Abstract
Cochlear implants (CIs) process sounds electronically and then transmit electric stimulation to the cochlea of individuals with sensorineural deafness, restoring some sensation of auditory perception. Many congenitally deaf CI recipients achieve a high degree of accuracy in speech perception and develop near-normal language skills. Post-lingually deafened implant recipients often regain the ability to understand and use spoken language with or without the aid of visual input (i.e. lip reading). However, there is wide variation in individual outcomes following cochlear implantation, and some CI recipients never develop useable speech and oral language skills. The causes of this enormous variation in outcomes are only partly understood at the present time. The variables most strongly associated with language outcomes are age at implantation and mode of communication in rehabilitation. Thus, some of the more important factors determining success of cochlear implantation are broadly related to neural plasticity that appears to be transiently present in deaf individuals. In this article we review the expected outcomes of cochlear implantation, potential predictors of those outcomes, the basic science regarding critical and sensitive periods, and several new research directions in the field of cochlear implantation.
1. Overview of cochlear implant function
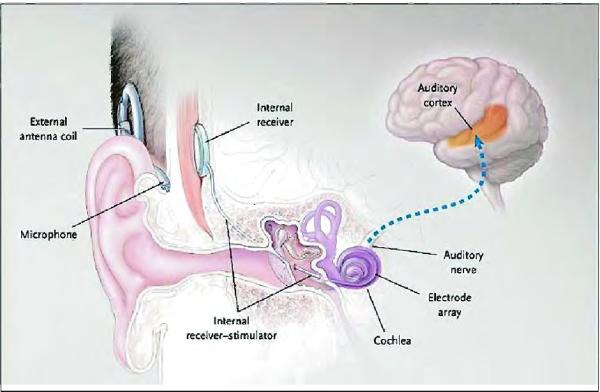
Cochlear implants (CIs) are implantable biomedical devices that are capable of providing some degree of auditory perception to patients with sensorineural hearing loss. Although some differences do exist between devices produced by the four different manufacturers (three are approved for use in the United States), the basic components and operations are essentially the same for all devices. All devices consist of external components worn by the patient that can be removed for sleep, bathing, etc., and internal components that are surgically implanted. The external and internal components communicate transcutaneously via radio frequency (RF) signals. Figure 1 shows a pictorial representation of the placement and function of a cochlear implant (Gates and Miyamoto, 2003).
Fig. 1.
Diagram from Gates and Miyamoto (2003) of placement of a cochlear implant and schematic representation of its relationship to the cochlea, auditory nerve, and auditory cortex.
The external components consist of a microphone, speech processor, and radio transmitter. The microphone and speech processor are worn behind the ear of the patient. Sound is collected by the microphone and converted into electric signals; these signals are then processed through a series of band-pass filters according to the specific parameters of the device's speech encoding strategy. After processing the incoming signals, information is transmitted across the skin to a receiver in the implanted device. The transmission coil is held in place over the receiver by magnets at the center of each coil, one internal and one external. This trans cutaneous (as opposed to percutaneous) transmission of data occurs across intact skin (ASHA, 2004).
The internal device further processes the signals and converts them into a series of bipolar square-wave signals that are carried down the electrode array to stimulate the remaining fibers of the auditory nerve in the modiolus. All currently used cochlear implants have multiple (16–22) stimulating electrodes. These electrodes are spaced along the electrode array that is inserted into the cochlea. Each square-wave signal transmitted down the electrode array is sent to a specific electrode based on the band-pass filtering in the speech processing strategy, thus taking advantage of the tono-topic encoding of frequency in the cochlea (ASHA, 2004).
2. Outcomes and outcome predictors
2.1. Pediatric CI recipients
The primary goal of cochlear implantation is open-set auditory-only speech understanding in every day listening environments. For the majority of implant recipients this goal is achievable, although wide variation in individual outcomes has been reported at all implant centers around the world. Some unilateral CI recipients develop the ability to talk on the telephone, while others gain only improvements in speech reading abilities (Cohen et al., 1999). An understanding of the neurobiological and neurocognitive factors that contribute to a favorable outcome after cochlear implantation would potentially allow clinicians to predict the expected results for an individual CI patient. Such knowledge could also allow adjustment of treatment and post-implantation rehabilitation to maximize the benefits of receiving a CI. Several studies have been undertaken to identify the factors that influence speech and language outcomes after cochlear implantation.
Although age at implantation was widely believed to be a major factor influencing outcome after cochlear implantation, no large studies examining this variable were conducted in the first several years after cochlear implantation became accepted; groups that were studied were often small and heterogeneous, including both adults and children (e.g. Snik et al., 1997). Nikolopoulos et al. (1999) performed a prospective trial with 126 prelingually deafened children, all implanted before the age of 7 years. Closed-set speech perception was tested using the Iowa Matrix Sentence test, in which the child hears a three-word sentence and is then presented sets of pictures arranged in 2×3-picture matrices. The child responds by pointing to the picture that is best identified by the three-word sentence. Connected discourse tracking (De Filippo and Scott, 1978) was used to measure open-set speech perception. In this test, the examiner reads an age-appropriate story to the child, who repeats the story back to the examiner in a phrase-by-phrase fashion.
As a measure of real-world performance in auditory tasks, the Categories of Auditory Performance (CAP) score was used. The CAP score is a categorical, nonlinear scale that ranges from 0 to 7. All levels are determined by ability to perform every-day auditory tasks, with 0 representing no awareness of environmental noise, and 7 representing the ability to talk on a telephone. Children were followed for up to four years after implantation. In this large study, every speech and language outcome was negatively correlated with age at implantation. This was interpreted as strong evidence for the importance of implanting profoundly deaf children at a young age, when considerable neural plasticity is still present. However, the authors of this study noted large variation between individual children in all tests. Thus, age of implantation alone should not be used in a predictive manner when considering cochlear implant candidacy.
Osberger and Fisher (2000) analyzed speech perception scores in implanted children using a variety of pre-implant demographic and hearing-related measures. Fifty-seven prelingually deafened children, with a mean implant age of 5.4 years, were given the Glendonald Auditory Screening Procedure (GASP) preoperatively and at 3-month intervals post-operatively for 12 months. The GASP is an open-set test of speech perception in which children repeat back single words of differing numbers of syllables and stress patterns. The GASP was selected because of the wide variability of cochlear implant recipients' scores on the measure. Scores on the GASP administered 12 months post-operatively were analyzed and correlated with: socioeconomic status, gender, communication mode (oral vs. total communication), age at onset of deafness, age at fitting of hearing aids, age at cochlear implantation, and duration of deafness at time of implantation. Pre-operative residual hearing was also assessed by several measures: unaided pure-tone average in both the ear to be implanted and the contralateral ear, score on Early Speech Perception Monosyllable Identification test in each ear independently, and score on the GASP in the best aided condition. Pure-tone average (PTA) is the mean value of hearing loss at 500, 1000, and 2000 Hz, as measured by pure-tone audiometry. Of these demographic and audiologic variables, only communication mode and the pre-operative GASP score were found to be significantly associated with a child's post-operative GASP test scores.
Oral communication (OC) children had better speech perception skills than children who used total communication (TC, the use of sign plus oral language). Even very low pre-operative open-set speech perception abilities were found to be beneficial for improved post-implantation speech perception. Oral communication children with pre-operative GASP scores of > 1% correct had a mean post-operative GASP score of 77% (SD = 21%) correct, whereas those who scored below 1 % correct had a 12-month post operative mean score of 48% (SD = 33%) correct. The difference was even more pronounced in total communication users at 61 ± 53% correct (for those with preoperative GASP scores > 1%) vs. 14 ± 20% correct (for preoperative GASP scores < 1%). As these numbers demonstrate, even when a variable has a significant effect on a CI user's outcome there is a large amount of variability in individual outcomes; although the overall influence of a preoperative or rehabilitative variable may be ascertained, individual performance may diverge widely from the general trend. Lastly, Osberger and Fisher failed to observe an effect from age at implantation, but did note that their 12-month follow-up period may have been too short for such effects to become apparent (Osberger and Fisher, 2000).
In further study of CIs in prelingually deafened recipients, Kirk et al. (2002) performed a longitudinal study of 74 children implanted before the age of 5 years. Speech and language abilities of the children were analyzed with respect to age at implantation – before or after 3 years old – and mode of communication – OC vs. TC. The Reynell Developmental Language Scales (RDLS) were used to measure both receptive and expressive language. These tests required children to perform language-based tasks that ranged in complexity from labeling of individual objects to comprehension (receptive language) and expression (expressive language) of complex multi-clause instructions. All children in the study had marked improvement in receptive and expressive language skills post implantation. Receptive language developed at a significantly higher rate in children implanted before 3 years old than in children implanted after age 3 years. Although there was a trend toward more rapid development of expressive language in younger implant recipients, it was not statistically significant. Receptive language development as measured by the RDLS was approximately equal for the OC and TC groups. Oral-only communication did provide an advantage over total communication in expressive language. The reason for this advantage was unclear at the time of the study; although it may have been intrinsic to the mode of communication and education, it could also have been a by-product of an impoverished language environment in households that used both manual and spoken language. Such a language environment could be the result of a household in which only a very few people know sign language or do not know sign language well, thus limiting the linguistic exposure of the child (Kirk et al., 2002; Moeller and Luetke-Stahlman, 1990).
Based on accumulating evidence that early implantation positively influenced the final outcome, some investigators began performing cochlear implantation on profoundly deaf children less than 12 months old. Miyamoto and colleagues (2003) reported on a single infant implanted at the age of 6 months. Testing of language skills by the Reynell Developmental Language Scales showed that by the age of two years the child had achieved nearly age-appropriate language abilities. This report was followed two years later by studies of Colletti, et al. (2005) and Miyamoto et al. (2005) reporting small series of infant cochlear implantations. Colletti et al. (2005) measured outcome with the Categories of Auditory Performance (CAP) scale. They reported significant gains in CAP scores at 12 to 24 months post-implantation for all 10 infants implanted, and a median time to onset of babbling of 4 months after CI activation. They further reported that CAP scores in children implanted between the ages of 12 and 36 months were delayed approximately 1 year relative to CAP scores for children implanted in the first year of life. The authors noted the need to determine whether such advantages are long-lasting or if the difference between groups was only temporary. They also mentioned the need for development of new infant-specific testing procedures to better evaluate audiologic outcomes in very young CI listeners.
Miyamoto et al. (2005) reported data from a series of 13 infants, all implanted prior to 12 months of age. In this paper, the authors also described the development of two new tests for the evaluation of infant speech perception abilities, both based on the time an infant spends looking at an object presented in association with a sound. A visual habituation procedure was designed in which a child was presented with an auditory stimulus of either a prolonged vowel sound (“aah”) or eight repetitions of the word “hop” while looking at a visual image of a checker board. The presentation of the visual and auditory stimuli was repeated until the child showed evidence of habituation to the stimulus, as evidenced by a decrease in time spent looking at the visual stimulus (the checker board image). The image was then displayed again with a novel auditory stimulus. An increase in looking time at the checker board when the auditory stimulus was changed was interpreted by these researchers as evidence that infants with CIs were capable of discriminating between continuous (“aah”) and discontinuous (“hop, hop, hop”) speech sounds.
In another test procedure, infants were shown two different videos, each associated with a specific speech stimulus. After an exposure period, the infants were presented with one of the two auditory stimuli and shown both videos. Infant CI recipients in this test preferentially looked at the video associated with the sound (the target video) instead of the non-target video (Miyamoto et al., 2005). This preference for looking at the target video indicated that early-implanted deaf children were capable of forming word-object associations.
Holt and Svirsky (2008) also addressed the question of early implantation. They examined the speech and language development in prelingually deafened children implanted before the age of 4 years. They sought to specifically address the question of very early implantation – defined in their paper as implantation at less than 12 months of age – and whether early implantation provided benefits appropriate to the possible increased risk of performing the operation on infants. Word recognition skills were measured using the Mr. Potato Head task, in which children build a Mr. Potato Head toy according to verbal instructions, e.g. “He wants green shoes.” The test is a closed-set procedure with chance performance of 5%. Receptive and expressive language were measured using the Reynell Developmental Language Scales. Ninety-six CI listeners were tested at 6-month intervals for 2 to 2.5 years after implantation; children were divided into 4 groups based on age at implantation, with each group representing a one-year span.
The authors found that nearly all children, no matter when they received their CI, had some delay in speech and language abilities compared to normal-hearing, typical-developing children. Contrary to other researchers, Holt and Svirsky (2008) found that for the group implanted between 6 and 12 months of age there were no significant differences in spoken word recognition and expressive language development as compared to the children implanted between 13 and 24 months of age. They did find a statistically significant advantage to implantation prior to 12 months of age in receptive language development. Children implanted at later ages (25–36 months and 37–48 months old) had slower rates of development in receptive and expressive language than the groups who received their implant before their second birthday. However, the rate of improvement in word recognition was similar in all 4 groups.
The lack of a significant difference in language scores between the earliest-implanted children and those implanted before age 2 should be interpreted with caution, however. As the authors point out, the infant implant group contained only 6 children, thus lowering the power of that comparison. The authors concluded that there is some sensitive period for the development of language that ends between the ages of 2 and 3.5 years, but the sensitive period for the development of word recognition extends to the age of 4 years and possibly beyond. This discrepancy between the end of the sensitive periods for the development of oral language and word recognition is an indication that these two aspects of oral communication may reflect different underlying processes (Holt and Svirsky, 2008). The issue of critical and sensitive periods is addressed in more detail in the next section.
Other possible predictors were also considered by Holt and Svirsky in this study. These included preoperative better-ear pure tone average, family's estimated income, communication mode, and gender. Better-ear PTA, communication mode, and family income did account for statistically significant sources of variation; gender did not. When the contributions of these additional influencing factors were statistically accounted for by treating them as covariates, the statistically significant differences in performance based on age at implantation were still present between the groups. The relationship between family income and language outcomes, though statistically significant, was ambiguous. Family income was positively correlated with the rate of receptive language development, but was negatively correlated with expressive language developmental rate (Holt and Svirsky, 2008).
Several authors have noted that different subsystems of language comprehension are processed in different parts of the brain and may therefore have distinct developmental timelines. Imaging studies in adults indicate that syntax and semantics appear to be processed in different areas, though the specific areas involved are not fully agreed upon by all investigators (Caplan, 1999). Functional magnetic resonance imaging and evoked response potential studies of the brains of bilingual adults have shown not only activation of distinct regions for grammar and semantics, but also that the regions activated differed according to age of acquisition of the second language (Thierry and Wu, 2007; Wartenburger et al., 2003). By measuring evoked response potentials as subjects listened to sentences, Osterhout (1997) found that syntactically and semantically anomalous words elicit different evoked potentials (ERP), although significant differences were observed between individuals. These differences are highly repeatable and have been found by other researchers (Hagoort et al., 1993; Osterhout and Nicol, 1999), with the existence of a semantic error-specific ERP waveform being documented as early as 1980 (Kutas and Hillyard, 1980). Further, the differences in ERP waveforms elicited by syntactically and semantically anomalous words generalize across a variety of languages (Balconi and Pozzoli, 2004; Hagoort et al., 1993). This could explain the pattern observed by Holt and Svirsky (2008), who used behavioral measures to document a differential pattern in speech and language outcomes suggesting the presence of separate developmental trajectories for vocabulary and oral language.
Several factors have been reported to have an impact on the ability of prelingually deaf children to develop oral language skills after cochlear implantation. Of these, the most important and consistently reported variable that influences the ability to use auditory-only communication is the age at which the child is implanted. Communication mode post-implantation has also been frequently reported to be a factor that contributes to final speech and language outcome, with oral-only communication producing speech and language results superior to those observed in children who use a combination of signing and spoken language (total communication). Better pre-implant residual hearing, measured by pure-tone perception or conventional speech discrimination scores, is predictive of better speech and language outcomes (Holt and Svirsky, 2008; Osberger and Fisher, 2000). Other factors, including socioeconomic status and gender, have been found to have inconsistent effects on speech and language outcomes following cochlear implantation. Although some factors that contribute to a CI listener's ability to use spoken language for communication have been identified, these variables do not account for all sources of variability; considerable individual differences are present in all the published literature and preclude the use of any one known factor as a reliable pre-implant predictor of post-implant success.
2.2. Adult CI recipients
Many children have been able to develop spoken language with the aid of a cochlear implant, and earlier implantation has been shown to result in better language outcomes. What, then, should be the approach to prelingually deafened adults and older children? Can they be expected to develop the ability to use oral language for communication if they receive a CI? If not, what degree of improvement can be reasonably expected – and what are the limitations – in auditory performance in the case of a person who has had long-term auditory deprivation?
Waltzman et al, (2002) published a report of 49 patients, all congenitally deaf who received a cochlear implant later in life – 14 as adults (ages 18–36) and 35 as children at least 8 years old, CI listeners were tested on open set word recognition (consonant-vowel-consonant words) and sentence perception in quiet and in noise (Hearing In Noise Test and City University of New York sentences, both open-set, sentence repetition tasks). Testing was performed preoperatively and annually after implantation. A majority of the adult implant recipients – 50% to 71%, depending on the test – showed some degree of improvement in the open-set speech understanding tests. Several implant recipients showed no gains over preoperative results, and the degree of improvement varied widely among those individuals who did improve. Some of the CI listeners that were reported as showing improvement actually made very modest gains (less than 5% improvement on a given test), and it is unclear in the article if these gains were statistically significant. However, other CI users had impressive increases in their ability to understand spoken language. The authors also noted that of the 14 adult participants in the study, five had their implants for more than one year. Of these five, none showed improvement in speech understanding after the first year of implant use. This finding suggested an earlier plateau, and thus limitation on the degree, of improvement in auditory function after implantation as compared to the gains seen in prelingually deaf children who receive CIs.
The children who received a cochlear implant at later than 8 years of age showed a pattern similar to the adult implant recipients (Waltzman et al., 2002); the majority of children showed improvement, though that improvement varied widely between individual CI users. A difference between the late pediatric and adult groups was the length of time after implantation for which improvement in auditory capabilities continued to increase. There were 8 children who had their implants for 2 years; the open-set speech perception and word recognition scores of these children continued to increase throughout the duration of the study. Although all recipients received their implant after the age of 8 years, age at implantation continued to have an effect on advantage gained from the CI, with younger age at implantation (and thus shorter duration of deafness) being associated with better outcomes.
In a retrospective review of 103 adult patients who received a CI after long-term deafness, Teoh et al. (2004a) found several trends in this population of CI recipients. Most importantly, the maximum performance achieved by the adult CI users was significantly below the level of speech understanding attained by early pediatric and postlingually deafened adult implant recipients. Most patients who received a CI after long term deafness obtained only limited closed-set speech perception and at most minimal useful open-set speech understanding. Age at implantation affected the final ability for the CI listener to understand and use speech. The performance asymptote was gradually lowered with each additional year of auditory deprivation. Implantation after the age of 12 years typically resulted in only limited ability to understand speech in closed-set tasks. This finding of limited, maximum benefit for adult recipients regardless of length of CI use is consistent with the hypothesis that a sensitive period exists for the development of the auditory system.
Although the overall performance of CI users who received their implants as adults was substantially lower than their earlier-implanted counterparts, there was still wide variation between individual CI users. A few late-implanted adults were able to achieve significant open-set speech understanding, but these CI listeners were the exception to the norm. This variation appears to be a result of patient characteristics rather than device type or processing strategy used, provided the CI used a modern processing strategy. Results across device types were similar to each other, and within CI users of each device type there was wide variability in individual results (Teoh et al., 2004a).
The performance plateau is not only lowered with adult implantation, it is also achieved more quickly. Consistent with the results of Waltzman et al. (2002), Teoh et al. (2004a) found in their review of 103 implant recipients that the maximal level of performance with a CI is achieved in the first 12 months after implantation following long-term deafness. This finding is in contrast to some of the earliest case series (e.g. Manrique et al. (1997) and Snik et al. (1997)) that suggested long periods of time may be required to achieve maximum benefit in prelingually deaf adult CI recipients.
Cochlear implantation of adolescents and adults who have congenital or acquired prelingual deafness very rarely results in the ability to use oral/aural language alone for communication. Any benefit the CI user gains from his/her implant will likely be evident within the first year after implantation. However, many adult CI recipients do obtain some benefit from implantation, so age alone should not be an absolute contraindication to implantation. The limitation of auditory abilities of later-implanted CI users suggests the presence of some sensitive or critical period within which development of the auditory system must occur, and after which the plasticity of the system is significantly limited. The evidence for such period(s) of development will be discussed next.
3. Critical and sensitive periods for prelingually deafened CI recipients
A major research question in the field of cochlear implantation has been whether there is some critical period of auditory pathway development within which prelingually deafened patients should be implanted. That is, in addition to the general findings discussed above that earlier implantation yields better results, does there exist some age (or duration of deafness) after which implantation can be expected to produce results much less favorable than implantation carried out prior to that age? The limits of the putative critical period differ depending on the methods used for its determination and when the research was conducted. More recent research generally reports a critical period ending at an earlier age than older research.
Some of the previously mentioned studies concluded that some “sensitive period” – generally defined as similar to a critical period, but with a less precise and less precipitous cut-off – is likely to exist, variously placing the upper limit of the period at 4 to 12 years of age, depending on the outcome being considered. Based on their study of 96 children implanted between the ages of 6 months and 4 years. Holt and Svirsky (2008) concluded that the capacity for acquisition of receptive and expressive language was significantly diminished after the age of 2 to 3.5 years of age. However, they note that the window for development of word recognition was not as restrictive and appeared to last to at least 4 years of age and possibly longer. Teoh et al. (2004a) in their study of later-implanted children and adults concluded that a sensitive period exists and noted that almost all CI users implanted after the age of 12 years rarely were able to develop open-set speech understanding. The wide discrepancy between the ages proposed by Holt and Svirsky (2008) and Teoh et al. (2004a) is in large part due to the differing standard of what constitutes a significant drop in performance and thus defines the end of the sensitive period; Holt and Svirsky based the end of the sensitive period on a statistically significant decrease in language outcome measures, whereas Teoh, et al. relied on a final outcome measure – the inability to develop open-set speech understanding.
A large study of speech and language outcomes of congenitally deaf children implanted at The Hospital for Sick Children (Toronto) over a wide range of ages (1–15 years) found that there was no true critical period after which implantation was only minimally useful, but did reconfirm the advantages of early implantation (Harrison et al., 2005). This conclusion was based on the Test of Auditory Comprehension, a closed-set task, and the GASP and Phonetically Balanced Kindergarten words, both open-set tests of single word repetition. These authors, then, concluded that implantation age is related to speech and language outcomes in a continuous fashion, rather than by discrete, rigidly fixed periods of time. They further state that in regard to functional outcomes of speech and language as determined by behavioral measures, it is unlikely that a clearly defined critical period could exist. Their argument for the absence of a cleanly demarcated critical period is based on the fact that any behavioral outcome is the product of multiple neural and cognitive mechanisms, each of which has its own developmental timeline. Although the development of each distinct mechanism may have a critical period, the overlap of these periods and complex integration of the many parts of the system make the presence of a single overarching critical period unlikely. This line of reasoning from Harrison et al. (2005) highlights an important difference in definitions of the term “critical period.” Whether such a period is detected depends on what measures are used to define the limits of the critical or sensitive period. Researchers who primarily investigate a subset of the complex auditory system (the auditory cortex, for example) have been more likely to discover a critical period than those scientists who study behavioral outcomes that represent the combined output of many underlying processes. Nonetheless, there have been some researchers who have argued that sensitive periods exist for the maturation of the auditory system as a whole.
In a follow-up article to their initial paper, Teoh et al. (2004b) examined the reasons for the limitation of final outcome in adult CI recipients and what factors might influence their audiologic performance. They note that each part of the auditory system – peripheral auditory system, cochlear nucleus and auditory midbrain, and the auditory cortex – undergoes degradation if not stimulated. Animal models have shown that dendritic processes of the spiral ganglion cells undergo extensive degeneration after hair cell damage (Hardie and Shepherd, 1999). However, in human temporal bone studies, the number of remaining spiral ganglion cells had no correlation to performance with a CI (Fayad et al., 1991). Thus, the redundancy within the auditory nerve, as compared to the relatively crude spacing of the implant electrodes, is great enough that spiral ganglion cell survival is not the limiting factor in determining performance with a cochlear implant.
The ultrastructural organization of the auditory brainstem is also affected by prolonged deafness (Teoh et al., 2004b). Changes shown to occur include reduced synaptic vesicle density, decreased terminal branching of neurons, and enlarged synaptic size. Physiologic changes included adverse affects on the synchrony of excitatory post-synaptic potentials and elevation of the response threshold of the cochlear nucleus. These anatomic and physiologic changes have all been shown to be at least partly reversible with renewed stimulation of the cochlear nerve.
According to Teoh et al. (2004b), the most important limiting factor in the development of useable audition in later-implanted children and adult CI recipients is the reorganization of auditory cortex. Because of the potential for other sensory modalities to occupy the auditory portions of the cortex, Teoh et al. (2004b) urge the use of aural based therapies with hearing aids prior to implantation and aural-only rehabilitation after cochlear implantation. Although these therapeutic interventions may help minimize cortical colonization by other modalities, the auditory input they provide to severely hearing impaired individuals is quite limited. Thus, the overall prognosis for auditory system development after prolonged deafness is poor.
Ponton et al. (1996) studied auditory evoked potentials (AEPs) in children with cochlear implants. The children's cochlear implants were directly stimulated with pulse trains of ten 200-μs biphasic pulses. Normal hearing controls heard a train of 100-μs clicks, presented monaurally. The latency of the positive peak P1 decreased exponentially throughout childhood, with an asymptotic value of approximately 50 ms in normal hearing controls. The latency of the P1 wave in cochlear implanted children was delayed by an amount equivalent to their time of sound deprivation (age of implantation). The authors concluded that (1) the auditory cortex does not develop without stimulation; (2) the plasticity of the cortical auditory system is maintained during sensory deprivation (deafness); and (3) maturation of the auditory system begins upon initiation of stimulation, in this case cochlear implantation.
Further research by Eggermont and Ponton (2003; Ponton and Eggermont, 2001) examined AEPs in two children with cochlear implants. The stimuli used were trains of four to ten 200-μs biphasic pulses, with 2 ms between the onsets of pulses within a train. Based on their longitudinal observations of the maturation of the P1-N1 complex, they concluded that the auditory system in the upper cortical layers has a maturational critical period that likely ends between 3 and 6 years of age and is strongly dependent on auditory stimulation for development. Late stimulation (i.e. cochlear implantation) led to early signs of development of the system, with P1 latency that was appropriate for hearing age (time exposed to sound). However, the latency of P1 stopped decreasing 6–8 years after implantation and N1 never developed. Thus maximal development was reached relatively quickly and was well short of the full development experienced by normal hearing children. These findings indicate that the final maturity of at least some parts of the central auditory system is dependent on the duration of deafness prior to the onset of stimulation (Ponton et al., 1999).
Findings strongly corroborating the initial results of Ponton, et al. (Eggermont and Ponton, 2003; Ponton et al., 1999) have been reported more recently by Sharma et al. (2005) in a longitudinal study of 21 children with unilateral cochlear implants. Children were classified as early CI recipients if they received their CI before 3.5 years of age or late CI recipients if cochlear implantation occurred after the age of seven years. The P1 response latency and morphology were observed. The P1 latencies of the early-implanted children rapidly approached age-appropriate levels, whereas the latencies of the P1 waveform in children implanted after age 7 decreased much more slowly and showed aberrant morphology. This initial work was extended two years later (Dorman et al., 2007) in a larger study of 245 children with cochlear implants. Nearly all of the children in this study who were implanted by the age of 3.5 years obtained normal P1 latencies, usually within 3–6 months after initial stimulation. Approximately half of the children implanted between ages 3.5 and 7 years had P1 latencies that decreased to within age-appropriate norms for normal hearing children. However, the children who experienced 7 or more years of auditory deprivation had an elevated P1 latency, even in measurements taken years after onset of stimulation. Additionally, the morphology of the P1 waveform remained abnormal, and the N1 response never developed in late-implanted children (Sharma et al., 2007). The differences between P1 morphology in early and late CI recipients led Sharma and Dorman to hypothesize that the P1 response in late-implanted children is generated from a source that is different from the generator in normal hearing and early-implanted children (Dorman et al., 2007).
More recently, Gilley et al. (2008) examined cortical auditory evoked potentials (AEP) in children with normal hearing, deaf children who underwent implantation before 4 years old, and deaf children implanted after 7 years old. They used current source reconstruction to estimate source generators for the P1 AEP response generated in response to hearing a single synthetic syllable. Like the normal hearing children, the early implanted children showed activation in the inferior temporal gyrus and the superior temporal sulcus. Two of the early implanted children also showed neural activity in the parietotemporal cortex, an area outside the auditory cortex. This activity – although minor and limited to only two of the children – may represent some neural reorganization after even relatively short periods of deafness.
The late implanted children studied by Gilley et al. (2008) showed focused activity in the parietotemporal cortex contralateral to the side of stimulation. The authors note that there is no reported instance of this pattern of stimulation in normal hearing listeners. Although there were late implanted children who did not show parietotemporal cortex excitement, no children in this group had activation of regions associated with the auditory cortex when performing basic auditory tasks. This finding of stimulation in portions of the cortex usually associated with the dorsal visual stream is cited by the authors as an indication of the degree to which cortical reorganization can occur, with traditionally visual areas being activated and/or colonized by auditory functions.
The sensitive period for speech and language development documented by these AEP studies does not perfectly agree with the sensitive period generally determined by behavioral studies. The discrepancy in sensitive periods can be attributed to two important differences in exactly what each type of study examined. The electrophysiological studies typically used a single syllable or even a brief non-speech sound (e.g. a click or direct stimulation of a CI electrode) to elicit a neurologic response. The speech and language studies on the other hand depend on perception of whole words and integration of words into meaningful sentences. These more complex processes are inherently more variable. Secondly, the AEP studies analyzed the response of a specific portion of the auditory system, such as the evoked cortical potential change at a particular generator site, but speech perception tests assess the processing of entire speech and language pathways functioning as an integrated system. Thus, scores on the speech and language tasks represent the end product of many interrelated processes, introducing greater variability in outcomes.
In a recent review, Kral (2007) also addressed the issue of sensitive periods and cross-modal reorganization. Based on evoked potential data and times of increasing dendritic complexity, he concluded that cross-modal reorganization could hinder the processing of auditory signals after implantation. This barrier to processing results from higher-order auditory areas being occupied by visual functions; descending modulation from higher auditory areas is also de-coupled from primary areas in the brain that develop in the absence of aural stimulation. The good agreement between the histological and electrophysiological data led Krai to conclude that a sensitive period for auditory development exists and that it ends between the 2nd and 4th years of life.
Cross-modal neuroplasticity in postlingually deaf adults has been shown to underlie their auditory rehabilitation. Adult CI recipients have been shown by positron emission tomography (PET, an imaging procedure that indicates cortical metabolism of glucose) to recruit visual cortex when hearing single words (Giraud et al., 2001). Further, the recruitment of visual cortex documented for words and syllables was not present when listeners were presented with non-speech noises. Additionally, recruitment of visual cortex was positively correlated with speech perception measures and lip-reading scores. These findings suggested that rehabilitation of adult CI recipients may be facilitated by visual cortex plasticity, and that in cases of limited auditory cortex plasticity, the plasticity of the visual cortex may be critical for the ability to learn to use additional visual cues to aid in speech comprehension. Bergeson and Pisoni's (Bergeson and Pisoni, 2004; 2005) work with prelingually deaf CI recipients further supported the importance of visual processing to CI rehabilitation. They tested deaf children prior to implantation using a visual-only version of the Common Phrases test. Strong positive correlations were found between pre-implant scores on the visual-only Common Phrases test and 3-year post-implantation measures of speech perception, speech intelligibility and language.
Other authors have found evidence that in the case of prelingually deaf implant recipients, visual/auditory cross-modal plasticity may not be as advantageous as for adult recipients. In a recent study by Lee et al. (2007), twenty-two children were examined preoperatively by PET and their results compared with post-operative performance on an open-set sentence repetition task. Hypometabolism of the auditory cortex was predictive of better speech perception post-implantation. This effect remained intact after age at implantation was taken into account as a covariable. Increased metabolism (and thus neuronal activity) of the auditory cortex implicates cortical colonization by other processes as a source for long-term auditory deficits in cochlear implant recipients (Lee et al., 2001). Increased activity in the left refrontal cortex preoperatively was also associated with better speech perception after implantation. These results may indicate a possible avenue for novel rehabilitation and intervention techniques. Specifically, broader rehabilitative efforts that focus on domain-general cognitive functions may be superior to highly targeted therapies that focus on specialized auditory circuits (Lee et al., 2007).
Taken together, the data from AEPs, functional imaging studies, animal models, and audiologic outcomes indicate that there appears to be a sensitive period – though perhaps not a true critical period – for implantation that goes beyond the simple notion that earlier is better. That is, neural plasticity of the auditory system, and thus the ability to construct meaningful percepts from the impoverished information provided by a cochlear implant, does not simply decrease linearly over time. At some point, between the ages of 2 and 4 years, the plasticity of the auditory system begins to decline sharply; thereafter the benefits of cochlear implantation are greatly reduced. This does not mean that implantation is not indicated after this time, as several studies discussed here have documented that later implant recipients can achieve auditory improvement over their unimplanted state. It does mean that realistic goals need to be explained to the patient, and the potential benefits and risks of cochlear implantation in such situations need to be carefully weighed. The existence of a sensitive period for auditory system development also points to the importance of early identification and cochlear implantation by the age of 2 years for deaf children who meet candidacy requirements.
4. New research directions
For the development of oral/aural communication in prelingually deafened individuals, the importance of early implantation has been clearly established, and considerable progress has been made in understanding some of the causes of decreased performance in patients who receive their implants after prolonged periods of deafness. However, there is much in the world of electrical hearing that is not yet well understood (NIH, 1995). The topics of new research are primarily in the following areas: the application of cochlear implants to the perception of non-speech sounds; predicting individual outcomes and understanding the fundamental causes of the wide individual variation that is common between cochlear implant recipients; and exploring novel behavioral and rehabilitative interventions that may help CI recipients with suboptimal outcomes. We will now briefly discuss some of these promising new directions of investigation.
As speech perception with cochlear implants has steadily improved over the last two decades, more attention has been turned to the cochlear implant user's perception of non-speech sounds. Perception of environmental sounds from everyday life is now being explored. Reed and Delhorne first reported on environmental sound recognition in cochlear implant recipients using a closed-set selection task (Reed and Delhorne, 2005). They found that word recognition and environmental sound identification were correlated. But they also reported wide inter-subject variability. Sounds that were confused tended to have similar temporal envelopes, and sounds with very distinctive temporal characteristics were the signals most often correctly identified. A current study at our center is examining the open-set identification of environmental sounds and the advantage of bimodal hearing (a CI plus a hearing aid in the contralateral ear) in environmental sound perception. Other work in this area has focused on the use of CI simulations of environmental sounds with normal hearing listeners and the effect of training and perceptual learning to better identify such spectrally-degraded input (Loebach and Pisoni, 2008; Shafiro, 2008).
Music perception by CI listeners is another rapidly growing field of research (McDermott, 2004). As with all other areas of cochlear implant performance, the variability in music perception tasks is quite large, with some CI listeners able to differentiate between pitches separated by a single semi-tone and others unable to identify a difference of greater than one octave (Drennan and Rubinstein, 2008; Galvin et al., 2007). Rhythm and lyrics are better preserved than melody and timbre (Drennan and Rubinstein, 2008; Galvin et al., 2007; Gfeller et al., 2006). Gfeller, et al. (2006) compared cochlear implant users, hybrid device (CI plus hearing aid) listeners, and normal hearing adults in a melody and instrument identification task. The CI recipients performed significantly worse than the hybrid and normal hearing listeners in these tasks. Low-frequency information is not conveyed well by cochlear implants, and this is believed to be the reason for many of the difficulties observed by these researchers. Other research using CI simulations with normal hearing adults has demonstrated that speech perception requires appreciably fewer channels (6–8 channels) than does accurate perception of music (up to 32 channels) (Kong et al., 2004). Several new processing methods to address these issues are being developed, including encoding the fundamental frequency with signal rate modulation (Laneau et al., 2006), MP3-like processing strategies, and current steering (Firszt et al., 2007). Current steering involves stimulating adjacent electrodes simultaneously to give the perception of stimulation at some point between the two electrodes on the array (Firszt et al., 2007). Processing strategies using this technique are being explored with the hope of increasing the number of functional channels from the present 16–22 to over 100. Such an increase could potentially be very helpful in the perception of acoustically complex sounds like music (Koch et al., 2007).
Since the earliest cochlear implants, great variability between CI users has been observed. This wide variation in performance has continued with the newer generations of implants and has been noted in the perception of both speech and non-speech sounds. Such variability between cochlear implant users makes predicting individual outcomes very difficult. Understanding the causes of this variation would allow clinicians to offer better prognoses to CI candidates preoperatively. As discussed earlier, the duration of deafness and rehabilitative communication mode are two factors that have been consistently shown to influence an implant recipient's language outcomes, but these variables do not address all of the variation seen in cochlear implant users. Several possible preoperative predictors of performance with a cochlear implant are now being investigated. Pre-implant visual-motor integration skills have been shown to be correlated with CI performance (Horn et al., 2007). However, these data are preliminary, and the authors note that larger sample sizes are needed to determine the amount of inter-user variation that can be predicted by pre-implant visual-motor integration skills assessment.
Another study by Horn, et al. used the Vineland Adaptive Behavior Scales to examine the relationship between pre-implant behavioral assessment and CI outcomes (Horn et al., 2005). This longitudinal study of 42 children found that motor development proceeds typically in deaf children, but other domains – most notably socialization and daily living skills – are noticeably delayed relative to norms for typically developing children. Pre-implant motor development, especially fine motor development and control, was also shown to be predictive of word recognition and language abilities after implantation, for the duration of the 3-year follow-up period of the study.
Other researchers are studying the role pre-implant functional imaging can play in predicting cochlear implant performance outcomes. Giraud and Lee (2007) measured resting metabolism using FDG-PET imaging prior to implantation and compared these images with speech perception scores 3 years after implantation. They found that those children who performed best with a CI exhibited the highest pre-implant resting metabolism in the dorsal brain regions – the prefrontal and parietal cortices. Pre-implant FDG-PET imaging of resting metabolism revealed both the cross-modal reorganization of the auditory cortex and activity consistent with higher cognitive mechanisms. Further investigation into the role of higher cognition in sensory adaptation is planned.
Wu et al. (2008) examined the utility of preoperative imaging and genetic screening in predicting outcomes with cochlear implants after rehabilitation. In their study, 67 pediatric cochlear implant users were imaged preoperatively with high-resolution computed tomography of the temporal bone and were screened for 3 genetic mutations that commonly cause congenital hearing loss: GJB2, SLC26A4, and the gene for mitochondrial 12S ribosomal RNA. The results of these screening procedures were compared with speech perception tasks 3 years after implantation. The genetic mutation SLC26A4 was found to be highly correlated with improved outcomes in implant performance, whereas the radiographic finding of a narrowed internal auditory canal was found to be correlated with poorer outcomes after rehabilitation. No other temporal bone radiologic findings were associated with language outcomes. The mutation GJB2 was not significantly correlated with hearing outcome due to the low number of GJB2 mutations identified in the study; however, all patients (four) with GJB2 mutations developed excellent open-set speech understanding. These authors reasoned that since the genetic mutations studied were specific to the cochlea, children with these mutations had auditory systems that were normally developed with the exception of the cochlear ultrastructure, leading to excellent results after early cochlear implantation.
The ability to predict speech and language outcomes after cochlear implantation is improving. However, this slowly improving prognostic ability has not been matched with an increased capacity to offer rehabilitative interventions in cases in which a patient has a suboptimal outcome after implantation. Ideally, patients at risk for such an outcome would be identified preoperatively, and appropriate interventions could be planned in advance. Ongoing work at our center is focused on the identification of cognitive diagnoses and impairments in CI recipients and providing interventions that address these areas. For example, working memory – the ability to retain and manipulate information concurrently with other mental processing activities – is of potential importance in development of language skills (Pisoni and Cleary, 2003). One study at our center is examining the effect of working memory training on working memory and other domains in CI recipients. Such broadening of the outcomes measured could aid in helping CI listeners with a variety of diagnoses – attention deficit/hyperactivity disorder, learning disabilities, etc. – to obtain better speech and language outcomes.
The new directions of research in the field of cochlear implantation are increasingly diverse and interdisciplinary (Pisoni et al., 2008). As these and other studies move forward, a greater understanding of the extent to which electrical hearing can aid deaf individuals, and the reasons for the highly variable outcomes of cochlear implantation, will be attained. Furthermore, patients who experience, or are at high risk for, a suboptimal outcome after cochlear implantation may be able to be helped with novel therapeutic interventions that are targeted to the specific underlying cause(s) for their difficulties in acquiring spoken language with electrical stimulation.
5. Conclusions
The introduction of cochlear implants has created a unique opportunity to gain an understanding of the development of the auditory system. Speech and hearing scientists have so far obtained considerable insight into the importance of sound in normal auditory development and the detrimental results of long-term auditory deprivation. The impressive plasticity of the auditory system at birth is the deaf patient's greatest advantage, but it can also be to his disadvantage. Such plasticity is responsible for the auditory system's ability to learn to interpret the degraded and impoverished information conveyed to it by a cochlear implant; it is also the reason that unused portions of the auditory system are reorganized and colonized by other sensory systems, rendering late implantation much less useful. In clinical practice, early implantation has now become widespread, resulting in better overall outcomes for CI recipients. Further study of the plasticity of the auditory system and higher cortical centers – from functional imaging, evoked potentials, behavioral studies, genetics, and other fields – will serve not only to enhance patient outcomes, but also to extend our basic knowledge of this complex system and how it interacts with other sensory and cognitive systems to support spoken language processing.
Acknowledgement
This research was supported by the following grants from the National Institutes of Health/National Institute of Deafness and Communication Disorders: training grant T32 DC 00012 to DBP; R01 DC 0064 to RTM; and R55 DC 009581 to DBP.
References
- ASHA Cochlear implants [Technical Report] 2004 [Google Scholar]
- Balconi M, Pozzoli U. N400 and P600 or the role of the ERP correlates in sentence comprehension: some applications to the Italian language. J Gen Psychol. 2004;131(3):268–302. doi: 10.3200/GENP.131.3.268-303. [DOI] [PubMed] [Google Scholar]
- Bergeson T, Pisoni DB. Audiovisual speech perception in deaf adults and children following cochlear implantation. In: Stein BE, Spence C, Calvert G, editors. The handbook of multisensory processes. MIT Press; Cambridge: 2004. pp. 749–771. [Google Scholar]
- Bergeson T, Pisoni DB, Davis RAO. Development of audiovisual comprehension skills in prelingually deaf children with cochlear implants. Ear Hear. 2005;26(2):149–164. doi: 10.1097/00003446-200504000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D. Activating brain systems for syntax and semantics. Neuron. 1999;24(2):292–293. doi: 10.1016/s0896-6273(00)80843-0. [DOI] [PubMed] [Google Scholar]
- Cohen NL, Waltzman SB, Roland JT, Staller SJ, Hoffman RA. Early results using the Nucleus CI24M in children. Amer J Otol. 1999;20:198–204. [PubMed] [Google Scholar]
- Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino F. Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope. 2005;115:445–449. doi: 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- De Filippo CL, Scott BL. A method for training and evaluating the reception of ongoing speech. J Acoust Soc Amer. 1978;63(4):1186–1192. doi: 10.1121/1.381827. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Sharma A, Gilley PM, Martin K, Roland P. Central auditory development: evidence from CAEP measurements in children fit with cochlear implants. J Commun Disord. 2007;40(11):284–294. doi: 10.1016/j.jcomdis.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, Rubinstein JT. Music perception in cochlear implant users and its relationship with psychophysical capabilities. J Rehabil Res Dev. 2008;45:775–790. doi: 10.1682/jrrd.2007.08.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123:249–252. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- Fayad J, Linthicum FH, Otto SR. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–811. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Koch DB, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otol Neurotol. 2007;28:629–636. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, Fu QJ, Nogaki G. Melodic contour identification by cochlear implant users. Ear Hear. 2007;28:302–319. doi: 10.1097/01.aud.0000261689.35445.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Miyamoto RT. Cochlear implants. New Engl J Med. 2003;349:421–423. doi: 10.1056/NEJMp038107. [DOI] [PubMed] [Google Scholar]
- Gfeller KE, Olszewski C, Turner C, Gantz B. Music perception with cochlear implants and residual hearing. Audiol Neurotol. 2006;11(suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Lee HJ. Predicting cochlear implant outcome from brain organisation in the deaf. Restor Neurol Neurosci. 2007;25:381–390. [PubMed] [Google Scholar]
- Giraud AL, Price CJ, Graham JM, Truy E, Frackowiak RSJ. Cross-modal plasticity underpins language recovery after cochlear implantation. Neuron. 2001;30(3):657–663. doi: 10.1016/s0896-6273(01)00318-x. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown C, Groothusen J. The syntatic positive shift as an ERP measure of sentence processing. Lang Cognit Proc. 1993;8:439–483. [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46:252–261. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- Holt RF, Svirsky MS. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29:492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Fagan MK, Dillon CM, Pisoni DB, Miyamoto RT. Visual-motor integration skills of prelingually deaf children: implications for pediatric cochlear implantation. Laryngoscope. 2007;117:2017–2025. doi: 10.1097/MLG.0b013e3181271401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Pisoni DB, Sanders M, Miyamoto RT. Behavioral assessment of prelingually deaf children before cochlear implantation. Laryngoscope. 2005;115:1603–1611. doi: 10.1097/01.mlg.0000171018.97692.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, Ying E, O'Neill T, Fears B. Effects of age at implantation in young children. Ann Otol Rhinol Laryngol. 2002;111(suppl 189)(5):69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Koch DB, Downing M, Osberger MJ, Litvak L. Using current steering to increase spectral resolution in CII and HiRes 90K users. Ear Hear. 2007;28(suppl 2):38S–41S. doi: 10.1097/AUD.0b013e31803150de. [DOI] [PubMed] [Google Scholar]
- Kong YY, Cruz R, Jones JA, Zeng FG. Music perception with temporal cues in acoustic and electric hearing. Ear Hear. 2004;25:173–185. doi: 10.1097/01.aud.0000120365.97792.2f. [DOI] [PubMed] [Google Scholar]
- Kral A. Unimodal and cross-modal plasticity in the 'deaf' auditory cortex. Int J Audiol. 2007;46:479–493. doi: 10.1080/14992020701383027. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Laneau J, Wouters J, Moonen M. Improved music perception with explicit pitch coding in cochlear implants. Audiol Neurotol. 2006;11:38–52. doi: 10.1159/000088853. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409(6817):149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Giraud AL, Kang E, Oh SH, Kang H, Kim CS, et al. Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex. 2007;17(4):909–917. doi: 10.1093/cercor/bhl001. [DOI] [PubMed] [Google Scholar]
- Loebach JL, Pisoni DB. Perceptual learning of spectrally-degraded speech and environmental sounds. J Acoust Soc Amer. 2008;123:1126–1139. doi: 10.1121/1.2823453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique M, Cervera-Paz FJ, Huarte A. Cerebral suditory plasticity and cochlear implants. Int J Ped Otorhinolaryngol. 1997;49(suppl. 1):S193–S197. doi: 10.1016/s0165-5876(99)00159-7. [DOI] [PubMed] [Google Scholar]
- McDermott HJ. Music perception with cochlear implants: a review. Trends Ampl. 2004;8(2):49–82. doi: 10.1177/108471380400800203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto RT, Houston DM, Bergeson T. Cochlear implantation in deaf infants. Laryngoscope. 2005;115:1376–1380. doi: 10.1097/01.mlg.0000172039.26650.9b. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Houston DM, Kirk KI, Perdew AE, Svirsky MS. Language development in deaf infants following cochlear implantation. Acta Otolaryngol. 2003;123:241–244. doi: 10.1080/00016480310001079. [DOI] [PubMed] [Google Scholar]
- Moeller MP, Luetke-Stahlman B. Parents' use of Signing Exact English: a descriptive analysis. J Speech Hear Disord. 1990;55:327–338. doi: 10.1044/jshd.5502.327. [DOI] [PubMed] [Google Scholar]
- NIH Cochlear implants in adults and children. NIH Consens Statement. 1995;13(2):1–30. [PubMed] [Google Scholar]
- Nikolopoulos TP, O'Donoghue GM, Archbold SM. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope. 1999;109(4):595–599. doi: 10.1097/00005537-199904000-00014. [DOI] [PubMed] [Google Scholar]
- Osberger MJ, Fisher L. Preoperative predictors of postoperative implant performance in children. Ann Otol Rhinol Laryngol. 2000;109(suppl 185):44–46. doi: 10.1177/0003489400109s1218. [DOI] [PubMed] [Google Scholar]
- Osterhout L. On the brain response to syntactic anomalies: manipulations of word position and word class reveal individual differences. Brain and Lang. 1997;59:494–522. doi: 10.1006/brln.1997.1793. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Nicol L. On the distinctiveness, independence, and time course of the brain responses to to syntactic and semantic anomalies. Lang Cognit Proc. 1999;14:283–317. [Google Scholar]
- Pisoni DB, Cleary M. Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear Hear. 2003;24(1 suppl):106S–120S. doi: 10.1097/01.AUD.0000051692.05140.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Conway CM, Kronenberger WG, Horn DL, Karpicke J, Henning SC. Efficacy and effectiveness of cochlear implants in deaf children. In: Marsharck M, Hauser PC, editors. Deaf cognition: foundations and outcomes. Oxford University Press; New York: 2008. pp. 52–101. [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MD, Kwong B, Masuda A. Auditory system plasticity in children after long periods of complete deafness. NeuroReport. 1996;8:61–65. doi: 10.1097/00001756-199612200-00013. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurotol. 2001;6:363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Moore JK, Eggermont JJ. Prolonged deafness limits auditory system developmental plasticity: evidence from an evoked potentials study in children with cochlear implants. Scandin Audiol. Suppl. 1999;51:13–22. [PubMed] [Google Scholar]
- Reed CM, Delhorne LA. Reception of environmental sounds through cochlear implants. Ear Hear. 2005;26:48–61. doi: 10.1097/00003446-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Shafiro V. Development of a large-item environmental sound test and the effects of short-term training with spectrally-degraded stimuli. Ear Hear. 2008;29:775–790. doi: 10.1097/AUD.0b013e31817e08ea. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1–2):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46:494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- Snik AF, Makhdoum MJ, Vermeulen AM, Brokx JP, van den Broek P. The relation between age at the time of cochlear implantation and long-term speech perception abilities in congenitally deaf subjects. Int J Ped Otorhinolaryngol. 1997;41(2):121–131. doi: 10.1016/s0165-5876(97)00058-x. [DOI] [PubMed] [Google Scholar]
- Teoh SW, Pisoni DB, Miyamoto RT. Cochlear implantation in adults with prelingual deafness. Part I. Clinical results. Laryngoscope. 2004a;114:1536–1540. doi: 10.1097/00005537-200409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh SW, Pisoni DB, Miyamoto RT. Cochlear implantation in adults with prelingual deafness. Part II. Underlying constraints that affect audiological outcomes. Laryngoscope. 2004b;114:1714–1719. doi: 10.1097/00005537-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry G, Wu YJ. Brain potentials reveal unconscous translation during foreign-language comprehension. Proc Natl Acad Sci USA. 2007;104(30):12530–12535. doi: 10.1073/pnas.0609927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltzman SB, Roland JT, Cohen NL. Delayed implantation in congenitally deaf children and adults. Otol Neurotol. 2002;23:333–340. doi: 10.1097/00129492-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Abutalebi J, Cappa SF, Villringer A, Perani D. Early setting of grammatical processing in the bilngual brain. Neuron. 2003;37:159–170. doi: 10.1016/s0896-6273(02)01150-9. [DOI] [PubMed] [Google Scholar]
- Wu CC, Lee YC, Chen PJ, Hsu CJ. Predominance of genetic diagnosis and imaging results as predictors in determining the speech perception performance outcome after cochlear implantation in children. Arch Fed Adol Med. 2008;162:269–276. doi: 10.1001/archpediatrics.2007.59. [DOI] [PubMed] [Google Scholar]