Abstract
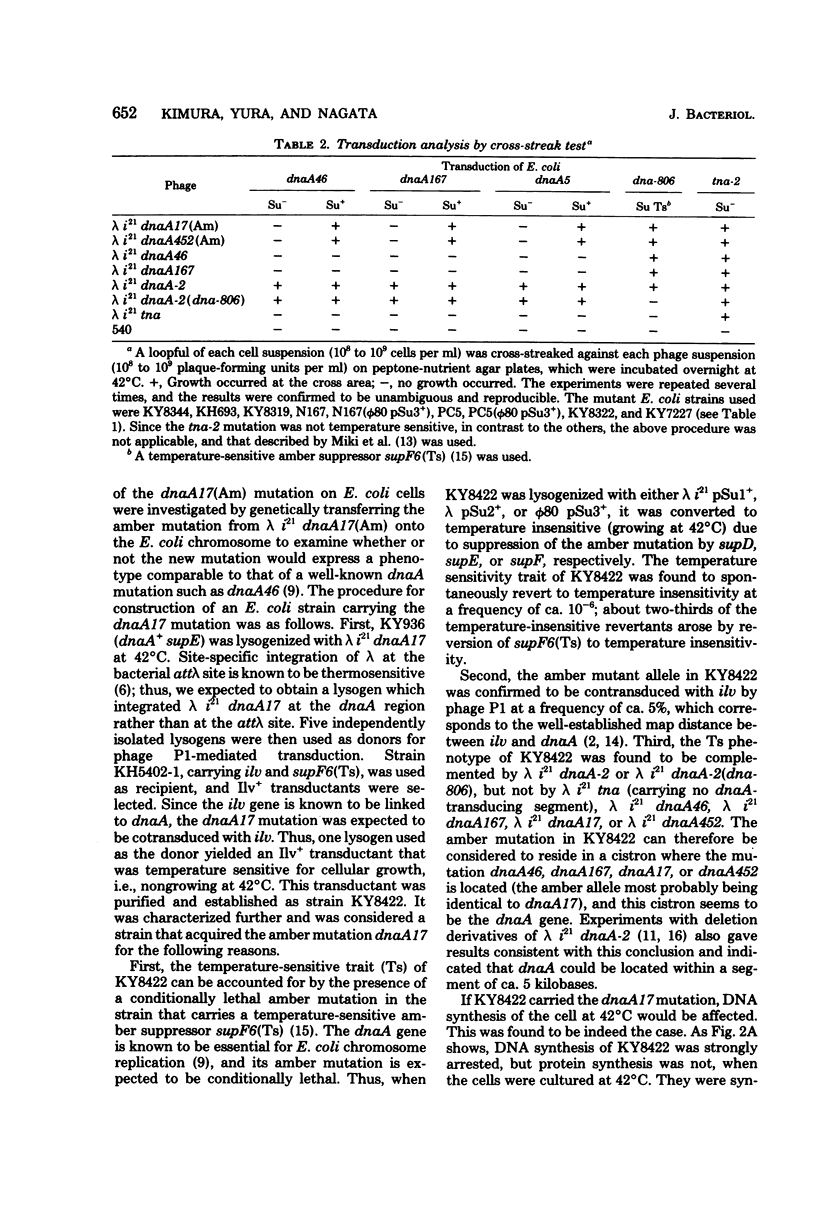
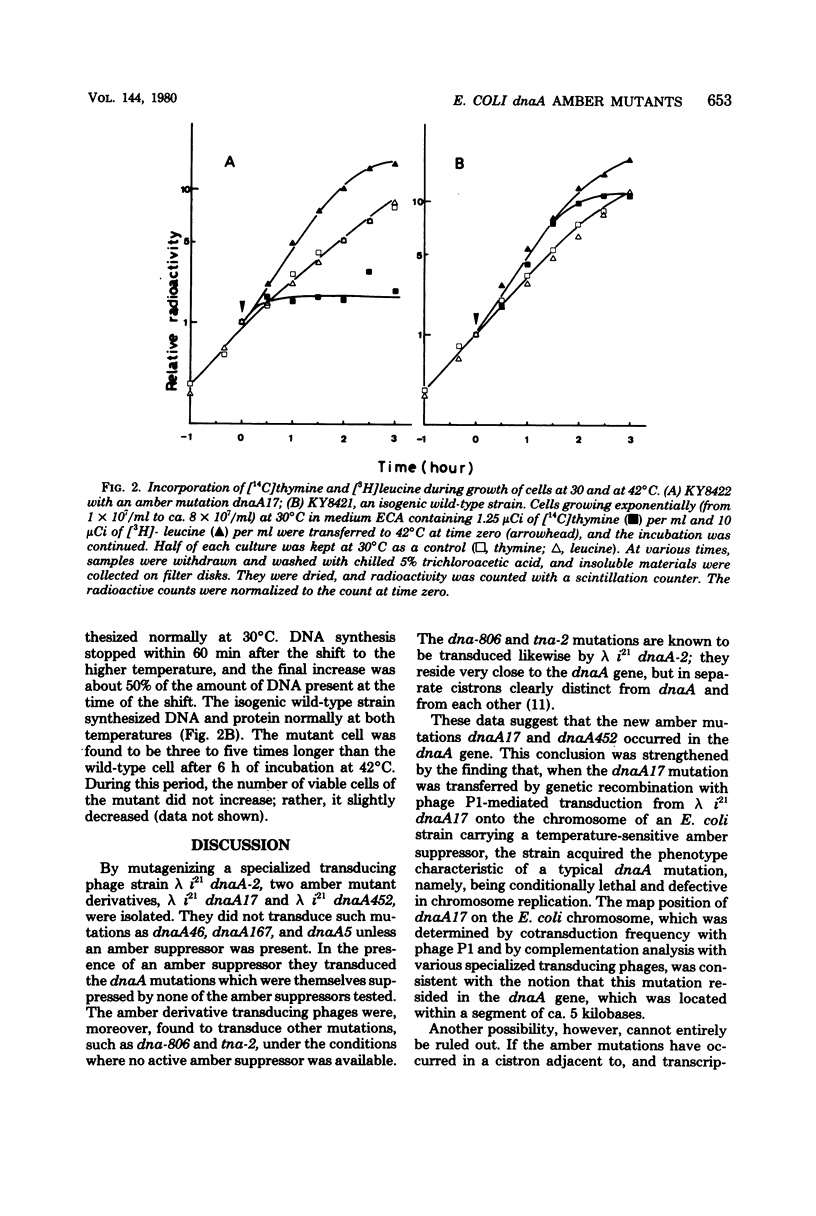
Specialized transducing phage lambda (formula, see text) dnaA-2 was mutagenized, and two derivatives designated lambda (formula) dnaA17(Am) and lambda (formula) dnaA452(Am) were obtained. They did not transduce such mutations as dnaA46, dnaA167, and dnaA5 when an amber suppressor was absent, but they did so in the presence of an amber suppressor. By contrast, they transduced the dna-806 and tna-2 mutations in the absence of an active amber suppressor. The dna-806 and tna-2 mutations are known to be located very close to the dnaA gene, but in separate cistrons. When ultraviolet light-irradiated uvrB cells were infected with the derivative phages and proteins specified by them were analyzed by gel electrophoresis, a 50,000-dalton protein was found to be specifically missing if an amber suppressor was absent. This protein was synthesized when an amber suppressor was present. The dnaA17(Am) mutation on the transducing phage genome was then transferred by genetic recombination onto the chromosome of an Escherichia coli strain carrying a temperature-sensitive amber suppressor supF6(Ts), yielding a strain which was temperature sensitive for growth and deoxyribonucleic acid replication. The temperature-sensitive trait was suppressed by supD, supE, or supF. We conclude that, most likely, the derivative phages acquired amber mutations in the dnaA gene whose product is a 50,000-dalton protein as identified by gel electrophoretic analysis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Tomizawa J. Chromosome replication in Escherichia coli K12 mutant affected in the process of DNA initiation. Genetics. 1971 Sep;69(1):1–15. doi: 10.1093/genetics/69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G., Echols H. Thermal asymmetry of site-specific recombination by bacteriophage lambda. Virology. 1973 Mar;52(1):30–38. doi: 10.1016/0042-6822(73)90395-4. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Rasmussen K. V. Regulation of the dnaA product in Escherichia coli. Mol Gen Genet. 1977 Oct 20;155(2):219–225. doi: 10.1007/BF00393163. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., von Meyenburg K. Characterization of the dnaA, gyrB and other genes in the dnaA region of the Escherichia coli chromosome on specialized transducing phages lambda tna. Mol Gen Genet. 1979 Sep;175(2):135–144. doi: 10.1007/BF00425529. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Kellenberger-Gujer G., Podhajska A. J., Caro L. A cold sensitive dnaA mutant of E. coli which overinitiates chromosome replication at low temperature. Mol Gen Genet. 1978 Jun 1;162(1):9–16. doi: 10.1007/BF00333845. [DOI] [PubMed] [Google Scholar]
- Kimura M., Miki T., Hiraga S., Nagata T., Yura T. Conditionally lethal amber mutations in the dnaA region of the Escherichia coli chromosome that affect chromosome replication. J Bacteriol. 1979 Dec;140(3):825–834. doi: 10.1128/jb.140.3.825-834.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Hiraga S., Nagata T., Yura T. Bacteriophage lambda carrying the Escherichia coli chromosomal region of the replication origin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5099–5103. doi: 10.1073/pnas.75.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Kimura M., Hiraga S., Nagata T., Yura T. Cloning and physical mapping of the dnaA region of the Escherichia coli chromosome. J Bacteriol. 1979 Dec;140(3):817–824. doi: 10.1128/jb.140.3.817-824.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Mizukami T. A temperature-sensitive Escherichia coli mutant defective in DNA replication: dnaN, a new gene adjacent to the dnaA gene. Mol Gen Genet. 1980;178(3):541–553. doi: 10.1007/BF00337859. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Temporal sequence of events during the initiation process in Escherichia coli deoxyribonucleic acid replication: roles of the dnaA and dnaC gene products and ribonucleic acid polymerase. J Bacteriol. 1977 Mar;129(3):1466–1475. doi: 10.1128/jb.129.3.1466-1475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




