Abstract
Background
The aim of this study was to compare the outcomes of the management of perianal fistulas in Crohn disease between infliximab, surgery or a combination of surgery and infliximab.
Methods
We prospectively subdivided 35 consecutive patients with Crohn disease with complex perianal fistulas into 3 groups: 11 patients received infliximab (5 mg/kg intravenously at 0, 2 and 6 wk; group A), 10 underwent surgery (group B) and 14 received a combination of surgery and postoperative infliximab (group C). We evaluated the rate and time of healing of perianal fistulas, the rate of recurrences and time to relapse at a median follow-up of 18.8 (standard deviation [SD] 10.8, range 8–38) months.
Results
The time to healing of fistulas was significantly shorter among patients who received surgery and infliximab than among those who received surgery alone (p < 0.05) and was close to statistically shorter among those who received both treatments than among those who received infliximab alone (p = 0.06). Patients who received surgery and infliximab had a significantly longer mean time to relapse (p < 0.05) than those who received infliximab (mean 2.6 [SD 0.7] mo) or surgery alone (mean 3.6 [SD 0.5] mo).
Conclusion
We found better outcomes among patients who received a combination of surgery and infliximab therapy. These patients experienced a short time to healing of fistulas and significantly longer mean time to relapse of complex fistulas.
Abstract
Contexte
Cette étude avait pour but de comparer les résultats du traitement des fistules périanales dans les cas de maladie de Crohn par administration d’infliximab, intervention chirurgicale ou une combinaison des 2.
Méthodes
Nous avons subdivisé de façon prospective, en 3 groupes, 35 patients consécutifs atteints de la maladie de Crohn qui avaient une fistule périanale complexe : 11 patients ont reçu de l’infliximab (5 mg/kg par voie intraveineuse à 0, 2 et 6 semaines; groupe A), 10 ont subi une intervention chirurgicale (groupe B) et 14 ont subi une intervention chirurgicale et ont reçu de l’infliximab après l’intervention (groupe C). Nous avons évalué le taux et la durée de la guérison des fistules périanales, le taux de récidive et la période écoulée avant la récidive à un suivi médian de 18,8 (écart-type [ET] 10,8, intervalle de 8 à 38) mois.
Résultats
La période de guérison des fistules a été beaucoup plus brève chez les patients qui ont subi une intervention chirurgicale et ont reçu de l’infliximab que chez ceux qui ont subi une intervention chirurgicale seulement (p < 0,05) et elle a été presque statistiquement plus brève chez ceux qui ont reçu les 2 traitements que chez ceux qui ont reçu de l’infliximab seulement (p = 0,06). Chez les patients qui ont subi une intervention chirurgicale et ont reçu de l’infliximab, la période moyenne qui s’est écoulée avant la rechute a été beaucoup plus longue (p < 0,05) que chez ceux qui ont reçu de l’infliximab (moyenne de 2,6 [ET, 0,7] mois) ou qui ont subi une intervention chirurgicale seulement (moyenne de 3,6 [ET, 0,5] mois).
Conclusion
Nous avons constaté de meilleurs résultats chez les patients qui ont subi une intervention chirurgicale combinée à un traitement à l’infliximab. Chez ces patients, la guérison des fistules a pris moins de temps et la période moyenne qui s’est écoulée avant la réapparition des fistules complexes a été beaucoup longue.
Perianal fistulas affect up to 20%–30% of patients with Crohn disease.1,2 The treatment of anorectal fistula associated with Crohn disease is difficult, particularly when the rectum is diseased, and optimal management is still a matter of debate. Therapeutic strategies for fistulizing Crohn disease involve both medical and surgical approaches but have largely unsatisfactory results, leading to proctectomy in 10% to 18% of cases.3–6
Infliximab, a murine chimeric monoclonal antibody against tumour necrosis factor (TNF)-α, has been reported to heal 46% of fistulas in Crohn disease7 but is associated with an increased rate of abscesses formation.2 Although the response to infliximab therapy may be dramatic, the median duration of fistula closure following an induction regimen of infliximab is 3 months, and repeated infusions are often required.8
Examination under anesthesia is the most common procedure performed to assess fistulous tracks.9,10 Surgical options include conventional fistulotomy, long-term seton drainage and advancement flap repair, whereas total proctectomy with a terminal stoma is reserved for patients with a severe rectal or perianal Crohn disease. Comparisons between operative treatment alone or in association with infliximab are limited.11 Investigators have reported that a combination of seton placement and infliximab results in an earlier initial response, lower recurrence rates and longer time to relapse than infliximab alone.8 Our aim in this study was to compare the outcomes of management of fistulizing perianal Crohn disease with infliximab, surgery or a combination of both infliximab and surgery in a prospective, nonrandomized study.
Methods
Between October 2001 and July 2007, 35 consecutive patients, all of European descent, with perianal complex fistulas because of Crohn disease were referred from gastroenterologists to our institution. Their disease had not responded to conventional medical treatments, including antibiotics (metronidazole 750–1500 mg/d or ciprofloxacin 1000 mg/d for 3 mo)12 and immunosuppressant therapy (azathioprine 2–2.5 mg/kg/d or methotrexate 25 mg/wk for at least 2 mo).13 All patients underwent clinical examinations, magnetic resonance imaging (MRI) or endoscopic ultrasound14 to classify the type of fistula and endoscopy for the evaluation of rectal inflammation. We defined the types of fistulas as simple or complex. Simple fistulas were defined as low (subcutaneous, low intersphincteric or low transphincteric) with a single external opening, no pain or fluctuation and no evidence of either rectovaginal fistulas or rectal strictures. Complex fistulas were defined as high (high intersphincteric, high transphincteric, suprasphincteric or extrasphincteric) with either multiple external openings associated with such pain or fluctuation to suggest a perianal abscess or with a rectovaginal fistula or anorectal stricture.12 We excluded patients with simple fistulas from this study. We recorded the Perianal Crohn’s Disease Activity Index score (PDAI) for all patients before treatment.15
Intervention
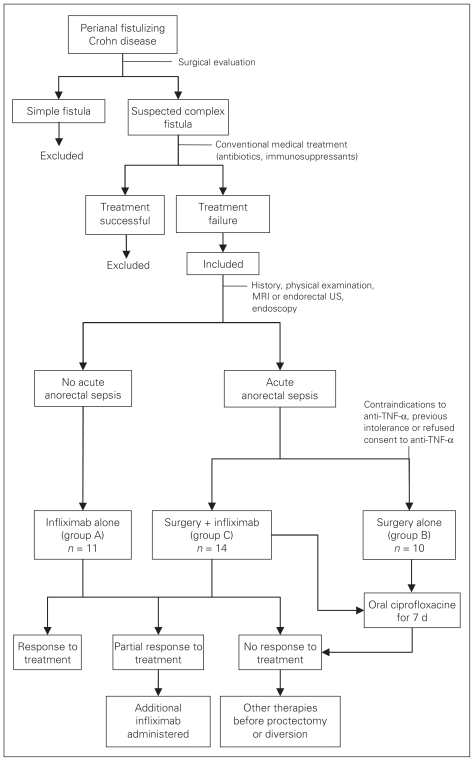
Patients received one of the following treatments: infliximab alone (group A), surgery alone (group B) or surgery and infliximab (group C). In our pretreatment evaluation, no significant differences were observed among the 3 groups by either endoscopic investigation or PDAI assessment. All patients were screened for contraindications to anti-TNF-α therapy. Women in groups A and C also underwent β-HCG evaluation every 3 weeks. In the absence of undrained acute anorectal sepsis (defined as areas of fluctuation, tenderness or induration), patients received infliximab alone. In the presence of acute anorectal sepsis, patients underwent surgery before infliximab administration. All patients with evidence of acute anorectal sepsis and a history of tuberculosis, known hypersensitivity to any murine proteins or other component of infliximab and those who did not consent to receiving biological therapy underwent surgery alone. The treatment algorithm is shown in Figure 1.
Fig. 1.
Study algorithm. MRI = magnetic resonance imaging; TNF = tumour necrosis factor; US = ultrasonography.
Patients in groups B and C were given 500 mg metronidazole intravenously. They received 1000 mg cefuroxime intraoperatively and were continued postoperatively on oral ciprofloxacin (1000 mg/d) for 7 days.16 Infliximab was infused using a standard protocol (groups A and C; 5 mg/kg intravenously after intravenous infusion of 100 mg of steroids at 0, 2 and 6 wk).8,13
Patients with a partial response were given additional infliximab (5 mg/kg intravenously every 8 wk);8,13 infliximab was not continued in nonresponders, and other therapies were carried out before eventually proceeding to fecal diversion or proctectomy.
For patients in group C, the first infusion of infliximab (time 0) was started within 24 hours after surgery.13 All patients given infliximab were monitored for adverse reactions. Patients in groups B and C underwent examination under anesthesia in the lithotomy position. Fistula tracks were treated by curettage, irrigation with saline and apposition of loose seton (soft silastic drain) to facilitate drainage. In group B patients, the seton was removed in the office if there was evidence of good healing both in the wounds and around the seton and complete cessation of drainage after 2 consecutive follow-up visits. If there was incomplete healing, the seton was used for long-term drainage. In group C patients, the seton drain was removed after the second infliximab administration if sepsis was not present. If there was no response, the seton was left in place.2,8
Postintervention evaluation
All patients were clinically evaluated during an outpatient visit every 2 weeks for 3 months and subsequently once a month, and local health status was recorded. We assessed the patients’ response to therapy 6 months after treatment during follow-up. We defined clinical response as complete if there was closure of all external openings and cessation of fistula drainage for more than 3 months. A reduction in the size, number, drainage or discomfort associated with a fistula was defined as a partial response. Patients with a persistent fistula at follow-up were considered to be nonresponders. We defined recurrence as the reopening of external fistula tracks with active drainage or the development of a perianal abscess at the site of the original fistula.
Statistical analyses
The results are expressed as mean and standard deviation (SD). We compared the differences in means between subgroups by use of the 2-tailed Student t test. Comparisons between categorical variables were made using the Fisher exact test or the Kruskal–Wallis test as appropriate. We considered p < 0.05 to be statistically significant.
Results
Eleven patients received infliximab alone (group A), 10 underwent surgery alone (examination under anesthesia and seton placement; group B) and 14 received a combination of surgery and postoperative infliximab therapy (group C). The patient characteristics are shown in Table 1. Twelve patients were also classified by MRI and 23 by endoscopic ultrasound. No deaths or major adverse side effects were observed during infliximab infusion. One patient complained of headache during the third administration of the drug, and one experienced light chest pain during the second infusion, but, despite this, the infliximab induction protocol was successfully carried out. We did not find significant differences in treatment efficacy between the patients who were previously given infliximab and those who were not. There were no adverse events associated with surgery. The median follow-up after treatment was 18.8 (SD 10.8, range 8–38) months.
Table 1.
Characteristics of patients
| Characteristic | Infliximab (group A), n = 11 | Surgery (group B), n = 10 | Infliximab + surgery (group C), n = 14 |
|---|---|---|---|
| Sex, male:female ratio | 4:7 | 3:7 | 6:8 |
| Current regular smoker, no. of patients | 4 | 3 | 6 |
| Median (range) | |||
| Age, yr | 36.3(19–63) | 33.1 (16–58) | 35.3 (18–65) |
| Duration of disease, yr | 8.8(1–14) | 7.9 (0.9–18) | 7.4 (0.8–21) |
| Duration of fistula, mo | 13.8(6–33) | 14.5 (4–29) | 12.1 (5–42) |
| Preoperative PCDAI score | 10.7(7–13) | 11.2 (7–15) | 11.07 (9–16) |
| Disease location, no. of patients | |||
| Small bowel | 2 | 2 | 3 |
| Colon | 4 | 3 | 4 |
| Small bowel and colon | 5 | 5 | 6 |
| Perianal only | 0 | 0 | 1 |
| Previous procedure, no. of patients | |||
| Resection for Crohn disease | 5 | 3 | 0 |
| Perianal surgery | 3 | 2 | 8 |
| Immunomodulator therapy | 11 | 10 | 14 |
| Antibiotics | 11 | 10 | 14 |
| Anti-TNF-α therapy | 2 | 3 | 1 |
| Magnetic resonance imaging | 4 | 3 | 5 |
| Endoscopic ultrasonography | 7 | 7 | 9 |
PCDAI = Perianal Crohn’s Disease Activity Index; TNF = tumour necrosis factor.
The outcomes of the interventions are shown in Table 2. There were no statistically significant differences found in the overall treatment response among the 3 groups (p = 0.74). No statistically significant differences were found in the rate of proctectomy or diversion among the 3 groups. Specifically, after an initial partial response, 1 patient in group A with proctitis developed a severe rectal stricture 5 weeks after infliximab therapy (2 administrations) and underwent proctectomy with a terminal colostomy because of obstruction. Two patients in group B received a proctectomy or diversion, either for fistula recurrence or worsened clinical symptoms. When we considered only the patients who completely responded to treatment, there was no statistically significant difference in the rate of recurrent fistula and persistent healing among the 3 groups. In patients who responded completely to treatment, the time to healing of fistulas was significantly shorter in group C than in group B (p = 0.041). There was no statistically significant difference in time to healing between groups A and C (p = 0.06). Among patients who had a recurrence after treatment, those in group C had a significantly longer mean time to relapse than those in groups A (p = 0.012) and B (p = 0.016).
Table 2.
Outcome of interventions
| Outcome | Infliximab (group A), n = 11 | Surgery (group B), n = 10 | Infliximab + surgery (group C), n = 14 | p value |
|---|---|---|---|---|
| Treatment response, no. of patients* | 0.74 | |||
| No response | 1 | 1 | 1 | |
| Partial response | 3 | 2 | 2 | |
| Complete response | 7 | 7 | 11 | |
| Proctectomy or diversion | 1/11 | 2/10 | 0/14 | 0.2 |
| Healed† | 4/7 | 4/7 | 9/11 | 0.9 |
| Healing time of fistula,† mean (SD), mo | 3.0 (0.8) | 4.2 (1.3) | 3.1 (0.8)‡ | |
| Recurrent fistula | 3/7 | 3/7 | 2/11 | 0.2 |
| Time to relapse, mean (SD), mo | 2.6 (0.7)§ | 3.6 (0.5)§ | 10.1 (2.4) | |
SD = standard deviation.
Patients assessed at 6-month follow-up visit.
Refers to patients with a complete response.
p < 0.05 v. group B.
p < 0.05 v. group C.
Discussion
The best treatment for fistulizing perianal Crohn disease is unclear and controversial.17 All conventional medical therapies have previously showed limited efficacy,2,18–20 with roughly one-third of patients with Crohn disease requiring surgical treatment of associated septic complications.1
An appropriate surgical choice depends on the type of fistula, disease activity and the presence or absence of either concomitant intestinal disease and undrained sepsis.21 In a study comparing endoscopic ultrasonography, MRI and examination under anesthesia for the evaluation of Crohn perianal fistulas, Schwartz and colleagues14 found that all 3 modalities had similar accuracy rates. High or complex fistulas require a conservative surgical approach to reduce the risk of incontinence, and noncutting setons or endorectal advancement flap procedures are the treatment of choice for patients who do not respond to medical therapy. The outcomes of surgery for complex perianal diseases are contradictory. An American Gastroenterological Association review12 of 29 studies involving patients with high or complex fistulas reported largely heterogeneous results with postoperative healing rates ranging from 0% to 100%, recurrence rates from 0% to 75% and proctectomy rates from 0% to 60%.
The advent of the anti-TNF-α antibody represents a major advance in the medical treatment of Crohn anal fistulas and has been shown in placebo-controlled trials to be effective in reducing the number of draining fistulas in the long term.7,13,22 Previous studies evaluating the efficacy of infliximab in the treatment of fistulizing Crohn disease have reported a 68% clinical response7 and healing rates ranging between 24% and 55%.7,23–26 Infliximab therapy has been reported to be associated with an increased rate of abscess formation in up to 11% of patients compared with 3% who received placebo.7 In the ACCENT II trial, Sands and colleagues22 reported an overall rate of abscess formation of 14.9% during a 52-week period, but the rate was slightly lower in patients receiving maintenance infliximab therapy rather than placebo. In patients who showed a clinical response, abscess development is presumably due to either closure of the external os or by the persistence of fistula tracks with varying degrees of residual inflammation, as shown by MRI and endoscopic ultrasound studies.27,28
Seton placement is indicated when an external orifice is at risk of closing prematurely; this risk is more likely with infliximab therapy.2 Hyder and colleagues13 demonstrated that a protocol-based approach using examination under anesthesia before infliximab therapy complemented selectively by MRI and seton drains may be associated with either a zero rate of abscess formation or exacerbation of anal infection. Davies and colleagues11 maintained that the risk of abscess development persists even when infliximab is used along with surgery. A recently reported observational study suggested that many patients with fistulas treated by infliximab still require surgical intervention.29 Regueiro and colleagues8 reported that patients who underwent examination under anesthesia before infliximab infusion had a better initial response (100% v. 82.6%), lower recurrence rates (44% v. 79%) and a longer time to recurrence (13.5 v. 3.6 mo) compared with those who received infliximab alone. A similar retrospective study carried out by Topstad and colleagues2 reporting on the use of seton placement, infliximab infusion and maintenance immunosuppressive therapy showed a long-term complete response in 67% of patients and a partial response (defined as decreased drainage) in 19% of patients. Gaertner and colleagues,30 in the largest published series of perianal Crohn fistulas treated with surgery and infliximab, reported that operative treatment resulted in complete fistula healing in about 60% of patients and that preoperative infliximab infusion did not affect overall healing rates.
Based on previous reported experience,2,13,31 we hypothesized that seton placement in association with infliximab therapy may produce uniform healing of fistulous tracks and reduce perianal sepsis. Our results suggest that appropriate surgical drainage in association with infliximab administration may be more advantageous than their use alone. This may be either because of the biological effect of earlier healing derived from infliximab therapy or because of the reduced risk of abscess development associated with noncutting seton placement. Indeed, seton placement is likely to reduce the risk of perianal abscess formation by maintaining constant fistula drainage,12 but the use of a loose seton should be time-limited to ease fistula healing. In our study, patients receiving infliximab had shorter time to healing fistulas; however, there was only a statistically significant difference between those who received infliximab and surgery compared with those who received only surgery.
Hyder and colleagues13 showed that infliximab in combination with surgery is a safe and effective short-term treatment in patients with fistulizing anal CD, but sustained long-term clinical fistula healing (median follow-up 21 mo) was achieved by only 18% of patients. Indeed, we did not find a statistically significant difference in the rate of recurrent fistulas and persistent healing among the 3 groups (Table 2), but we did find a significantly longer mean time to relapse in patients who received a combination of surgery and infliximab compared with those who received either therapy alone. A similar finding was reported by Regueiro and colleagues,8 who found that the mean time to recurrence of complex fistulas in patients who received infliximab plus examination under anesthesia and seton placement was longer than in patients who received infliximab alone (13 v. 2.14 mo, p = 0.001).
In our study, patients who received surgery and infliximab had their rectum spared in cases of persistent perianal disease, whereas 10% of patients overall (1 in group A, 2 in group B, 0 in group C) required proctectomy or diversion. According to Davies and colleagues,11 this finding, although not statistically significant, may suggest that patients who receive a combined approach, even in the case of persisting fistulas, are more likely to have the rectum preserved.
Although in the ACCENT II study,22 long-term treatment with infliximab significantly prolonged the duration of complete closure of fistulas, our study only evaluated outcomes after a short course of infliximab, and we did not routinely use it as maintenance therapy. Indeed, we observed no substantial improvement in patients undergoing repeated infusions. Maintenance treatment with infliximab is expensive and, if prolonged, may require concomitant use of immunosuppressive drugs to counteract the development of human antichimeric antibodies that may lead to infusion reactions, loss of efficacy and, rarely, serious infections.12
This study, because of its nonrandomized prospective design, has a possible selection bias related to the choice of treatment on the basis of the patient’s clinical features and different grades of sepsis. Our findings must be further confirmed, possibly by a controlled study involving a larger number of patients, before drawing definitive conclusions.
In conclusion, we found a better outcome of management of fistulizing Crohn disease in patients who received a combination of infliximab and surgery. These patients experienced a shorter time to healing and a significantly longer mean time to relapse of fistulas.
Footnotes
Competing interests: None declared.
Contributors: Drs. Sciaudone, Riegler and Selvaggi designed the study. Drs. Di Stazio, Guadagni, Pellino, Coscione and Selvaggi acquired the data, which Drs. Sciaudone, Limongelli, Riegler and Selvaggi analyzed. Drs. Sciaudone, Di Stazio, Limongelli, Guadagni, Pellino and Selvaggi wrote the article, which Drs. Riegler, Coscione and Selvaggi reviewed. All author approved publication of the article.
References
- 1.Schwartz DA, Loftus EV, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–80. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 2.Topstad DR, Panaccione R, Heine JA, et al. Combined seton placement, infliximab infusion, and maintenance immunosoppressives improve healing rate in fistulizing anorectal Crohn’s disease. A single center experience. Dis Colon Rectum. 2003;46:577–83. doi: 10.1007/s10350-004-6611-4. [DOI] [PubMed] [Google Scholar]
- 3.White RA, Eisenstat TE, Rubin RJ, et al. Seton management of complex anorectal fistulas in patients with Crohn’s disease. Dis Colon Rectum. 1990;33:587–9. doi: 10.1007/BF02052212. [DOI] [PubMed] [Google Scholar]
- 4.Wolff BG, Culp CE, Beart RW, Jr, et al. Anorectal Crohn’s disease. A long-term perspective. Dis Colon Rectum. 1985;28:709–11. doi: 10.1007/BF02560279. [DOI] [PubMed] [Google Scholar]
- 5.Levien DH, Surrel J, Mazier WP. Surgical treatment of anorectal fistula in patients with Crohn’s disease. Surg Gynecol Obstet. 1989;169:133–6. [PubMed] [Google Scholar]
- 6.Williams JG, Rothenberger DA, Nemer FD, et al. Fistula-in-ano in Crohn’s disease. Results of aggressive surgical treatment. Dis Colon Rectum. 1991;34:378–84. doi: 10.1007/BF02053687. [DOI] [PubMed] [Google Scholar]
- 7.Present DH, Rutgeers P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 8.Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn’s disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis. 2003;9:98–103. doi: 10.1097/00054725-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Steinhart AH, McLeod RS. Medical and surgical management of perianal Crohn’s disease. Inflamm Bowel Dis. 1996;2:200–10. [PubMed] [Google Scholar]
- 10.Williamson PR, Hellinger MD, Larach SW, et al. Twenty year review of the surgical management of perianal Crohn’s disease. Dis Colon Rectum. 1995;38:389–92. doi: 10.1007/BF02054227. [DOI] [PubMed] [Google Scholar]
- 11.Davies MM, Larson DW, Wolff BG, et al. Surgery versus surgery with infliximab for perianal fistulae in Crohn’s patients: a case matched study [abstract] Colorectal Dis. 2005;7(Suppl 1):1–2. [Google Scholar]
- 12.Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–30. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Hyder SA, Travis SPL, Jewell DP, et al. Fistulating anal Crohn’s disease: results of combined surgical and infliximab treatment. Dis Colon Rectum. 2006;49:1837–41. doi: 10.1007/s10350-006-0656-5. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz DA, Wiersema MJ, Dudiak KM, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas. Gastroenterology. 2001;121:1064–72. doi: 10.1053/gast.2001.28676. [DOI] [PubMed] [Google Scholar]
- 15.Sostegni R, Daperno M, Scaglione N, et al. Review article: Crohn’s disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17:11–7. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 16.Turunen U, Farkila M, Seppala K. Long term treatment of perianal or fistulous Crohn’s disease with ciprofloxacin. Scand J Gastroenterol. 1989;24(Suppl):144. [Google Scholar]
- 17.Singh B, Mortensen NJ, Jewell DP, et al. Perianal Crohn’s disease. Br J Surg. 2004;91:801–14. doi: 10.1002/bjs.4613. [DOI] [PubMed] [Google Scholar]
- 18.Present DH, Lichtier S. Efficacy of cyclosporine in treatment of fistula of Crohn’s disease. Dig Dis Sci. 1994;39:374–80. doi: 10.1007/BF02090211. [DOI] [PubMed] [Google Scholar]
- 19.Hanauer SB, Smith MB. Rapid closure of Crohn’s disease fistulas with continuous intravenous cyclosporine A. Am J Gastroenterol. 1993;88:646–9. [PubMed] [Google Scholar]
- 20.Lowry PW, Weaver AL, Tremaine WJ, et al. Combination therapy with oral tacrolimus (FK506) and azathioprine or 6-mercaptopurine for treatment refractory Crohn’s disease perianal fistulae. Inflamm Bowel Dis. 1999;5:239–45. doi: 10.1097/00054725-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Bayer I, Gordon PH. Selected operative management of fistula-in-ano in Crohn’s disease. Dis Colon Rectum. 1994;37:760–5. doi: 10.1007/BF02050138. [DOI] [PubMed] [Google Scholar]
- 22.Sands BE, Blank MA, Diamond RH, et al. Maintenance infliximab does not result in increased abscess development in fistulizing Crohn’s disease: results from the ACCENT II study. Aliment Pharmacol Ther. 2006;23:1127–36. doi: 10.1111/j.1365-2036.2006.02878.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen RD, Tsang JF, Hanauer SB. Infliximab in Crohn’s disease: first anniversary clinical experience. Am J Gastroenterol. 2000;95:3469–77. doi: 10.1111/j.1572-0241.2000.03363.x. [DOI] [PubMed] [Google Scholar]
- 24.Sample C, Bailey RJ, Todoruk D, et al. Clinical experience with Infliximab for Crohn’s disease: the first 100 patients in Edmonton, Alberta. Can J Gastroenterol. 2002;16:165–70. doi: 10.1155/2002/379307. [DOI] [PubMed] [Google Scholar]
- 25.Arnott ID, McDonald D, Williams A, et al. Clinical use of infliximab in Crohn’s disease: the Edinburgh experience. Aliment Pharmacol Ther. 2001;15:1639–46. doi: 10.1046/j.1365-2036.2001.01092.x. [DOI] [PubMed] [Google Scholar]
- 26.Ricart E, Panaccione R, Loftus EV, et al. Infliximab for Crohn’s disease in clinical practice at the Mayo Clinic. The first 100 patients. Am J Gastroenterol. 2001;96:722–9. doi: 10.1111/j.1572-0241.2001.03612.x. [DOI] [PubMed] [Google Scholar]
- 27.van Assche G, Vanbeckevoort D, Coremans G, et al. MRI imaging of the effects of Infliximab in perianal fistulazing Crohn’s disease. Gastroenterology. 2001;120(Suppl 1):A68. doi: 10.1111/j.1572-0241.2003.07241.x. [DOI] [PubMed] [Google Scholar]
- 28.van Bodegraven AA, Sloots CE, Felt-Bersma RJ, et al. Endosonographic evidence of persistence of Crohn’s disease-associated fistulas after infliximab treatment, irrespective of clinical response. Dis Colon Rectum. 2002;45:39–45. doi: 10.1007/s10350-004-6111-6. [DOI] [PubMed] [Google Scholar]
- 29.Poritz LS, Rowe WA, Koltun WA. Remicade does not abolish the need for surgery in fistulizing Crohn’s disease. Dis Colon Rectum. 2002;45:771–5. doi: 10.1007/s10350-004-6296-8. [DOI] [PubMed] [Google Scholar]
- 30.Gaertner WB, Decanini A, Mellgren A, et al. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas. Dis Colon Rectum. 2007;50:1754–60. doi: 10.1007/s10350-007-9077-3. [DOI] [PubMed] [Google Scholar]
- 31.Talbot C, Sagar PM, Johnston MJ, et al. Infliximab in the surgical management of complex fistulating anal Crohn’s disease. Colorectal Dis. 2005;7:164–8. doi: 10.1111/j.1463-1318.2004.00749.x. [DOI] [PubMed] [Google Scholar]