 0.88 | 0.03337 | 0.97 | 0.10148 |
| Similar to elastin | Tmem43 | 5.7 | 1.10 | 0.00318 | 1.16 | 0.00158 | 1.20 | 0.00599 |
| Splice isoform short of thymosin beta-4 | Tmsb4x | 3.51 | 1.32 | 0.00490 | 0.98 | 0.80410 | 1.05 | 0.47081 |
| Precursor tenascin protein precursor | Tnc | 8.7 | 1.21 | 0.02554 | 1.10 | 0.09750 | 1.16 | 0.04027 |
| Testis-specific serine kinase 4 | Tssk4 | 2 | 0.93 | 0.02715 | 1.25 | 0.00351 | 1.19 | 0.01167 |
| Tubulin, alpha 1a | Tuba 1a | 15.83 | 2.36 | 0.01315 | 2.05 | 0.01677 | 1.72 | 0.06347 |
| Tubulin, beta 2 | Tubb2a | 2.02 | 1.00 | 0.99946 | 1.05 | 0.41712 | 0.76 | 0.00163 |
| Tubulin beta-2C chain | Tubb2c | 25.23 | 1.35 | 0.02188 | 1.40 | 0.01263 | 1.14 | 0.13922 |
| Ubiquitin-activating enzyme E1 1 | Uba1 | 8 | 1.34 | 0.00793 | 1.25 | 0.01538 | 1.01 | 0.68045 |
| Vimentin | Vim | 50.95 | 1.23 | 1.8E–10 | 1.16 | 8.9E–09 | 0.96 | 0.12721 |
| Splice Isoform 2 of 14-3-3 protein theta | Ywhaq | 17.16 | 1.26 | 0.01944 | 1.07 | 0.29184 | 0.97 | 0.49853 |
Fig. 5.
A four-way Venn diagram reveals proteins that are contraregulated. By using only proteins reaching a 99% confidence level of identification as a database, a Venn diagram was generated to indicate with highest confidence those proteins that were differentially expressed between gentamicin and UcnII treatments. For both conditions, the majority of differentially expressed proteins were up-regulated. Only four proteins were up-regulated under UcnII treatment while also being down-regulated by gentamicin treatment. Conversely, one protein was down-regulated by UcnII treatment while being up-regulated by gentamicin. Identities are listed in Table II.
TABLE II.
True Contraregulated Proteins (Gent vs. UcnII): Unused ProtScore ≥2.0
| Symbol | 115:114 | P | 116:114 | P | 117:114 | P | |
|---|---|---|---|---|---|---|---|
| Gent down/Ucn up Prothymosin α | Ptma | 0.8 | 0.102 | 1.7 | 0.002 | 1.0 | 0.746 |
| Parathymosin | Ptms | 0.5 | 0.007 | 1.2 | 0.027 | 0.8 | 0.034 |
| Nucleolin | Ncl | 0.6 | 0.028 | 1.3 | 0.002 | 1.0 | 0.360 |
| v-Type proton ATPase | Atp6v0c | 0.5 | 0.002 | 1.3 | 0.044 | 1.3 | 0.042 |
| Gent up/Ucn down | |||||||
| Synaptosomal-associated protein 23 | Snap23 | 1.3 | 0.012 | 0.9 | 0.281 | 1.1 | 0.250 |
Functional clustering
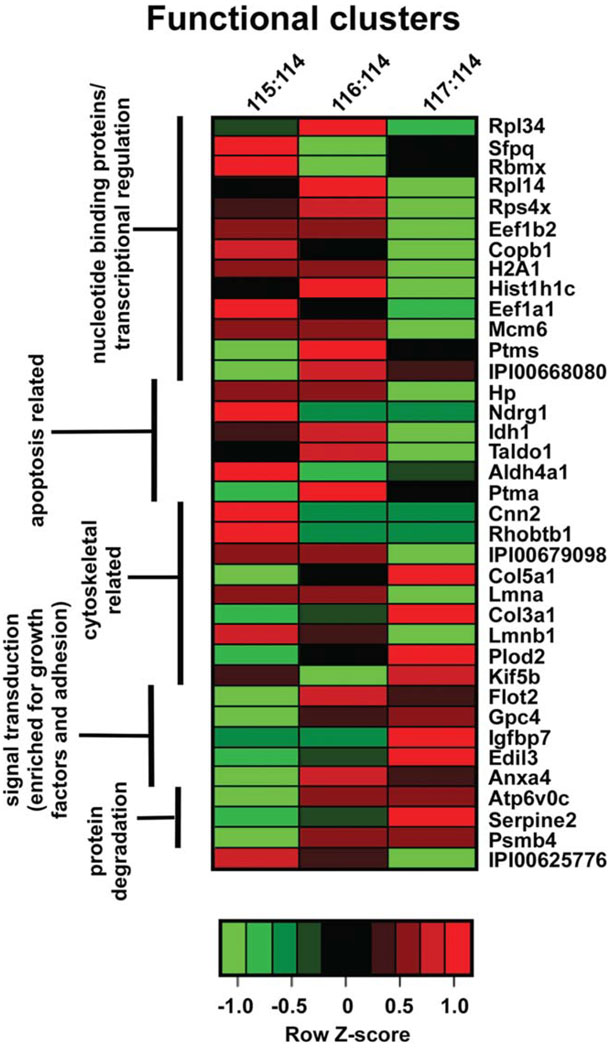
Results from the iTRAQ-based analysis were examined to determine whether functional clusters could be revealed that would be useful in better understanding potential mechanisms whereby UcnII exerts its protective effects over gentamicin-induced damage. Data from Table I were further refined and selected for functional clustering if the absolute log2 (-fold change) P ≤ 0.01. Although these criteria were highly stringent along both the expression and identification dimensions, they nonetheless returned 37 proteins that met or exceeded this overall metric. Expression level ratios relative to baseline were assessed for each protein, and a Z-score was generated for each row and used to map the maximal expression ratio for each protein between gentamicin only (115:114) and UcnII pretreatment followed by gentamicin (117:114) conditions. Proteins were manually curated into biologically relevant functional groups, clustered by this annotation, and visualized using a heat map (Fig. 6). The 37 proteins separated into five mutually exclusive functional clusters that represented distinct biological processes. These involved protein degradation and signal transduction, which were decreased in the gentamicin condition relative to baseline, while being significantly up-regulated following UcnII pretreatment followed by gentamicin challenge. Proteins related to cytoskeletal processes also clustered, and, although the data indicated contraregulated expression between the two main conditions, as a cluster the proteins did not behave similarly within each condition (i.e., some were up- and some were down-regulated, but always opposite the other treatment paradigm). Apoptosis-related proteins also formed a cluster, and most of these proteins were overexpressed under gentamicin conditions and suppressed with UcnII pretreatment. Finally, the largest cluster generated was composed of proteins involved in nucleotide binding and transcriptional regulation, most of which were up-regulated following gentamicin exposure and suppressed following UcnII pretreatment, indicating the normalizing effects of UcnII pretreatment.
Fig. 6.
Hierarchical clustering of manually curated functional groups of proteins that are differentially expressed across conditions reveals significant and concordant changes in five functional sets. Proteins were identified using the highest confidence filter for both protein identification (99%) and P value of expression change (≤0.01). Each protein was then annotated for functionality and submitted for clustering. Manual curation identified five main protein sets, as defined on the left hand margin of the heat map. The color of each heat map cell represents the Z-score of expression along the row, allowing a comparison of expression of any protein along across conditions. Both nucleotide binding/transcriptional regulation and apoptosis-related protein sets are up-regulated under gentamicin conditions (115:114) while being comparatively down-regulated under ther pretreatment with UcnII (117:114) condition. Signal transduction (especially concerning growth factors) and protein-degradation-related proteins are more highly expressed following pretreatment with UcnII than under gentamicin conditions. Identity of members of the final list is indicated on the right margin of the heat map. Column headers: 115:114, expression under gentamicin stimulation normalized to control, untreated condition; 116:114, expression under urocortin II stimulation normalized to control, untreated condition; 117:114, expression under urocortin II pretreatment stimulation followed by gentamicin challenge, normalized to control, untreated condition. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Expression of the corticotropin-releasing hormone family of peptides and their receptors has previously been demonstrated in the inner ear (Vetter et al., 2002; Graham et al., 2010). Although the classically understood role of the CRF system involves activation of the hypothalamic-pituitary-adrenal axis, recent work has demonstrated its involvement more globally in oxidative stress-related biology. In particular, peptides of the CRF family have been shown to be neuro- and cardio-protective. Thus, the family is involved in neurodegenerative disorders such as Alzheimer’s disease (Bayatti and Behl, 2005), excitotoxicity (Elliott-Hunt et al., 2002), and ischemia-reperfusion injury, especially of cardiac tissue (Brar et al., 1999, 2000, 2002, 2004; Scarabelli et al., 2002; Burley et al., 2007). The cardiac ischemia-reperfusion injury model demonstrated that the CRF system is involved in cardioprotection via inhibition of free radical activity (Liu et al., 2005).
A myriad of ROS-generating stimuli impinge on the inner ear, including exposure to intense sound and ototoxic drugs. Because mammalian hair cells do not regenerate, significant effort from many groups has gone into understanding the consequences of ROS production in the inner ear in the hopes of neutralizing cellular damage and protecting hearing. However, little is currently known about how the inner ear normally handles the significant everyday stress it is under and what endogenous signaling mechanisms are expressed that allow this very active system to avoid/lessen metabolically related damage over the lifetime of an individual. Hints that an endogenous system not only exists but is capable of significantly altering the response of the inner ear to the effects of oxidative stress has been underscored by a series of findings reporting the ability of low-level sound exposure (termed pretreatment, or conditioning) to 1) increase resistance to oxidative stress-related loss of hair cells and hearing function (Harris et al., 2006), 2) increase antioxidant enzyme activity (Jacono et al., 1998), 3) inhibit apoptotic activity (Niu et al., 2003), and finally 4) decrease gentamicin-induced hair cell loss (Suryadevara et al., 2009). Understanding the endogenous defenses mounted against cellular stress and stress-related signaling in the inner ear may reveal novel methods by which one may intervene against environmentally induced damage as well as against age-related hearing loss or that associated with therapeutically necessary exposures to drugs with ototoxic side effects.
The role(s) of SOD and related proteins in protection of the inner ear is well understood. Loss of SOD1 from the cochlea potentiates both noise-induced and age-related hearing loss (McFadden et al., 1999a,b, 2001). Our experiments suggest that mechanisms by which CRF2-mediated protection against ROS-related damage is established may occur via previously recognized SOD mechanisms as well as by novel, apparently non-SOD-related, mechanisms. With regard to SOD-related protection, our data demonstrate that, in the presence of gentamicin, SOD activity was significantly decreased (almost three times over baseline values), but combining UcnII pretreatment with gentamicin exposure induced a significant up-regulation of SOD activity compared with gentamicin-only treatment. The ability of CRF2 activity to inhibit numerous other cellular responses linked to oxidative stress, such as caspase-3 activation, suggests that there are clearly other signaling pathways that can be activated to generate cytoprotective responses besides those previously described involving SOD.
In light of the data demonstrating the protective effects of prior activation of CRF2 on gentamicin-induced ROS generation and activation of cell death pathways, we initiated a top-down proteomics screen. The goal of this procedure was to begin defining novel therapeutic targets useful in combating ROS production and consequent ototoxin-induced damage. We began a directed search using only the most highly reliably identified proteins to define novel cochleoprotective actions mediated by CRF2 activity. These data represent the first demonstration of novel protective mechanisms invoked by the CRF system of the inner ear. Unexpectedly, very few proteins were contraregulated between UcnII exposure and gentamicin exposure despite the ability of UcnII pretreatment to block cellular response to gentamicin. Only five proteins were contraregulated between UcnII and gentamicin stimulation. Among these, prothymosin α (Ptma) did not change in a statistically relevant manner from baseline under either gentamicin-only or UcnII + gentamicin treatment (P = 0.102 and P = 0.746, respectively) but underwent a highly significant change in expression from baseline under UcnII exposure (P = 0.002). We interpret these findings as suggestive of an early rise in Ptma expression during the UcnII pretreatment phase that is protective against gentamicin exposure and that was subsequently down-regulated to normal levels upon gentamicin challenge. Ptma may therefore still represent a protein of some interest for future validation, especially given that Ptma has been shown to switch cell death mode pathways from necrosis (generally considered to be an irretrievable pathway once initiated) to apoptosis (Ueda, 2008, 2009). The apoptotic pathway is fully rescuable in vitro and in vivo by application or overexpression/release of neurotrophins or growth factors. Interestingly, Figure 6 demonstrates just such an up-regulation of a growth-factor-enriched expression cluster while also revealing a down-regulation of apoptosis-related proteins. Thus, UcnII pretreatment may render cells more capable of initiating a rapid switch between cell death modes while at the same time inducing protein expression levels favorable to surviving apoptosis. Such a response to Ptma during ischemic stroke has been demonstrated (Fujita et al., 2009). Finally, it has been previously demonstrated that Ptma blocks apoptosome formation, thereby inhibiting caspase activation (Jiang et al., 2003). Our biochemical assays also demonstrated an inhibition of caspase-3 activity following UcnII pretreatment. All of these data suggest that the ability of UcnII to up-regulate Ptma can serve as a survival mechanism in the face of cellular stress and that CRF2 activation may be crucial for inducing this mechanism in the inner ear.
Interestingly, mechanisms of aminoglycoside-induced hair cell death may be dependent on the model being examined. In vitro assays employing explant cultures have implicated caspase activation (Cunningham et al., 2002) and classical apoptotic pathways (Li et al., 1995; Vago et al., 1998) in cell death and have also demonstrated that caspase inhibitors prevent aminoglyco-side-induced hair cell death (Matsui et al., 2002, 2003). However, it has been suggested that aminoglycoside-induced cell death pathways in vivo are caspase independent (Jiang et al., 2006), and few data exist for classic apoptotic events being induced by chronic aminoglyco-side exposures. Our data may help shed light on this dichotomy. If an intact CRF signaling system functioning in vivo switches the death pathways away from apoptosis, this may explain the paucity of data implicating apoptosis as a cell death mechanism in vivo. This would also suggest that there is a loss of some aspect of CRF signaling under in vitro conditions, thus allowing the hair cells to undergo apoptosis when explants are analyzed. If this hypothesis is true, manipulating the in vitro models by activating the CRF system exogenously should inhibit the apoptosis pathways previously described under these conditions.
Only parathymosin and nucleolin (Ncl) exhibited statistically relevant expression changes in the opposite direction between gentamicin challenge (both down-regulated) and UcnII challenge (both up-regulated, although parathymosin did not reach original baseline values, and Ncl was lowered from the UcnII induced levels to expression levels that were not statistically different from baseline). Prothymosin a and parathymosin are essential for cell cycle progression and cellular proliferation in both normal and cancer cells (Tsitsiloni et al., 1993; Letsas et al., 2007). Parathymosin is also known to be involved in transient, cyclical binding with histone H1 and therefore has been suggested to be a modulator of histone–chromatin interactions in specific subnuclear domains (Martic et al., 2005). By extension, then, one possible effect of UcnII pretreatment is to modulate specific gene expression and initiation of protein translation, the specific products of which may be involved in apoptotic effects. Supporting this hypothesis is the number of ribosomal proteins that are also up-regulated with UcnII treatment/pretreatment as reported in Table I. Interestingly, numerous small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins are up-regulated following UcnII exposure, whereas canonical RNA proteins of the 60S and 40S RNA complexes seem to be down-regulated. Nucleolin is significantly up-regulated by UcnII exposure, and this is maintained at baseline levels in spite of gentamicin exposure, making it a protein of particular interest. Although Ncl has been well recognized for its role in also regulating chromatin structure, it has recently been found to have a strong antiapoptotic role under H2O2 challenge (Zhang et al., 2009). Thus, rescuing Ncl from its gentamicin-induced down-regulation represents a potential future target for intervention. Only the vacuolar v-type H+-ATPase (Atp6v0c) maintained a statistically up-regulated expression profile when exposed to UcnII, even under gentamicin challenge (which itself caused a highly significant decrease in expression; P = 0.002). Gentamicin-induced loss of Atp6v0c expression may contribute to build up of, and toxic response to, lysosomal debris. Cellular toxicity following loss of Atp6v0c has been demonstrated under neurodegenerative conditions such as Alzheimer’s disease (Liu et al., 2008). The ability of UcnII pretreatment apparently to inhibit this response to gentamicin may represent activation of multiple parallel pathways that together impart resistivity to otherwise ototoxic compounds.
Four mitochondrial proteins involved in ATP synthesis were significantly up-regulated following gentamicin exposure. Although these were also up-regulated following UcnII pretreatment, three of the four were expressed at lower levels than were induced by gentamicin. Most significantly, Atp5h, a protein involved in generating the pore structure of the ATP channel, is held to values insignificantly different from baseline. One may postulate that the gentamicin-induced up-regulation of these proteins represents an imbalanced or stressed mitochondrial fraction. If this is true, the ability of UcnII to negate even some of the overexpression across this short time frame may represent a key mechanism whereby UcnII/CRF2 activity establishes its protective effects. Because the mitochondria play an important role in the consequences following oxidative stress, this may still represent a fertile avenue to pursue.
It is possible that the expression level of any single protein could change in a small, yet still statistically meaningful, manner, making it more difficult to assign functional significance to such a change. However, large ensembles of such small changes clustered along major signaling pathways could represent major effectors for CRF2-based protection. Thus, we carried out a hierarchical clustering of hand-annotated functional groups to examine the dynamics of differential expression (Fig. 6). The major processes that are affected by CRF2 pretreatment in the face of gentamicin-induced oxidative stress were split into two ensembles of proteins moving with opposite trajectories. Expression of proteins related to apoptosis pathways and nucleotide binding were lower following UcnII pretreatment relative to levels induced by gentamicin exposure. Signal transduction pathways that are especially enriched for growth factors and adhesion and also protein degradation pathways were up-regulated by UcnII pretreatment with respect to gentamicin-only exposure. Thus, one may use such data to continue probing these pathways with novel drugs/substances in order to define better the efficacy of novel inhibitors of oxidative stress-related damage to the inner ear in vivo.
This work has demonstrated that CRF2 activation induces a protective effect against ROS production and associated signaling in cochlear-derived immortalized cells. The inner ear is composed of numerous cell types, and, insofar as it was unknown whether and by what mechanisms CRF2 activation might exert a protective effect against ROS-induction and associated damage, the OC-K3 cell line was used in this first report of CRFR-mediated protection. The cell line is useful for assessing potential signaling cascades induced by CRF2 activation, because the line is composed of a homogeneous cell population. However, it should be recognized that direct generalization of the results obtained here to the in vivo state should be made with caution until further experiments designed to define whether these processes are present in the intact cochlea, and which cell types are involved, are performed. However, the general use of cell lines as a tool for elucidating novel signaling cascades cannot be ignored and represents the best way to define novel protein interactions prior to establishing them in any in vivo state.
In conclusion, we have demonstrated that CRF2 activity provides significant protection from cytotoxic (oxidative) stress in an inner ear-derived cell line. We have gone on to show that the mechanisms whereby such protection is expressed are complex and multifaceted but are likely to involve protection of mitochondrial homeostasis and modulation of nuclear function that is at least partially involved with translational activity. Additionally, a switch from a necrotic to an apoptotic cell death pathway, coupled with an overexpression of a number of antiapoptotic proteins/pathways stimulated by UcnII, is likely to be of importance in the protective capacity of CRF2 activity. Our data suggest that activation of the endogenous CRF system of the inner ear (Vetter et al., 2002; Graham et al., 2010) may represent a novel avenue by which protection from oxidative stress can be further explored and underscore the potential importance of alternate signaling pathways that are distinct from the commonly exploited purely antioxidant pathways in establishing protection against cytotoxic agent exposures.
Supplementary Material
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: R01DC006258 (to D.E.V.); Contract grant sponsor: Genome Canada (to the University of Victoria Proteomics Centre).
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Bayatti N, Behl C. The neuroprotective actions of corticotropin releasing hormone. Ageing Res Rev. 2005;4:258–270. doi: 10.1016/j.arr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Brar BK, Stephanou A, Okosi A, Lawrence KM, Knight RA, Marber MS, Latchman DS. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol Cell Endocrinol. 1999;158:55–63. doi: 10.1016/s0303-7207(99)00183-5. [DOI] [PubMed] [Google Scholar]
- Brar B, Jonassen A, Stephanou A, Santilli G, Railson J, Knight R, Yellon D, Latchman D. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- Brar B, Stephanou A, Knight R, Latchman D. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol. 2002;34:483–492. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjos OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24–35. doi: 10.1210/en.2003-0689. discussion 21–23. [DOI] [PubMed] [Google Scholar]
- Burley DS, Hamid SA, Baxter GF. Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev. 2007;12:279–291. doi: 10.1007/s10741-007-9029-y. [DOI] [PubMed] [Google Scholar]
- Campo P, Subramaniam M, Henderson D. The effect of “conditioning” exposures on hearing loss from traumatic exposure. Hear Res. 1991;55:195–200. doi: 10.1016/0378-5955(91)90104-h. [DOI] [PubMed] [Google Scholar]
- Canlon B, Borg E, Flock A. Protection against noise trauma by pre-exposure to a low level acoustic stimulus. Hear Res. 1988;34:197–200. doi: 10.1016/0378-5955(88)90107-4. [DOI] [PubMed] [Google Scholar]
- Choe L, D’Ascenzo M, Relkin NR, Pappin D, Ross P, Williamson B, Guertin S, Pribil P, Lee KH. Eight-plex quantitation of changes in cerebrospinal fluid protein expression in subjects undergoing intravenous immunoglobulin treatment for Alzheimer’s disease. Proteomics. 2007;7:3651–3660. doi: 10.1002/pmic.200700316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott-Hunt CR, Kazlauskaite J, Wilde GJ, Grammatopoulos DK, Hill-house EW. Potential signalling pathways underlying corticotrophin-releasing hormone-mediated neuroprotection from excitotoxicity in rat hippocampus. J Neurochem. 2002;80:416–425. doi: 10.1046/j.0022-3042.2001.00712.x. [DOI] [PubMed] [Google Scholar]
- Fredelius L, Johansson B, Bagger-Sjoback D, Wersall J. Time-related changes in the guinea pig cochlea after acoustic overstimulation. Ann Otol Rhinol Laryngol. 1990;99:369–378. doi: 10.1177/000348949009900510. [DOI] [PubMed] [Google Scholar]
- Fujita R, Ueda M, Fujiwara K, Ueda H. Prothymosin-alpha plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition. Cell Death Differ. 2009;16:349–358. doi: 10.1038/cdd.2008.159. [E-pub November 2008]. [DOI] [PubMed] [Google Scholar]
- Graham CE, Basappa J, Vetter DE. A corticotropin-releasing factor system expressed in the cochlea modulates hearing sensitivity and protects against noise-induced hearing loss. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.01.014. doi: 10.1016/j.nbd.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Bielefeld E, Hu BH, Henderson D. Increased resistance to free radical damage induced by low-level sound conditioning. Hear Res. 2006;213:118–129. doi: 10.1016/j.heares.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Huizing EH, de Groot JC. Human cochlear pathology in aminoglycoside ototoxicity—a review. Acta Otolaryngol Suppl. 1987;436:117–125. doi: 10.3109/00016488709124984. [DOI] [PubMed] [Google Scholar]
- Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–38. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha S, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- Kalinec F, Kalinec G, Boukhvalova M, Kachar B. Establishment and characterization of conditionally immortalized organ of corti cell lines. Cell Biol Int. 1999;23:175–184. doi: 10.1006/cbir.1998.0339. [DOI] [PubMed] [Google Scholar]
- Kalinec G, Webster P, Lim D, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8:177–189. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- Kopke R, Allen K, Henderson D, Hoffer M, Frenz D, Van de Water T. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann NY Acad Sci. 1999;884:171–191. doi: 10.1111/j.1749-6632.1999.tb08641.x. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsas KP, Vartholomatos G, Tsepi C, Tsatsoulis A, Frangou-Lazaridis M. Fine-needle aspiration biopsy-RT-PCR expression analysis of prothymosin alpha and parathymosin in thyroid: novel proliferation markers? Neoplasma. 2007;54:57–62. [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Liu C, Yang C, Liu X, Li S. In vivo protective effects of urocortin on ischemia-reperfusion injury in rat heart via free radical mechanisms. Can J Physiol Pharmacol. 2005;83:459–465. doi: 10.1139/y05-033. [DOI] [PubMed] [Google Scholar]
- Liu QY, Lei JX, Sikorska M, Liu R. A novel brain-enriched E3 ubiquitin ligase RNF182 is up regulated in the brains of Alzheimer’s patients and targets ATP6V0C for degradation. Mol Neurodegener. 2008;3:4. doi: 10.1186/1750-1326-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martic G, Karetsou Z, Kefala K, Politou AS, Clapier CR, Straub T, Papamarcaki T. Parathymosin affects the binding of linker histone H1 to nucleosomes and remodels chromatin structure. J Biol Chem. 2005;280:16143–16150. doi: 10.1074/jbc.M410175200. [DOI] [PubMed] [Google Scholar]
- Matsui J, Ogilvie J, Warchol M. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Haque A, Huss D, Messana EP, Alosi JA, Roberson DW, Cotanche DA, Dickman JD, Warchol ME. Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo. J Neurosci. 2003;23:6111–6122. doi: 10.1523/JNEUROSCI.23-14-06111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Henderson D, Shen YH. Low-frequency “conditioning”provides long-term protection from noise-induced threshold shifts in chinchillas. Hear Res. 1997;103:142–150. doi: 10.1016/s0378-5955(96)00170-0. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999a;413:101–112. [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999b;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McFadden S, Ohlemiller K, Ding D, Shero M, Salvi R. The influence of superoxide dismutase and glutathione peroxidase deficiencies on noise-induced hearing loss in mice. Noise Health. 2001;3:49–64. [PubMed] [Google Scholar]
- Miyakita T, Hellstrom PA, Frimanson E, Axelsson A. Effect of low level acoustic stimulation on temporary threshold shift in young humans. Hear Res. 1992;60:149–155. doi: 10.1016/0378-5955(92)90017-h. [DOI] [PubMed] [Google Scholar]
- Niu X, Shao R, Canlon B. Suppression of apoptosis occurs in the cochlea by sound conditioning. Neuroreport. 2003;14:1025–1029. doi: 10.1097/01.wnr.0000070830.57864.32. [DOI] [PubMed] [Google Scholar]
- Oliveros JC. VENNY. An interactive tool for comparing lists with Venn diagrams. 2007 http://bioinfogp.cnb.csic.es/tools/venny/index.html.
- Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Drug ototoxicity. Annu Rev Pharmacol Toxicol. 1986;26:79–99. doi: 10.1146/annurev.pa.26.040186.000455. [DOI] [PubMed] [Google Scholar]
- Santi PA, Duvall AJd. Stria vascularis pathology and recovery following noise exposure. Otolaryngology. 1978;86:ORL354–ORL361. doi: 10.1177/019459987808600229. [DOI] [PubMed] [Google Scholar]
- Scarabelli T, Pasini E, Stephanou A, Comini L, Curello S, Raddino R, Ferrari R, Knight R, Latchman D. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J Am Coll Cardiol. 2002;40:155–161. doi: 10.1016/s0735-1097(02)01930-7. [DOI] [PubMed] [Google Scholar]
- Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- Slominski A, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system”? Ann NY Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton E, Mazurkiewicz J, Wei E. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Henderson D, Campo P, Spongr V. The effect of ‘conditioning’ on hearing loss from a high frequency traumatic exposure. Hear Res. 1992;58:57–62. doi: 10.1016/0378-5955(92)90008-b. [DOI] [PubMed] [Google Scholar]
- Suryadevara AC, Wanamaker HH, Pack A. The effects of sound conditioning on gentamicin-induced vestibulocochlear toxicity in gerbils. Laryngoscope. 2009;119:1166–1170. doi: 10.1002/lary.20145. [DOI] [PubMed] [Google Scholar]
- Tsitsiloni OE, Stiakakis J, Koutselinis A, Gogas J, Markopoulos C, Yialouris P, Bekris S, Panoussopoulos D, Kiortsis V, Voelter W, et al. Expression of alpha-thymosins in human tissues in normal and abnormal growth. Proc Natl Acad Sci USA. 1993;90:9504–9507. doi: 10.1073/pnas.90.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H. Prothymosin alpha plays a key role in cell death modeswitch, a new concept for neuroprotective mechanisms in stroke. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:315–323. doi: 10.1007/s00210-007-0254-7. [E-pub January 2008]. [DOI] [PubMed] [Google Scholar]
- Ueda H. Prothymosin alpha and cell death mode switch, a novel target for the prevention of cerebral ischemia-induced damage. Pharmacol Ther. 2009;123:323–333. doi: 10.1016/j.pharmthera.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Vago P, Humbert G, Lenoir M. Amikacin intoxication induces apoptosis and cell proliferation in rat organ of Corti. Neuroreport. 1998;9:431–436. doi: 10.1097/00001756-199802160-00014. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J, Polak M, Malgrange B, Lefebvre PP, Staecker H, Balkany TJ. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol. 2004;25:627–632. doi: 10.1097/00129492-200407000-00035. [DOI] [PubMed] [Google Scholar]
- Vetter D, Li C, Zhao L, Contarino A, Liberman M, Smith G, Marchuk Y, Koob G, Heinemann S, Vale W, Lee K. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Basappa J, Turcan S. Methods in molecular biology. Clifton, NJ: Humana Press; 2009. Multiplexed isobaric tagging protocols for quantitative mass spectrometry approaches to auditory research; pp. 345–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Konishi K, Iguchi H, Nakagawa T, Shibata S, Kato A, Sunami K, Kawakatsu C. The emergence of free radicals after acoustic trauma and strial blood flow. Acta Otolaryngol Suppl. 1995;519:87–92. doi: 10.3109/00016489509121877. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang H, Jiang B, Liang P, Liu M, Deng G, Xiao X. Nucleolin/C23 is a negative regulator of hydrogen peroxide-induced apoptosis in HUVECs. Cell Stress Chaperones. 2009 doi: 10.1007/s12192-009-0138-5. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.