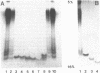
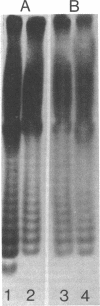
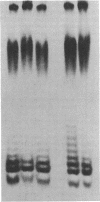
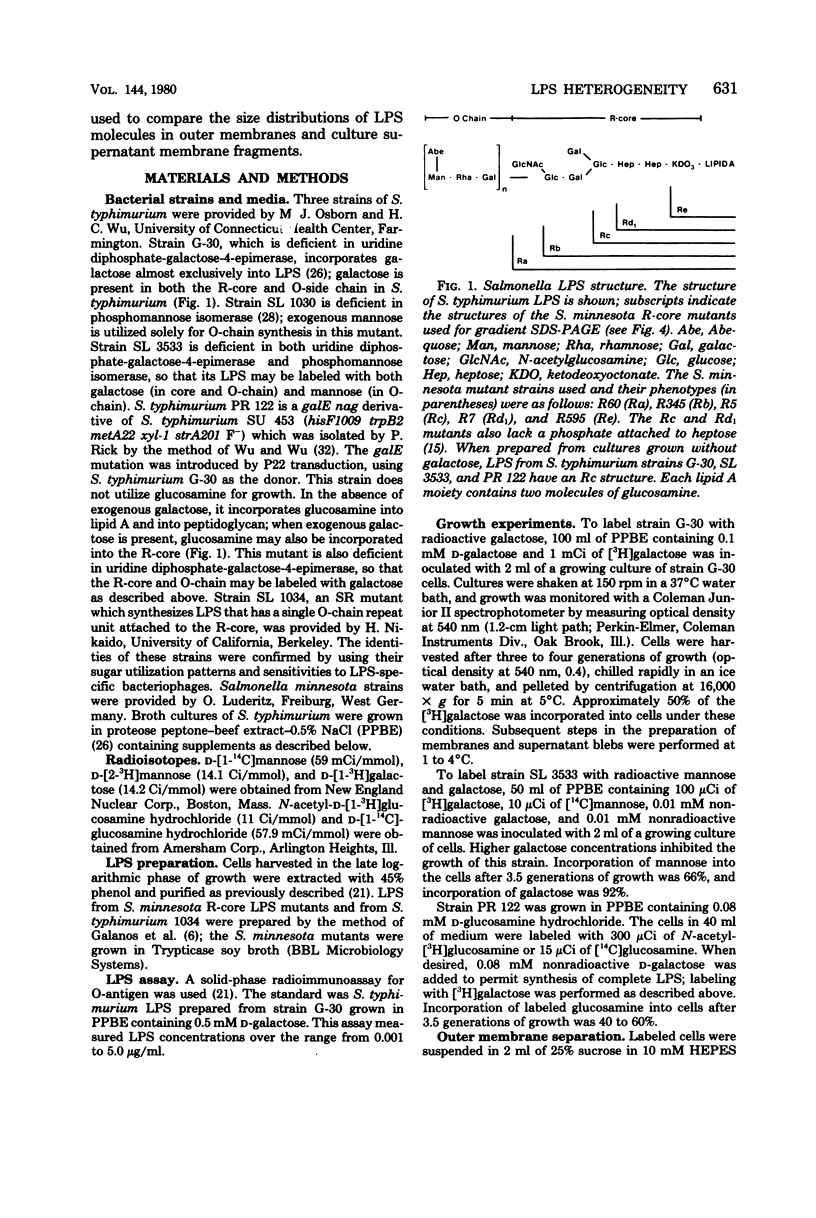
Abstract
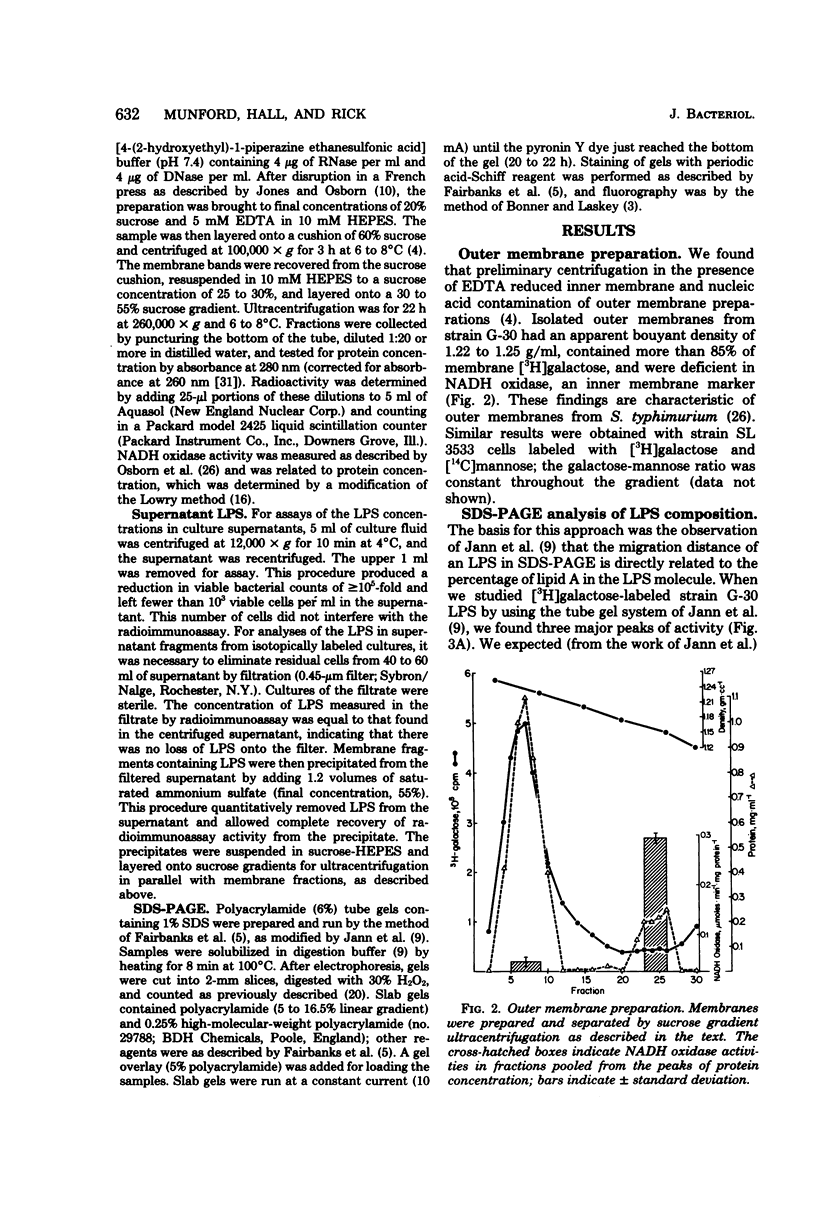
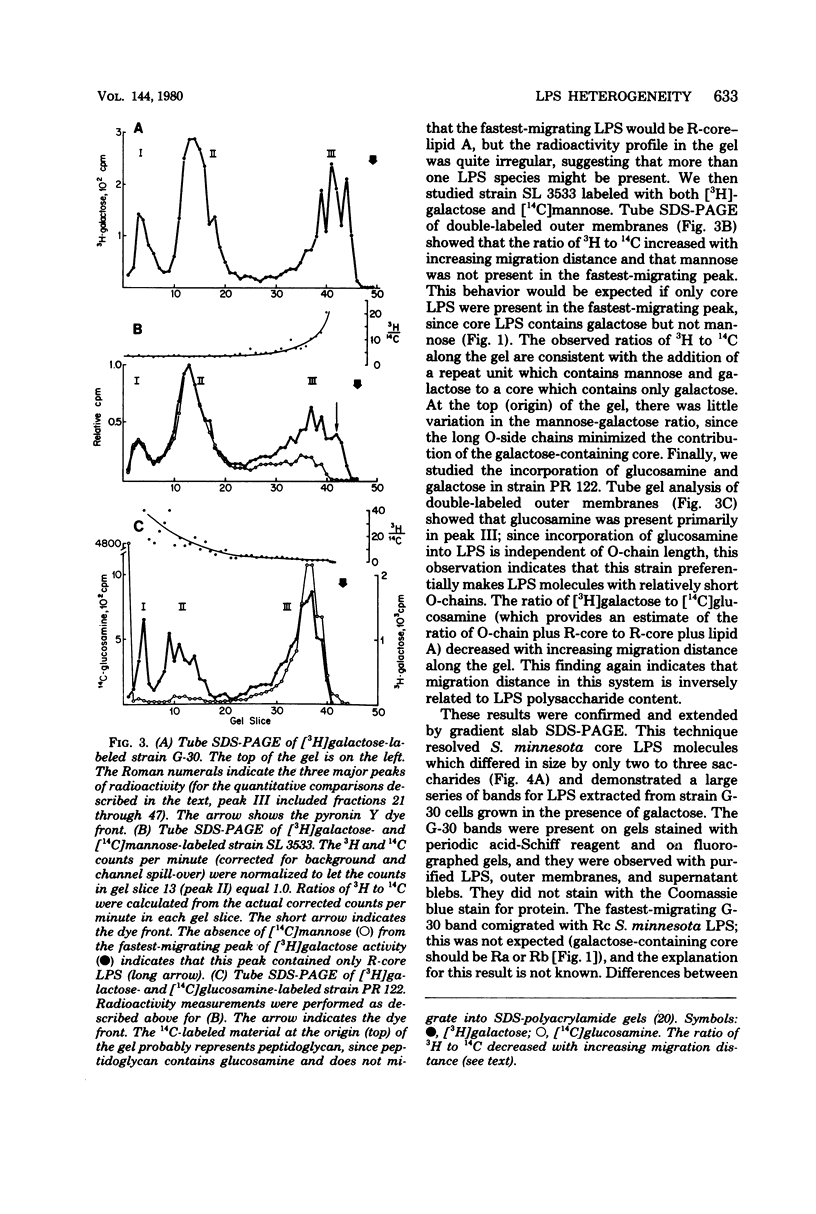
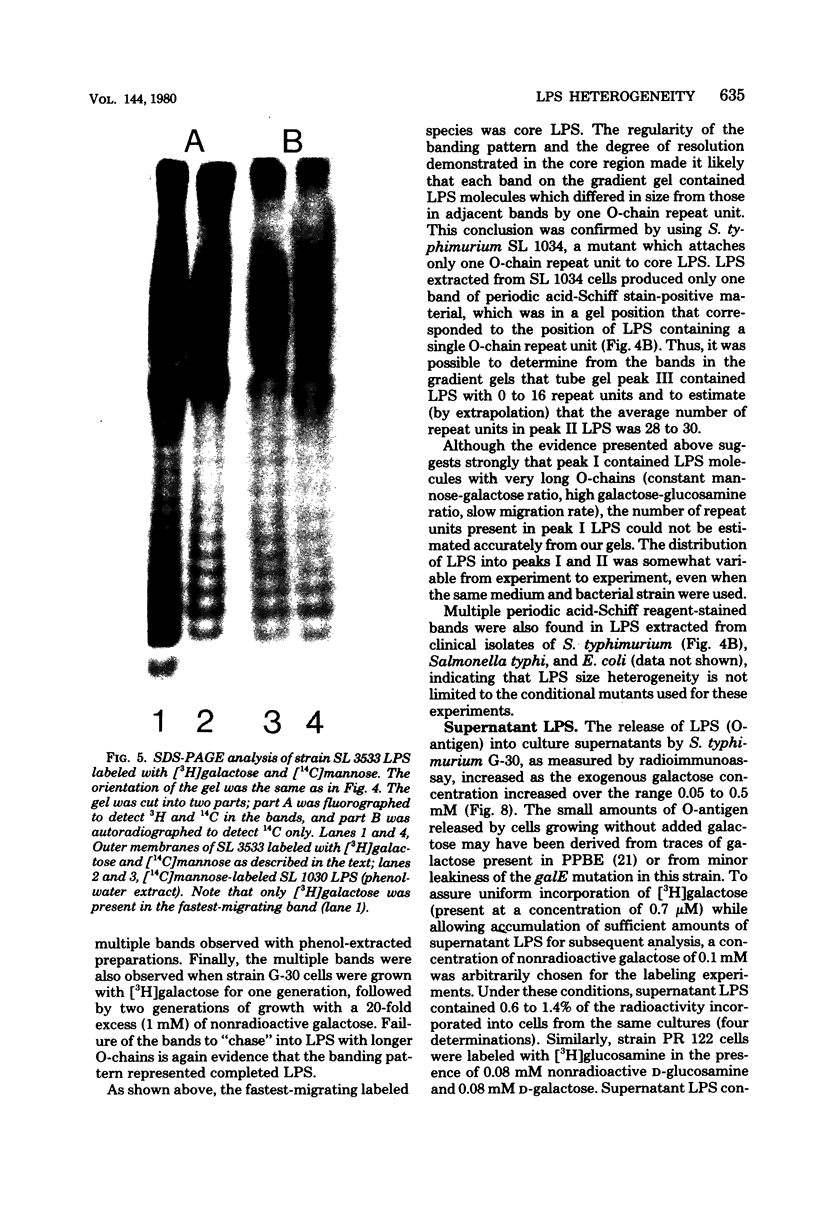
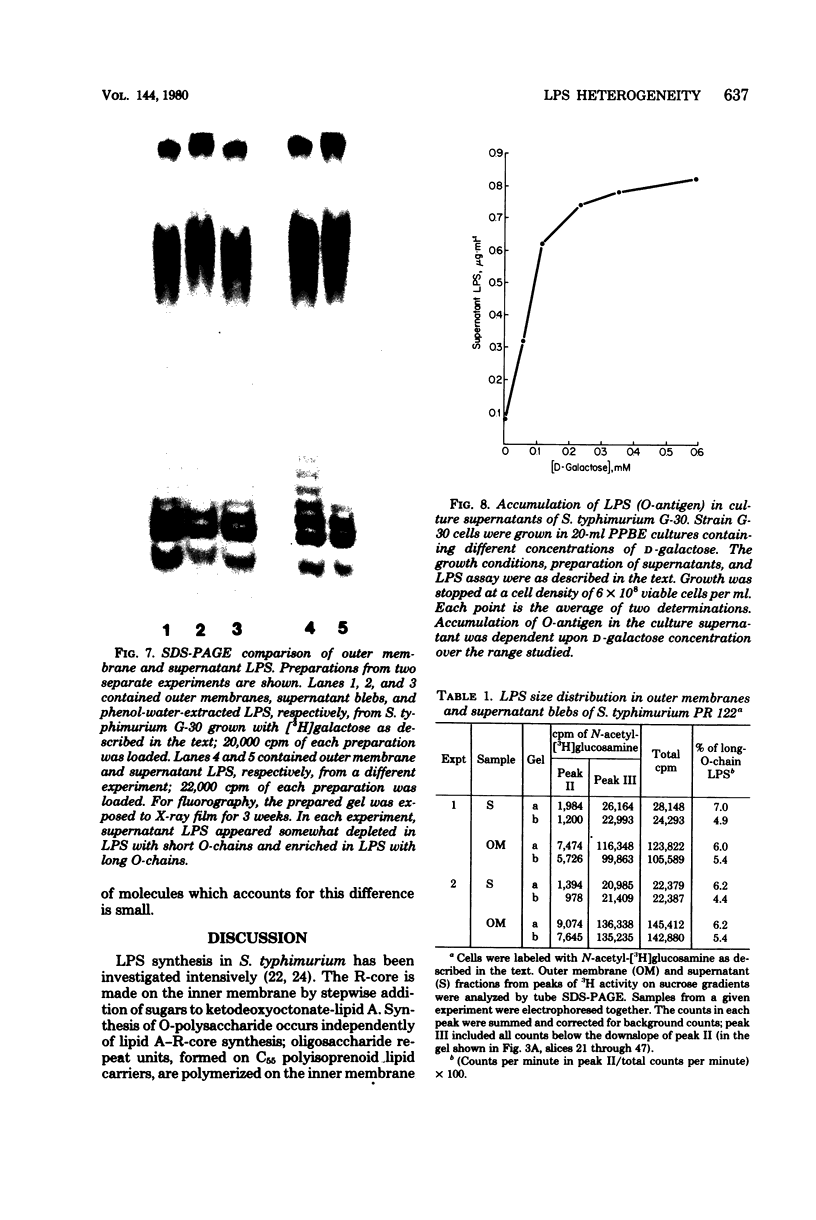
Enterobacteriaceae cells growing in liquid media shed fragments of their outer membranes. These fragments, which may constitute a biologically important form of gram-negative bacterial endotoxin, have been reported to contain proteins, phospholipids, and lipopolysaccharides (LPS). In this study we compared the sizes of LPS molecules in shed membrane fragments and outer membranes from cells growing in broth cultures. Using conditional mutants of Salmonella typhimurium which incorporate specific sugars into LPS, we analyzed radiolabeled LPS by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This technique revealed that S. typhimurium LPS are more heterogeneous than previously known; molecules possessing from 0 to more than 30 O-chain repeat units were identified in outer membranes, supernatant fragments, and purified LPS. The size distributions of LPS molecules in outer membranes and supernatant fragments were similar; supernatant fragments appeared to be slightly enriched in molecules with long O-polysaccharide chains. Our results indicate the LPS molecules of many sizes are synthesized, translocated to outer membranes, and released into culture supernatants. Since the hydrophilic O-polysaccharides extend from bacterial surfaces into the aqueous environment, our findings suggest that the cell surface topography of this bacterium may be very irregular. We also speculate that heterogeneity in the degree of polymerization of O-antigenic side chains may influence the interactions of the toxic moiety of LPS (lipid A) with host constituents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. C., Apirion D. Identification of lipopolysaccharides and phospholipids of Escherichia coli in polyacrylamide gels. J Bacteriol. 1977 Jul;131(1):347–355. doi: 10.1128/jb.131.1.347-355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Properties of the O-specific hapten formed in vivo by mutant strains of Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Evaluation of some extraction methods for the preparation of bacterial lipopolysaccharides for structural analysis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):751–759. doi: 10.1111/j.1699-0463.1972.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Gotschlich E. C. Iodination of Escherichia coli with chloramine T: selective labeling of the outer membrane lipoprotein. J Bacteriol. 1977 May;130(2):775–780. doi: 10.1128/jb.130.2.775-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Radioimmunoassay for Gram-negative bacterial lipopolysaccharide O antigens: influence of antigen solubility. Infect Immun. 1979 Oct;26(1):42–48. doi: 10.1128/iai.26.1.42-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H., Whang H. Y., Neter E. Participation of lipopolysaccharide genes in the determination of the enterobacterial common antigen: analysis of R mutants of Salmonella minnesota. J Bacteriol. 1974 Sep;119(3):760–764. doi: 10.1128/jb.119.3.760-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Hellerqvist C. G., Valtonen V. V., Mäkelä P. H. The smooth lipopolysaccharide character of 1,4,(5),12 and 1,9,12 transductants formed as hybrids between groups B and D of salmonella. Eur J Biochem. 1971 Oct 26;22(4):500–505. doi: 10.1111/j.1432-1033.1971.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Rosen S. M., Zeleznick L. D., Fraenkel D., Wiener I. M., Osborn M. J., Horecker B. L. Characterization of the cell wall lipopolysaccharide of a mutant of Salmonella typhimurium lacking phosphomannose isomerase. Biochem Z. 1965 Aug 19;342(4):375–386. [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]