Abstract
Background
Hepatic stellate cells, the main mediators in the pathogenesis of fibrosis, are triggered by free radicals and produce collagen. Melatonin is a powerful physiologic scavenger of hydroxyl radicals. It is also involved in the inhibitory regulation of the collagen content in tissue. There is no effective treatment available for liver fibrosis. Our objective was to evaluate the effects of melatonin on liver fibrosis induced by bile-duct ligation (BDL) in rats.
Methods
We divided male Wistar rats (n = 32) into 4 groups. Two groups received BDL and 2 groups received sham operations. One of the BDL groups and one of the sham groups were administered melatonin (100 mg/kg/day via intraperitoneal injection), and the controls were given vehicle only. After 1 month, we biochemically evaluated the changes in hepatic fibrosis by measuring tissue collagen levels and histopathologic examination. We evaluated the levels of malondialdehyde (MDA), glutathione (GSH), luminal and lucigenin in tissue homogenates, and we studied proinflammatory cytokines in serum using commercially available kits.
Results
Bile-duct ligation caused hepatic fibrotic changes, whereas melatonin suppressed these changes in 5 of 8 rats (p < 0.001). Bile-duct ligation resulted in increased collagen, MDA, luminal and lucigenin levels and decreased GSH levels, whereas melatonin reversed these effects.
Conclusion
We found that melatonin functions as an effective fibrosuppressant and antioxidant, and the results suggest that it can be used as a therapeutic option.
Abstract
Contexte
Les cellules de Kupffer dans le foie, qui sont les principaux médiateurs de la pathogenèse de la fibrose, sont déclenchées par des radicaux et produisent du collagène. La mélatonine est un puissant capteur physiologique des radicaux hydroxyles. Elle joue aussi un rôle dans la régulation inhibitrice du contenu en collagène des tissus. Il n’y a pas de traitement efficace disponible contre la fibrose hépatique. Nous voulions évaluer les effets de la mélatonine sur la fibrose hépatique causée par une ligature du canal biliaire (LCB) chez les rats.
Méthodes
Nous avons réparti des rats Wistar mâles (n = 32) en 4 groupes. Les sujets de 2 groupes ont subi une LCB et les sujets de 2 autres groupes, une opération factice. Un des groupes qui ont subi la LCB et 1 de ceux qui ont subi une intervention factice ont reçu de la mélatonine (100 mg/kg/jour par injection intrapéritonéale) et les sujets témoins ont reçu l’excipient seulement. Après 1 mois, nous avons évalué les changements biochimiques de la fibrose hépatique en mesurant les concentrations de collagène dans les tissus et nous avons procédé à un examen histopathologique. Nous avons évalué les concentrations de malondialdéhyde (MDA), de glutathion (GSH) et de luminol et de lucigénine dans des homogénats de tissus, et nous avons étudié les cytokines pro-inflammatoires dans le sérum au moyen de trousses vendues dans le commerce.
Résultats
La ligature du canal biliaire a causé des changements de la fibrose hépatique tandis que la mélatonine a supprimé ces changements chez 5 rats sur 8 (p < 0,001). La ligature du canal biliaire a entraîné une élévation de la concentration de collagène, de MDA, de luminol et de lucigénine et une diminution des concentrations de GSH, tandis que la mélatonine a inversé ces effets.
Conclusion
Nous avons constaté que la mélatonine est un fibrolytique et antioxydant efficace et les résultats indiquent qu’elle peut servir comme traitement possible.
Liver fibrosis is a major feature of many chronic liver injuries and is one of the main components of cirrhosis. It is characterized by the accumulation of collagen and extracellular matrix proteins in the space of Disse. Activation of hepatic stellate cells (HSCs) and the initiation of unbalanced synthesis of collagen are the main components in the pathogenesis that leads to fibrosis.1–3
Accumulation of bile acids and inflammatory cells in liver tissue causes free radical production in biliary obstruction.4,5 Several lines of evidence have suggested the important role of oxidative stress in the etiopathogenesis of liver fibrosis.1,2,5 Furthermore, oxidative stress aggravates liver fibrosis via HSC activation,2,6 and lipid peroxidation stimulates transcription of the collagen gene.7
Melatonin (N-acetyl-5-methoxytryptamine), a secretory product of the pineal gland, is a powerful endogenous antioxidant. Exogenous application of this molecule leads to a remarkable decline in oxidative stress and inflammation because of its ability to scavenge hydroxyl radicals and cytokines.2,8 In addition, melatonin also enhances the levels of potential antioxidants such as glutathione peroxidase, superoxide dismutase and glutathione (GSH) by an indirect mechanism.2,8 There are studies in the literature that report the antifibrotic effects of melatonin as well as its properties in controlling collagen levels in tissues.2,9–12 Further, the cytostatic effects of melatonin on polymorphonuclear leukocytes and HSCs may lead to the inhibition of both free radical damage and fibrogenic activity.2
At present, no clinically effective therapy for liver fibrosis is available. We hypothesize that melatonin could be a potential therapeutic candidate for fibrotic liver diseases. In this study, the possible fibrosuppressant effect of melatonin on liver fibrosis induced by bile duct ligation (BDL) in rats was evaluated as a novel therapeutic choice.
Methods
Materials
We purchased melatonin from Sigma Chemical Co., cytokine kits (enzyme-linked immunosorbent assay [ELISA]), rat interleukin (IL)-1b and tumour necrosis factor (TNF)-α from R&D Systems Europe, and ELISA and rat IL-6 from Diaclone Research.
Experimental animal procedures
We obtained full approval for this study from the local ethics committee on animal research, and the study was performed according to the criteria of international guidelines for animal research. The experimental protocol was performed at Marmara University Animal Research Laboratory. All animals received humane care in compliance with the National Institutes of Health criteria for laboratory animals and had free access to standard rat chow and water.
We obtained 32 Wistar rats (aged 3.5–4.5 mo, 200–240 g) from the Institutional Animal Research Laboratory. Animals were kept at a constant temperature (mean 22°C, standard deviation [SD] 1°C) with 12-hour light and dark cycles, in the same unit. The animals were allowed to acclimatize to their new conditions for 1 week before the study began.
Thirty-two male Wistar rats underwent BDL or sham operation under general pentobarbital anesthesia. Two groups of rats underwent BDL, and the control rats underwent sham operations. Briefly, the common bile duct was exposed after laparotomy. Two double knots were placed proximally and distally, and the part of the bile duct between the 2 double knots was excised. In the sham group, the abdomen was closed without BDL. In total, 16 rats underwent BDL and 16 received sham operations. One of each of the operation groups was given melatonin (100 mg/kg/day via intraperitoneal injection), and the controls were given the vehicle. The 4 groups were as follows: saline group (sham operation and injected with saline, n = 8); melatonin group (sham operation and injected with melatonin, n = 8); BDL group (BDL and injected with saline, n = 8); and BDL–melatonin group (BDL and injected with melatonin, n = 8).
After 1 month, the animals were fasted overnight and sacrificed. We obtained serum samples by centrifugation of the trunk blood (4024 g, 10 min, 4°C). We removed and weighed the livers. After obtaining samples for histologic assessment, the livers were snap frozen in liquid nitrogen. The serum and liver samples were stored at −80°C for laboratory assays. All histopathological and biochemical tests were performed blinded.
Tissue homogenization
We weighed the liver samples and homogenized them in 0.15 m NaCl to determine the amount of reactive oxygen species (ROS). The liver tissue homogenates were diluted with 0.15 m NaCl up to 20%. The tissue homogenates were sonicated 2 times for 30-second intervals at 4°C. After sonication, we centrifuged the homogenates at 4024 g for 10 minutes or at 20 124 g for 15 minutes. Aliquots of the supernatants were used for the subsequent studies. We determined the protein content of the tissue aliquots by the Lowry method.13
Biochemical parameters
We determined the plasma levels of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT) and bilirubin by use of a spectrophotometer (Roche 917 R autoanalyzer) and commercial kits.
Oxidative stress parameters
Malondialdehyde measurements
We performed measurements of thiobarbituric acid reactive species (TBARS) using the method described by Yagi.14 We homoginized the liver tissue in an icy 10% trichloroacetic acid solution and then centrifuged the mixture. The superficial liquid portion was mixed with equal volume of TBARS (0.67%) and heated at 90°C for 15 minutes. We measured TBARS in nmol/g of tissue according to the absorbance at 532 nm.
Chemiluminescence measurements
We measured reactive oxygen metabolites (ROM) at room temperature via a chemiluminescence technique using a Mini Lumat LB 9506 luminometer (EG&G). Samples were placed into 2 mL of a 0.02-mol HEPES buffer (pH 7.4) containing 0.5 mol of phosphate-buffered saline. For measurement of ROM, we used 0.2 nmol of concentrated lucigenin (specific for superoxide radicals) or luminal (HOCl−, H2O2, OH−). We performed serial measurements at 15-second intervals for 5 minutes, and we calculated the area under the curve and relative light units; we also corrected for fresh tissue weight (relative light units per mg tissue area under the curve).15,16
Glutathione level measurements
We measured the levels of glutathione (GSH) using a spectrophotometer with Boyne and Ellman’s reagent and method.17 We calculated the results as μmol GSH/g tissue.
Cytokine tests
We analyzed the serum levels of TNF-α, IL-1β and IL-6 using commercially available enzyme immunoassay kits. The principal method for the immunoassay was the same for all cytokines. The analyses of all samples, standards and controls were performed in duplicate. The coefficients of intra-assay variation for TNF-α, IL-1β, and IL-6 were 4.2%, 4.1% and 4.0%, respectively; the interassay variations were 6.7%, 7.9% and 6.9% (n = 10), respectively.
Biochemical collagen content determination
We assessed the liver collagen content by use of the colorimetric method described by Lopez de Leon and Rojkind.18 In this analysis, collagenous protein are coloured red by Sirius red (36554-8, 2610-10-8; Aldrich Chemical) and noncollagenous proteins are coloured green by fast green (14280; Merck). We obtained 15 μm–thick liver slices from each paraffin block and layered the slices on glass slides. We deparaffinized the slices and performed the assay as originally described.18 We calculated the collagen content using the formula described by the authors as μg collagen/mg protein.18
Liver tissue sampling
We explored the left, middle and right lobes of each liver. Six different slices (5 × 5 × 5 mm) were fixed in 10% buffered formalin, routinely processed and blocked in paraffin for the detection of the collagen content by image analysis.3
Histopathology
The histopathologic examinations were performed blinded. We stained 5-μm liver sections with hematoxylin–eosin and Masson trichrome–Gomori reticulin stains. The grading of necroinflammatory activity and the staging of fibrosis were performed according to Knodell’s criteria.19
Statistical analysis
All results are expressed as mean and standard errors. We analyzed numerical data using the Kruskal–Wallis test. Significant differences revealed by multigroup comparisons were further analyzed by use of the Dunn test. Nominal data were analyzed by the χ2 test or the Fisher exact test with Yates correction. We considered p values less than 0.05 to be significant.
Results
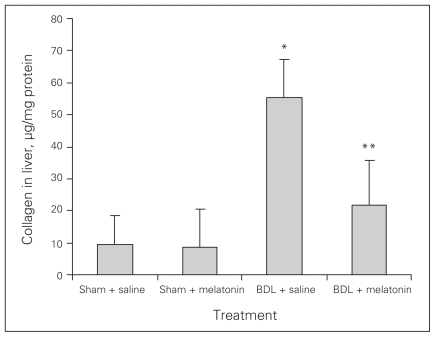
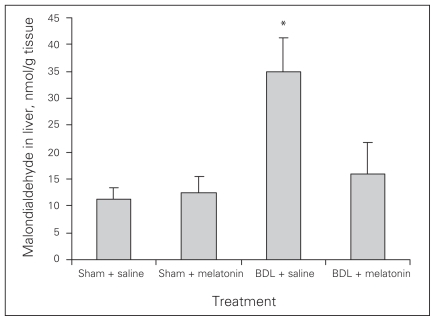
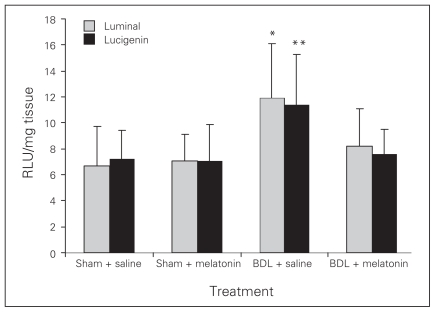
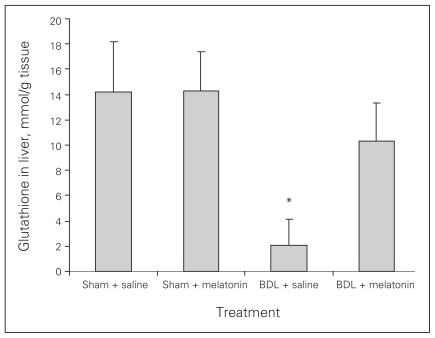
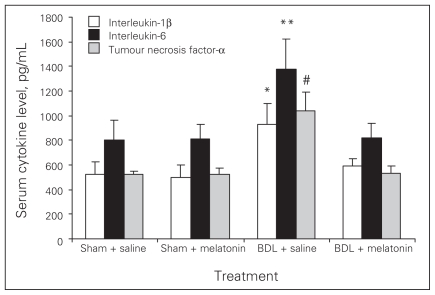
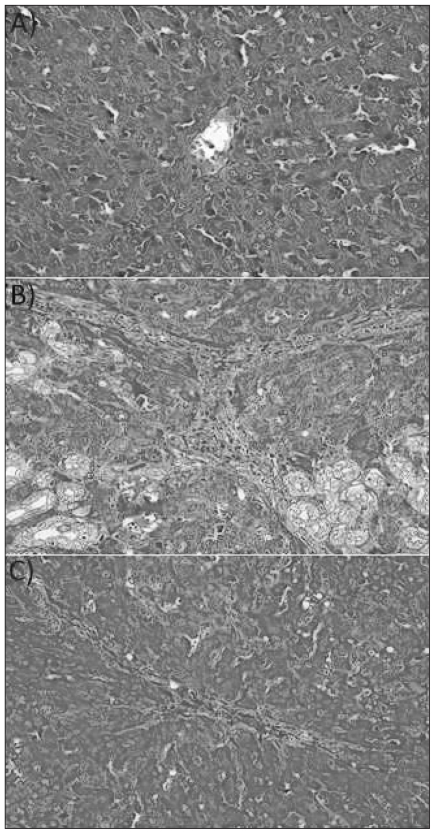
Melatonin lowered the levels of AST, ALT, ALP, GGT and bilirubin in the BDL–melatonin group compared with the BDL group (Table 1). The levels of albumin were not statistically different among the groups. The mean levels of liver tissue collagen, malondialdehyde (MDA), luminal and lucigenin in the BDL group were significantly higher than in the BDL–melatonin group; they were also higher than in the saline- and melatonin-injected groups (Figs. 1, 2 and 3). The mean hepatic level of GSH in the BDL group was significantly lower than in the BDL–melatonin, saline and melatonin groups (Fig. 4). The average levels of IL-1β, IL-6 and TNF-α in the BDL group were significantly higher than in the BDL–melatonin, saline and melatonin groups (Fig. 5). The rats in the saline and melatonin groups had normal liver histology (Fig. 6A). Bile-duct ligation caused prominent liver fibrosis in the untreated BDL group, and BDL alone led to grade 3 liver fibrosis. The bridging fibrosis in the portoportal and portocentral areas was prominent in the BDL group (Fig. 6B). Melatonin treatment improved fibrosis in 5 of 8 rats BDL–melatonin model (p < 0.001). These animals showed stage 2 fibrosis (Fig. 6C).
Table 1.
Biochemical parameters in rats that underwent bile-duct ligation or sham operation
| Parameter | Treatment; mean (standard error) | p value | |||
|---|---|---|---|---|---|
| Sham + saline | Sham + melatonin | BDL + saline | BDL + melatonin | ||
| Aspartate transaminase, U/L | 158 (14) | 162 (12) | 371 (36) | 261 (27) | 0.008 |
| Alanine transaminase, U/L | 66 (8) | 59 (6) | 97 (8) | 74 (7) | 0.006 |
| Alkaline phosphatase, U/L | 464 (214) | 377 (237) | 1747 (299) | 775 (365) | 0.007 |
| γ-glutamyl transpeptidase, U/L | 8 (2) | 10 (5) | 81 (23) | 43 (17) | 0.008 |
| Total bilirubin, mg/dL | 0.2 (0.04) | 0.2 (0.03) | 13.6 (4.1) | 7.7 (3.3) | < 0.001 |
| Direct bilirubin, mg/dL | 0.02 (0.01) | 0.02 (0.01) | 10 (2.2) | 5.3 (1.7) | < 0.001 |
| Albumin, g/dL | 3.9 (0.8) | 3.9 (0.7) | 3.8 (0.7) | 3.9 (0.8) | 0.65 |
BDL = bile-duct ligation.
Plasma aspartate transaminase, alanine transaminase, alkaline phosphatase, γ-glutamyl transpeptidase and bilirubin levels in the BDL rats were significantly higher than the BDL + melatonin rats (p < 0.05), the saline rats and the melatonin rats (p < 0.01, for both).
Fig. 1.
Levels of tissue collagen in rats after bile-duct ligation (BDL) or sham operation and treatment with saline or melatonin. Results are means and standard errors. *p < 0.001 versus BDL and melatonin, saline and melatonin-only treated rats. **p < 0.001 versus saline and melatonin-only treated rats.
Fig. 2.
Levels of tissue malondialdehyde after bile-duct ligation (BDL) or sham operation and saline or melatonin administration. Results are means and standard errors. *p < 0.001 versus other 3 groups.
Fig. 3.
Tissue luminal and lucigenin levels after bile-duct ligation (BDL) or sham operation and saline or melatonin administration. Results are means and standard errors. *p < 0.001 versus other 3 groups, **p < 0.001 versus the other 3 groups.
Fig. 4.
Tissue glutathione levels after bile-duct ligation (BDL) or sham operation and saline or melatonin administration. Results are means and standard errors. *p < 0.001 versus other 3 groups.
Fig. 5.
Serum cytokine levels after bile-duct ligation (BDL) or sham operation and saline or melatonin administration. Results are means and standard errors. *p < 0.001 versus other 3 groups, **p < 0.001 versus the other 3 groups; #p < 0.001 versus the other 3 groups.
Fig. 6.
(A) Normal liver architecture was observed in the saline and melatonin groups (hematoxylin and eosin staining, original magnification ×40). (B) Damage induced by bile-duct ligation (BDL) in the liver. Stage 3 fibrosis with prominent portoportal and portoseptal bridging was seen in the BDL rats (Trichrome, original magnification ×40) (C) Administration of melatonin improved liver damage induced by BDL. In this figure, only stage 2 fibrosis can be seen in the BDL–melatonin group (Trichrome, original magnification ×40).
Discussion
At present, there is no effective treatment of liver fibrosis available for clinical use. Some experimental studies have reported partial success with substances such as pegylated interferon20 and N-acetylcysteine1 in this setting. An effective therapeutic approach against the development of hepatic fibrosis is still needed.
Current data indicate that oxidative stress is related to the activation of HSCs, which are the central mediators in the pathogenesis of liver fibrosis.21,22 Hepatic stellate cells are activated by MDA, and this activation is blocked by antioxidants.2 Lipid peroxidation is an indicator of tissue damage and accelerates collagen synthesis by stimulating HSCs.23 Fibrosis caused by lipid peroxidation and its products decreases after the administration of antioxidants in animal models.2,21
Melatonin studies are ongoing into its antiaging and anticancer effects. Its best known effect is as a sleeping pill and a way to fight jet lag.24,25 Recently, our group reported that melatonin is a potent hepatoprotective agent in a non-alcoholic steatohepatitis rat model.8 In the present study, we aimed to study some of the basic oxidative stress markers and antioxidant levels to see if melatonin has an effect in an acceptable fibrosis model of BDL. Melatonin improved oxidative stress by increasing GSH levels and decreasing MDA, luminal and lucigenin levels in this model.
Cytokines are also involved in triggering and activating HSCs, which produce collagen.26 In addition to reducing oxidative stress parameters, melatonin significantly decreased the levels of proinflammatory cytokines (IL-1β, IL-6 and TNF-α) in the BDL group compared with the BDL–melatonin group.
Several lines of evidence suggest that melatonin has either a regulatory role in maintaining collagen levels or an inhibitory role in collagen accumulation.2,9,10 In patients with scleroderma, cutaneous collagen synthesis increases during summer, which has been related to a seasonal reduction in the activity of the pineal gland, thus suggesting a relation between fibrosis and melatonin.9
A previous study from our group was the first to show the partial protective effect of melatonin on liver fibrosis in an experimental model involving dimethylnitrosamine (DMN) in rats.2 We found significantly lower hydroxyproline levels in the DMN- and melatonin-treated rats compared with the rats that received only DMN. Histologic evaluation of liver specimens from all 14 rats in the BDL-only group revealed a central-to-central and central-to-portal bridging structure. The parenchymal loss and generation of repair tissue was minimal in 5 of 14 DMN–melatonin rats, suggesting a significant protective effect of melatonin on hepatic histology.2
Pastor and colleagues27 reported that decreased levels of GSH were significantly correlated with elevated levels of MDA in a BDL model. Our findings are in agreement with their results. In terms of liver biochemistry, the serum levels of AST, ALT, ALP, GGT and bilirubin were higher in the BDL group than in the other 3 groups.
In a recent study, Hong and colleagues11 showed protective antifibrotic effects of melatonin on hepatic fibrosis induced by carbon tetrachloride in experimental rats. They concluded that the protective effect of melatonin on hepatic fibrosis may be related to its antioxidant activities. In our study, we wanted to evaluate the free radical scavenging, anti-inflammatory and antifibrotic activities of melatonin in an acceptable fibrosis model of BDL. We evaluated formation of liver fibrosis both qualitatively by histology and quantitatively by measuring collagen levels. We found that the collagen levels were significantly decreased in all rats in the BDL–melatonin group, whereas histologic protection was evident in 5 of 8 rats. In the BDL–melatonin rats, we found no significant difference to biochemical parameters between the histologically responsive and unresponsive rats.
In conclusion, we found that daily melatonin injections at pharmacologic doses are effective in reducing liver damage in rats with BDL-induced hepatic fibrosis. The protective action of melatonin may be related to its antioxidant, anti-inflammatory and antifibrotic activities.
Acknowledgment
The authors thank Shringi Sharma and Kenneth Dorko from the University of Pittsburgh for their suggestions and critical review of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: All authors contributed to the acquisition of data, revising the content and the final version. Dr. G. Tahan was mainly responsible for the management of the study and performed the analysis and interpretation of data. Dr. Akin organized the design and feeding of animals and performed the acquisition of data and drug administration. Dr. Aydogan performed surgery and sacrificed the animals. Drs. Ramadan and Yapicier performed histopathologic examinations of the biopsies and took pictures of the tissues. Dr. V. Tahan followed the animals and contributed to the writing of the manuscript. Dr. Tarcin collected and stored the samples and interpreted the data. Dr. Uzun performed biochemical, free radical and cytokine tests. Dr. Zengin designed the study and statistics and managed the study. All authors read and approved the final version of the manuscript submitted for publication.
References
- 1.Tahan G, Tarcin O, Tahan V, et al. The effects of N-acetylcysteine on bile duct ligation–induced liver fibrosis in rats. Dig Dis Sci. 2007;52:3348–54. doi: 10.1007/s10620-006-9717-9. [DOI] [PubMed] [Google Scholar]
- 2.Tahan V, Ozaras R, Canbakan B, et al. Melatonin reduces dimethyl-nitrosamine-induced liver fibrosis in rats. J Pineal Res. 2004;37:78–84. doi: 10.1111/j.1600-079X.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 3.Tarcin O, Avsar K, Demirturk L, et al. In vivo inefficiency of pentoxifylline and interferon-alpha on hepatic fibrosis in biliary-obstructed rats: assessment by tissue collagen content and prolidase activity. J Gastroenterol Hepatol. 2003;18:437–44. doi: 10.1046/j.1440-1746.2003.03004.x. [DOI] [PubMed] [Google Scholar]
- 4.Sokol RJ, Devereaux M, Khandwala R, et al. Evidence for involvement of oxygen free radicals in bile acid toxicity to isolated rat hepatocytes. Hepatology. 1993;17:869–81. [PubMed] [Google Scholar]
- 5.Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 6.Svegliati Baroni G, D’Ambrosio L, Ferretti G, et al. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–6. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Houglum K, Trautwein C, et al. Stimulation of collagen alpha 1(I) gene expression is associated with lipid peroxidation in hepatocellular injury: A link to tissue fibrosis. Hepatology. 1994;19:1262–71. [PubMed] [Google Scholar]
- 8.Tahan V, Atug O, Akin H, et al. Melatonin ameliorates methionine choline deficient diet induced nonalcoholic steatohepatitis in rats. J Pineal Res. 2009;46:401–7. doi: 10.1111/j.1600-079X.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- 9.Cunnane SC, Manku MS, Horrobin DF. The pineal and regulation of fibrosis: pinealectomy as a model of primary biliary cirrhosis: roles of melatonin and prostaglandins in fibrosis and regulation of T lymphocytes. Med Hypotheses. 1979;5:403–14. doi: 10.1016/0306-9877(79)90107-5. [DOI] [PubMed] [Google Scholar]
- 10.Drobnik J, Dabrowski R. Melatonin suppresses the pinealectomy-induced elevation of collagen content in a wound. Cytobios. 1996;85:51–8. [PubMed] [Google Scholar]
- 11.Hong RT, Xu JM, Mei Q. Melatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2009;15:1452–8. doi: 10.3748/wjg.15.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogeturk M, Kus I, Pekmez H, et al. Inhibition of carbon tetrachloride-mediated apoptosis and oxidative stress by melatonin in experimental liver fibrosis. Toxicol Ind Health. 2008;24:201–8. doi: 10.1177/0748233708093725. [DOI] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 15.Davies GR, Simmonds NJ, Stevens TR, et al. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut. 1992;33:1467–72. doi: 10.1136/gut.33.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haklar G, Sayin-Ozveri E, Yuksel M, et al. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001;165:219–24. doi: 10.1016/s0304-3835(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 17.Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–53. doi: 10.1016/0003-2697(72)90335-1. [DOI] [PubMed] [Google Scholar]
- 18.Lopez de Leon A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed and paraffin-embedded sections. J Histochem Cytochem. 1985;33:737–43. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 19.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 20.Tasci I, Mas MR, Vural SA, et al. Rat liver fibrosis regresses better with pegylated interferon alpha2b and ursodeoxycholic acid treatments than spontaneous recovery. Liver Int. 2006;26:261–8. doi: 10.1111/j.1478-3231.2005.01210.x. [DOI] [PubMed] [Google Scholar]
- 21.Svegliati Baroni G, D’Ambrosio L, Ferretti G, et al. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–6. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- 22.Houglum K, Lee KS, Chojkier M. Proliferation of hepatic stellate cells is inhibited by phosphorylation of CREB on serine 133. J Clin Invest. 1997;99:1322–8. doi: 10.1172/JCI119291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geesin JC, Hendricks LJ, Falkenstein PA, et al. Regulation of collagen synthesis by ascorbic acid: characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys. 1991;290:127–32. doi: 10.1016/0003-9861(91)90598-d. [DOI] [PubMed] [Google Scholar]
- 24.Hoang BX, Shaw DG, Pham PT, et al. Neurobiological effects of melatonin as related to cancer. Eur J Cancer Prev. 2007;16:511–6. doi: 10.1097/CEJ.0b013e32801023dc. [DOI] [PubMed] [Google Scholar]
- 25.Shamseer L, Vohra S. Complementary, holistic, and integrative medicine: melatonin. Pediatr Rev. 2009;30:223–8. doi: 10.1542/pir.30-6-223. [DOI] [PubMed] [Google Scholar]
- 26.EL-Swefy S, Hassanen SI. Improvement of hepatic fibrosis by leukotriene inhibition in cholestatic rats. Ann Hepatol. 2009;8:41–9. [PubMed] [Google Scholar]
- 27.Pastor A, Collado PS, Almar M, et al. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27:363–70. doi: 10.1016/s0168-8278(97)80183-3. [DOI] [PubMed] [Google Scholar]