Abstract
Background
Intraoperative spinal cord and nerve root monitoring is used to identify an insult to the neural elements with the goal of preventing injury. There are 2 major categories of monitoring: evoked potentials (somatosensory evoked potentials and motor evoked potentials) and electromyography. The availability of intraoperative neuromonitoring and the indications for use vary widely. In this study, we aimed to document the current practices and opinions of Canadian spine surgeons with regards to intraoperative spinal monitoring.
Methods
We surveyed members of the Canadian Spine Society about the availability and use of various types of intraoperative neuromonitoring modalities for surgical procedures.
Results
We distributed 105 surveys and received 95 responses (90%). Somatosensory evoked potentials were the most commonly available form of intraoperative neuromonitoring, although it was available to only 65.3% of respondents. Surgeons in either full-time or part-time academic practice used monitoring more frequently than those in private practice (p < 0.001), but this association was not based on surgeon preference after controlling for availability. Years of practice and training background (orthopedic or neurosurgical) did not influence the use of monitoring. Canadian spine surgeons overwhelmingly reported that they use intraoperative neuromonitoring to reduce the risk of adverse operative events, rather than because of liability concerns. Most respondents believed that monitoring should be used in the correction of major deformity and scoliosis.
Conclusion
The availability of spinal monitoring in Canada is variable. Most surgeons believe that it is an important adjunct to improve patient safety.
Abstract
Contexte
La surveillance intraopératoire de la moelle épinière et des racines nerveuses sert à déterminer l’atteinte des éléments nerveux afin de prévenir des lésions. Il y a 2 grandes catégories de surveillance: les potentiels évoqués (potentiels évoqués somatosensoriels et potentiels évoqués moteurs) et l’électromyographie. La disponibilité de la neurosurveillance intraopératoire et les indications de son utilisation varient énormément. Au cours de cette étude, nous voulions documenter les pratiques et les opinions courantes des chirurgiens canadiens spécialistes du rachis en ce qui a trait à la surveillance intraopératoire de la moelle.
Méthodes
Nous avons sondé les membres de la Société canadienne du rachis au sujet de la disponibilité et de l’utilisation de diverses méthodes de neurosurveillance intraopératoire durant les interventions chirurgicales.
Résultats
Nous avons distribué 105 questionnaires et reçu 95 réponses (90 %). Les potentiels évoqués somatosensoriels constituaient la forme la plus répandue de neurosurveillance intraopératoire, même si 65,3 % seulement des répondants y avaient accès. Les chirurgiens de pratique universitaire à temps plein ou à temps partiel utilisaient la surveillance plus souvent que ceux qui exerçaient en pratique privée (p < 0,001), mais ce lien n’était pas basé sur la préférence du chirurgien compte tenu de la disponibilité. Le nombre d’années de pratique et la formation (en orthopédie ou en neurochirurgie) n’avaient pas d’effet sur l’utilisation de la surveillance. Les chirurgiens canadiens spécialistes du rachis ont déclaré par une majorité écrasante qu’ils utilisent la neurosurveillance intraopératoire afin de réduire le risque d’événements indésirables au cours de l’intervention plutôt qu’à cause de préoccupations liées à la responsabilité. La plupart des répondants sont d’avis qu’il faudrait utiliser la surveillance dans les interventions de correction d’une malformation majeure et d’une scoliose.
Conclusion
La disponibilité de la surveillance de la moelle au Canada est variable. La plupart des chirurgiens croient qu’il s’agit d’un outil d’appoint important pour améliorer la sécurité des patients.
As current methods of complex spinal surgery are applied to ever more challenging clinical scenarios, new or worsened neurologic deficits are becoming a greater inherent risk to patients. A recent study performed by Delank and colleagues1 found a low incidence of iatrogenic paraplegia following surgery for scoliosis (0.55%), short spinal fusion (0.14%) and cervical discectomy (0.07%). Although the incidence is low, spinal cord damage or nerve root injury may result in unfavourable surgical outcomes.
In 1991, a joint position statement from the Scoliosis Research Society and the European Spinal Deformity Society advocated the use of somatosensory evoked potentials (SSEPs) to limit neurologic damage during the correction of spinal deformities.2 Somatosensory evoked potentials were first used in the 1970s and were thought to detect not only injury to the posterior sensory columns but also to the entire spinal cord. It was later shown that injury to the more sensitive lateral and anterior columns could occur in the presence of normal intraoperative SSEPs. Somatosensory evoked potentials have limited use in patients with pre-existing abnormal spinal cord function.3 Despite the more localized functional monitoring provided by this modality, its use alone has been estimated to reduce the incidence of paraplegia by 60% in orthopedic spine surgery.4
Motor evoked potentials (MEPs) monitor the descending motor pathways, mainly represented by the corticospinal tracts, and may thus allow more accurate prediction of motor deficits. Transcortical MEPs are very sensitive, more so than SSEPs, in detecting ischemic injury due to the higher metabolic demand from the numerous synaptic junctions within the anterior horn grey matter.5 A number of studies have evaluated the efficacy of MEP in detecting impending neurologic dysfunction and have found sensitivities of 100% and specificities of 97% in cases of spinal deformity correction, especially when the threshold-level approach to determining injury detection is used.6–9 This high sensitivity can result in a number of false positives, increasing the chance of unnecessary interruptions of the surgical procedure.10 The complexity of the operation may also be increased with this form of monitoring because the anesthetic needs are more specialized, often requiring total intravenous anesthesia to maintain a stable concentration of anesthetic agent to optimize the MEP results.11
Transcranial electrical stimulation, the most common method used to generate MEPs, results in patient movement and therefore requires constant communication between the surgeon, anesthesiologist and neurophysiologist. Other risks of MEP monitoring include seizures, tongue and lip injury (especially if the bite block is improperly placed), C3 and C4 stimulation affecting respiration, and intraoperative awareness because of the specialized anesthetic requirements.12
The criteria for detecting an impending neurologic deficit by MEP varies by institution. There is a lack of defined criteria for the prediction of mild deficits versus complete neurologic deficits.10 The inter- and intrauser variability regarding the interpretation of intraoperative recordings is higher with MEP than SSEP.7
The addition of spontaneous electromyography (EMG) provides real-time examination of the corticospinal tracts and other descending pathways.13 Electromyography can be used in 3 different ways: evoked, spontaneous and combined. Spontaneous EMG is primarily used to monitor nerve root activity, but it can also show subtle signs of cord damage. Evoked EMG is most often used to determine if breach of the pedicle wall has occurred by stimulating the pedicle screw directly or by stimulating the canal made for the screw.14–19
Although there is no class I evidence,20 there is growing consensus that multimodal intraoperative monitoring is very helpful in spine surgery. To appropriately power a well-designed study, high patient enrolment would be required to prove the hypothesis that intraoperative monitoring reduces the incidence of neurologic injury secondary to the very low risk of injury. Combining SSEP, MEP and EMG provides the maximum information about spinal cord and nerve root integrity, including both the ascending and descending tracts and the anterior and posterior spinal arteries.21 Multimodal intraoperative monitoring provides a higher specificity and sensitivity for detecting neurologic injury than any modality used in isolation.3,10,20–28
Although intraoperative neuromonitoring is an attractive option to maximize the safety of spinal procedures and limit the risk of iatrogenic neurologic injury, the availability and type of monitoring varies among centres. In Canada, this is mostly owing to a lack of suitably trained technologists and neurophysiologists.29 Other factors that may influence the use of monitoring include surgeon experience, training background and resource availability. The objectives of this questionnaire study was to assess what types of intraoperative neuromonitoring are available and to evaluate which modalities are used in different spinal procedures. We surveyed only actively practising spine surgeons (orthopedic and neurosurgical) in Canada. This study was not intended to establish or propose standards of care, but rather to evaluate the availability and use of spinal neuromonitoring in Canada.
Methods
The questionnaire (Appendix 1, available online at www.cma.ca/cjs) was sent to all members of the Canadian Spine Society (CSS) by email. Members who did not respond were sent a follow-up questionnaire by regular mail. The CSS is composed of orthopedic and neurologic surgeons who practice in Canada and devote more than half of their practise to the treatment of spinal disorders.
Background questions included the type of residency training (orthopedics v. neurosurgery), years in practice and current practice type (full-time academic, part-time academic and private practice). Members were asked which monitoring modalities they use for which indications, who performs it and why they use monitoring (e.g., liability risks, hospital policy, reduce operative risk for the patient). A final question was whether or not respondents believed monitoring should be a standard of care for various types of cases.
We compiled the data in Excel, and analyses (χ2 tests and multivariate analysis) were carried out using SPSS for Windows (SPSS 13.0, SPSS Inc.).
Results
In total, 105 surveys were distributed, and 95 responses were received (90.5%). Most replies were to the online survey (60%) rather than the mailed survey (40%). The majority of respondents were orthopedic spine surgeons (73.7%). Most respondents (69.4%) were in either full-time (42.1%) or part-time (27.3%) academic practice, whereas the rest (30.6%) identified themselves as being in private practice. There were 15 surgeons in practice for less than 5 years, 21 in practice for 5–10 years, 34 in practice for 10–20 years and 25 in practice for more than 20 years.
Residency training (orthopedics or neurosurgery) and years in practice did not influence the use of monitoring (p = 0.15 and p = 0.58, respectively). Full-time or part-time academic practice significantly correlated with use (p < 0.001). After controlling for the availability of monitoring, private-practice spine surgeons were equally likely to use some type of spinal monitoring. The only factor found to significantly affect the use of intraoperative monitoring was availability (p < 0.001).
Respondents were asked to indicate the types of intraoperative neuromonitoring available at their institutions: SSEP, MEP, EMG or none. Somatosensory evoked potential was the most widely available type of spinal monitoring (65.3%), followed by EMG (44.2%) and MEP (28.4%). One-third of respondents did not have any type of intraoperative neuromonitoring available to them (34.7%).
Overall, 62.1% of respondents reported that they use at least some form of electrophysiologic monitoring during some spinal procedures, whereas 37.9% reported that they never use intraoperative neuromonitoring.
According to the respondents, most monitoring is performed and interpreted by electrophysiologists (78.0%), followed by surgeons (16.9%), neurologists (11.9%) and anesthesiologists (6.9%).
When asked “What is the main reason you use spine monitoring?” the overwhelmingly response was “to reduce the operative risk” (83.1%). Other responses included “liability risks” (11.9%) and “everyone else is using it” (5.0%). None of the respondents indicated that monitoring was used because of hospital policy.
Those who reported using spinal monitoring were asked to indicate the modality they use in different cases. The results are shown Table 1. Intraoperative neuromonitoring was most often selected for scoliosis and deformity correction, intradural tumours, instrumentation in the cervical and thoracic spine, cervical stenosis and thoracic discectomy. Monitoring was rarely performed for lumbar decompression.
Table 1.
Indications for spinal monitoring
| Indication | Modality, % of respondents | ||
|---|---|---|---|
| SSEP | MEP | EMG | |
| Scoliosis or major deformity | 42 | 36 | 15 |
| Spinal cord tumours | 30 | 19 | 6 |
| Cervical or thoracic instrumentation | 19 | 13 | 7 |
| Thoracic discectomy | 17 | 15 | 3 |
| Cervical stenosis | 16 | 13 | 3 |
| Intradural extramedullary tumour | 15 | 16 | 8 |
| Lumbar (below L2) instrumentation | 6 | 5 | 12 |
| Cervical discectomy | 11 | 7 | 7 |
| Metastasis with spinal cord compression | 8 | 7 | 3 |
| Lumbar stenosis | 3 | 4 | 4 |
| Lumbar discectomy | 1 | 1 | 4 |
EMG = electromyography; MEP = motor evoked potentials; SSEP = somatosensory evoked potentials.
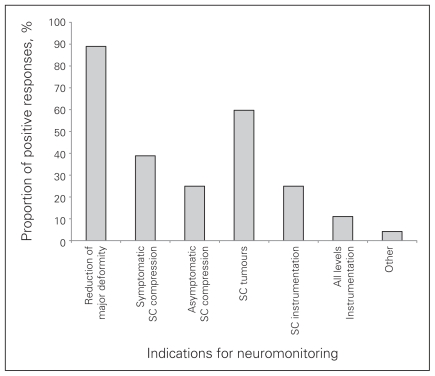
All respondents were asked, “Do you think intraoperative spinal cord and nerve root monitoring should be a standard of care in Canada?” Only 4.2% believed that monitoring should be a standard of care for all cases, whereas 20% responded “not at all.” Most (75.8%) chose “yes, for selected cases.” These respondents were asked to indicate the categories of cases they felt monitoring should be used (Fig. 1). The most common responses were for “reduction of a major deformity or scoliosis” (89.9%) and “spinal cord tumours” (59.7%). Seventy-two percent of orthopedic surgeons and 76% of neurosurgeons felt that deformity and scoliosis reduction was an indication for monitoring, whereas only 40% of orthopedic surgeons and 72% of neurosurgeons felt that spinal cord tumours would be appropriate cases to standardize the use of monitoring.
Fig. 1.
Case selection for neuromonitoring. SC = spinal cord.
The respondents’ opinions regarding “standard of care” were not related to residency training background (p ≤ 0.93), type of practice (p ≤ 0.37), number of years in practice (p ≤ 0.84), availability (p ≤ 0.46) or use of intraoperative neuromonitoring (p ≤ 0.92).
Discussion
Currently, there are no well-established North American practice guidelines about the use of intraoperative spinal cord and nerve root monitoring. The purpose of this study was to evaluate the availability and use of various modalities of neuromonitoring for spinal procedures in Canada.
Availability varies widely across Canada, with 62.6% of respondents indicating that some form of intraoperative spinal monitoring was available at their institutions. The most widely available neuromonitoring modality was SSEP (65.3%). In a survey of 139 spine surgeons mostly from the United States, Magit and colleagues30 also reported that SSEP was the most commonly used neuromonitoring modality, which was available to 95% of respondents.
Spontaneous and evoked EMG was available to 67% and 61% of respondents, respectively, in the American survey;30 however, only 44.2% of Canadian spine surgeons reported that intraoperative EMG was available at their institutions. Transcranial and spinal MEP was available to 41% and 58% of respondents in the American survey and only 28.4% in the Canadian survey.
These differences may be because of the critical shortage of clinical neurophysiologists and suitably trained technologists in Canada.29 At the senior author’s centre, a neurophysiology staff member is often not available for spine cases, and a simplified surgeon-driven monitor (NIM-Spine, Medtronic Inc.) is used to obtain spontaneous and evoked EMGs and transcranial MEPs. Of the respondents to our surgvey, 16.9% reported that the surgeon was responsible for carrying out the intraoperative neuromonitoring at their centre.
We found that the availability of monitoring was the only independent predictor of its use. Other factors such as practice setting (private or academic), training background (orthopedics or neurosurgery) and years in practice did not influence the use of monitoring. Magit and colleagues30 also found no significant differences between the use of intraoperative neuromonitoring and varying years of experience or orthopedic versus neurosurgical training. However, fellowship-trained surgeons were significantly more likely to use monitoring (93%) than non–fellowship trained surgeons (63%). Interestingly, the availability of spinal monitoring was also associated with surgeon self-reported satisfaction.30
One of the unique features of our survey was that we asked spinal surgeons who use monitoring to describe the main reason why they use it. Overwhelmingly, the response was to reduce the operative risk for the patient (83.1%). Liability risks were less of a concern (11.9%).
We anticipated that some respondents would not have monitoring available at their institution, and therefore we only asked users of monitoring which modalities they used for different surgical indications. Previous questionnaires30 have been designed to assess surgeon preference for different procedures regardless of their institutional availability, but this introduces significant bias because preference may be largely based on what type of monitoring is available. We found that intraoperative neuromonitoring was most often selected for scoliosis and deformity correction, intradural tumours, instrumentation in the cervical and thoracic spine, cervical stenosis and thoracic discectomy. Spinal monitoring was rarely performed for lumbar decompression (Table 1).
We wanted to know, independent of availability, whether respondents believed that spine monitoring should be a “standard of care” in Canada. This is a loaded term that is often discussed but poorly defined. There may be several reasons why intraoperative neuromonitoring is chosen by the surgeon. This ranges from availability, to preference, to past experiences, to the interpretation of data regarding its usefulness. We purposefully did not define the concept of standard of care for the purposes of this survey. Thus, the results cannot be used to establish a standard of care for spinal monitoring in Canada. At best, they reflect what the spine surgeon community believes would be ideal to maximize the safety of surgical procedures, if the resources were made available to them.
Most respondents indicated that intraoperative monitoring should be a standard of care for scoliosis or deformity cases and intramedullary spinal cord tumours. A literature review on these topics reveals a large volume of case series and cohort studies, many of which support the use of both unimodal and multimodal monitoring for both pathologies to help reduce the development of new-onset neurologic deficits.7–9,24,27,31–33 However, there remains no class I evidence to strongly support the use of monitoring. Also, inconsistencies in the injury-detection criteria among various modalities and institutions make it difficult to apply the data obtained from these studies in practice.
There were some limitations to our survey, particularly with regards to selection bias, with all the responses coming from the CSS. The possibility of selection bias raises the question of whether the results are applicable to the Canadian spine community in general. According to the CSS, almost all orthopedic spine surgeons in Canada are CSS members. However, just over 10% of Canadian neurosurgeons are CSS members. The validity of the use of intraoperative neuromonitoring for intradural tumours can also be questioned because the replies may reflect the fact that the CSS has fewer neurosurgeons relative to orthopedic surgeons, and it is neurosurgeons who perform most of these cases. When the question was broken down to compare the responses from orthopedic surgeons and neurosurgeons, a large discrepancy was found. More neurosurgeons than orthopedic surgeons felt that intraoperative neuromonitoring should be a standard of care for spinal cord tumour cases, which likely reflects the practice pattern in Canada.
Conclusion
In Canada, SSEP is the most commonly available form of intraoperative neuromonitoring, although it was available to only about two-thirds of respondents to our survey. Canadian spine surgeons overwhelmingly use intraoperative neuromonitoring to reduce the risk of adverse operative events, rather than for liability concerns. There were no significant differences in the use of spinal monitoring between orthopedic spine surgeons and neurosurgeons, and duration of practice also had no effect. Most respondents indicated that spine monitoring should be used for the reduction of major deformity and scoliosis, although a standard of care was not defined.
Footnotes
This work was presented in part at the Canadian Spine Society Eighth Annual Meeting, Mar. 12–15, 2008, in Kamloops, BC, and at the Canadian Neurological Sciences Federation 43rd Annual Congress, June 17–20, 2008, in Victoria, BC.
Competing interests: None declared. Although CSS members were included in this survey, the results do not represent a consensus statement of the Society.
Contributors: Drs. Peeling, Hentschel, Fox and Fourney designed the study. Drs. Peeling, Fox, Hall and Fourney acquired the data. Drs. Peeling, Fox and Fourney analyzed the data. Drs. Peeling, Hentschel and Fourney wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Delank KS, Delank HW, Konig DP, et al. Iatrogenic paraplegia in spinal surgery. Arch Orthop Trauma Surg. 2005;125:33–41. doi: 10.1007/s00402-004-0763-5. [DOI] [PubMed] [Google Scholar]
- 2.Dawson EG, Sherman JE, Kanim LE, et al. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine. 1991;16:S361–4. [PubMed] [Google Scholar]
- 3.Costa P, Bruno A, Bonzanino M, et al. Somatosensory- and motor-evoked potential monitoring during spine and spinal cord surgery. Spinal Cord. 2007;45:86–91. doi: 10.1038/sj.sc.3101934. [DOI] [PubMed] [Google Scholar]
- 4.Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-d. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–9. doi: 10.2106/JBJS.F.01476. [DOI] [PubMed] [Google Scholar]
- 6.Calancie B, Molano MR. Alarm criteria for motor-evoked potentials: What’s wrong with the “presence-or-absence” approach. Spine. 2008;33:406–14. doi: 10.1097/BRS.0b013e3181642a2f. [DOI] [PubMed] [Google Scholar]
- 7.Langeloo DD, Journee HL, de Kleuver M, et al. Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery. A review and discussion of the literature. Neurophysiol Clin. 2007;37:431–9. doi: 10.1016/j.neucli.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Hsu B, Cree AK, Lagopoulos J, et al. Transcranial motor-evoked potentials combined with response recording through compound muscle action potential as the sole modality of spinal cord monitoring in spinal deformity surgery. Spine. 2008;33:1100–6. doi: 10.1097/BRS.0b013e31816f5f09. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman JA, Lyon R, Feiner J, et al. The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine. 2008;33:E414–24. doi: 10.1097/BRS.0b013e318175c292. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki T, Kubota S. History of the development of intraoperative spinal cord monitoring. Eur Spine J. 2007;2007(Suppl 2):S140–6. doi: 10.1007/s00586-007-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang AC, Than KD, Etame AB, et al. Impact of anesthesia on transcranial motor evoked potential monitoring during spine surgery: a review of the literature. Neurosurg Focus. 2009;27:E7–10. doi: 10.3171/2009.8.FOCUS09145. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19:416–29. doi: 10.1097/00004691-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Skinner SA, Nagib M, Bergman TA, et al. The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Neurosurgery. 2005;56:299–314. doi: 10.1227/01.neu.0000156545.33814.8d. [DOI] [PubMed] [Google Scholar]
- 14.Danesh-Clough T, Taylor P, Hodgson B, et al. The use of evoked EMG in detecting misplaced thoracolumbar pedicle screws. Spine. 2001;26:1313–6. doi: 10.1097/00007632-200106150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Clements DH, Morledge DE, Martin WH, et al. Evoked and spontaneous electromyography to evaluate lumbosacral pedicle screw placement. Spine. 1996;21:600–4. doi: 10.1097/00007632-199603010-00013. [DOI] [PubMed] [Google Scholar]
- 16.Darden BV, II, Wood KE, Hatley MK, et al. Evaluation of pedicle screw insertion monitored by intraoperative evoked electromyography. J Spinal Disord. 1996;9:8–16. [PubMed] [Google Scholar]
- 17.Calancie B, Madsen P, Lebwohl N. Stimulus-evoked EMG monitoring during transpedicular lumbosacral spine instrumentation. Initial clinical results. Spine. 1994;19:2780–6. doi: 10.1097/00007632-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Glassman SD, Dimar JR, Puno RM, et al. A prospective analysis of intraoperative electromyographic monitoring of pedicle screw placement with computed tomographic scan confirmation. Spine. 1995;20:1375–9. [PubMed] [Google Scholar]
- 19.Welch WC, Rose RD, Balzer JR, et al. Evaluation with evoked and spontaneous electromyography during lumbar instrumentation: a prospective study. J Neurosurg. 1997;87:397–402. doi: 10.3171/jns.1997.87.3.0397. [DOI] [PubMed] [Google Scholar]
- 20.Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248–64. doi: 10.1016/j.clinph.2007.09.135. [DOI] [PubMed] [Google Scholar]
- 21.Deletis V, Sala F. The role of intraoperative neurophysiology in the protection or documentation of surgically induced injury to the spinal cord. Ann N Y Acad Sci. 2001;939:137–44. doi: 10.1111/j.1749-6632.2001.tb03620.x. [DOI] [PubMed] [Google Scholar]
- 22.Luk KD, Hu Y, Wong YW, et al. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26:1772–7. doi: 10.1097/00007632-200108150-00008. [DOI] [PubMed] [Google Scholar]
- 23.Herdmann J, Deletis V, Edmonds HL, Jr, et al. Spinal cord and nerve root monitoring in spine surgery and related procedures. Spine. 1996;21:879–85. doi: 10.1097/00007632-199604010-00023. [DOI] [PubMed] [Google Scholar]
- 24.Eggspuehler A, Sutter MA, Grob D, et al. Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J. 2007;16(Suppl 2):S188–96. doi: 10.1007/s00586-007-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggspuehler A, Sutter MA, Grob D, et al. Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J. 2007;16(Suppl 2):S209–15. doi: 10.1007/s00586-007-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutter M, Eggspuehler A, Grob D, et al. The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective study of 1,017 patients. Eur Spine J. 2007;16(Suppl 2):S162–70. doi: 10.1007/s00586-007-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutter M, Eggspuehler A, Grob D, et al. The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur Spine J. 2007;16(Suppl 2):S197–208. doi: 10.1007/s00586-007-0422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutter M, Eggspuehler A, Muller A, et al. Multimodal intraoperative monitoring: an overview and proposal of methodology based on 1,017 cases. Eur Spine J. 2007;16(Suppl 2):S153–61. doi: 10.1007/s00586-007-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KM, Warren S, Young GB. National manpower survey on clinical neurophysiology laboratories in Canada. Can J Neurol Sci. 2007;34:106. [Google Scholar]
- 30.Magit DP, Hilibrand AS, Kirk J, et al. Questionnaire study of neuromonitoring availability and usage for spine surgery. J Spinal Disord Tech. 2007;20:282–9. doi: 10.1097/01.bsd.0000211286.98895.ea. [DOI] [PubMed] [Google Scholar]
- 31.Accadbled F, Henry P, de Gauzy JS, et al. Spinal cord monitoring in scoliosis surgery using an epidural electrode. Results of a prospective, consecutive series of 191 cases. Spine. 2006;31:2614–23. doi: 10.1097/01.brs.0000240642.28495.99. [DOI] [PubMed] [Google Scholar]
- 32.Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–43. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 33.Wiedemayer H, Fauser B, Sandalcioglu IE, et al. The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg. 2002;96:255–62. doi: 10.3171/jns.2002.96.2.0255. [DOI] [PubMed] [Google Scholar]