Abstract
Non-small cell lung cancer (NSCLC) is the major cause of lung cancer-related deaths in the United States. We are developing cell-based vaccines as a new approach for the treatment of NSCLC. NSCLC is broadly divided into three histologic subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Since these subtypes are derived from the same progenitor cells, we hypothesized that they share common tumor antigens and vaccines that induce immune reactivity against one subtype may also induce immunity against other subtypes. Our vaccine strategy has focused on activating tumor-specific CD4+ T cells, a population of lymphocytes that facilitates the optimal activation of effector and memory cytotoxic CD8+ T cells. We now report that our NSCLC MHC II vaccines prepared from adeno, squamous, or large cell carcinomas each activate CD4+ T cells that cross-react with the other NSCLC subtypes and do not react with HLA-DR-matched normal lung fibroblasts or other HLA-DR-matched non-lung tumor cells. Using MHC II NSCLC vaccines expressing the DR1, DR4, DR7, or DR15 alleles, we also demonstrate that antigens shared among the different subtypes are presented by multiple HLA-DR alleles. Therefore, MHC II NSCLC vaccines expressing a single HLA-DR allele activate NSCLC-specific CD4+ T cells that react with the three major classes of NSCLC and the antigens recognized by the activated T cells are presented by several common HLA-DR alleles, suggesting that the MHC II NSCLC vaccines are potential immunotherapeutics for a range of NSCLC patients.
Keywords: Immunotherapy, cancer vaccine, CD4+ T cells
Introduction
Lung cancer is the leading cause of cancer related deaths in the United States 1. At the time of diagnosis, approximately 40% of lung cancer patients have metastatic disease and over time another 60% will develop metastases. Non-small cell lung cancer (NSCLC) accounts for 75–80% of all lung cancers, with small cell lung cancer accounting for the remaining 20–25% 2. NSCLC is broadly divided into three subtypes based on the cellular origin of the malignant cells: adenocarcinoma (35% of all NSCLC), squamous cell carcinoma (30%) and large cell carcinoma (10–15%) 3. Since the different subtypes of NSCLC have different responses to therapy, an accurate pathological diagnosis is essential before therapy can be initiated.
Although NSCLC is treated with various therapies, including surgery, radiotherapy, chemotherapy, and multimodalities therapies, the overall survival rate of patients remains low 4. Because the immune system has the ability to systemically recognize and eliminate malignant cells 5, novel immunotherapies aimed at NSCLC are being developed 6–12. Several of these immunotherapies have proven to be efficacious in preclinical settings in experimental animals, but have been minimally effective in NSCLC patients 13–15.
Because CD4+ T cells facilitate the activation of CD8+ T cells and the generation of long term immunological memory 16–20, vaccines that activate tumor-reactive CD4+ T cells may be useful for the treatment of both metastatic NSCLC as well as adjuvant therapy for patients who have undergone resection or definitive chemoradiotherapy. Therefore, we are developing cell-based vaccines that activate NSCLC-specific CD4+ T cells. These cell-based vaccines, called major histocompatibility complex (MHC) II NSCLC vaccines, consist of NSCLC cells that constitutively express MHC class I molecules and are transfected with the CD80 costimulatory gene and MHC class II genes that are syngeneic to the recipient 21. These vaccines are unique in that they are based on the concept that the genetically modified tumor cells will present novel MHC II-restricted tumor peptides because they lack the MHC II-associated accessory molecule Invariant chain (Ii) 22. Ii is present in professional antigen presenting cells (APC) where it binds in the endoplasmic reticulum to the peptide binding groove of newly synthesized MHC II molecules. Ii then directs MHC II molecules to the endocytic pathway where it is degraded and peptides derived from endocytosed, exogenously synthesized molecules are bound 23, 24. Because the MHC II vaccines lack Ii, their MHC II molecules are free to bind peptides derived from endogenously synthesized molecules and therefore present a different set of peptides than Ii+ APC 25. This scenario has led us to hypothesize that the MHC II molecules of the vaccines present a novel array of tumor-encoded peptides and in conjunction with vaccine-expressed CD80 activate a novel repertoire of CD4+ T cells. This hypothesis was recently confirmed by studies using an MHC II breast cancer vaccine 22. Because CD4+ T cells “help” CD8+ T cells either directly or through dendritic cells, interaction with patients’ tumor cells is not required.
In vivo studies in mice with sarcoma 26, melanoma 27, and mammary carcinoma 28 demonstrated that MHC II vaccines mediate rejection of established primary tumors and can significantly extend survival time of mice with established metastatic disease in a T cell dependent fashion. Subsequent in vitro studies with human MHC II breast cancer and uveal melanoma vaccines confirmed the ability of MHC II vaccines to activate tumor-specific CD4+ T cells 22, 29. MHC II NSCLC vaccines have also been shown to activate tumor-specific CD4+ T cells from both healthy donors and from NSCLC patients, despite the presence of immune suppressive myeloid-derived suppressor cells (MDSC) in the patients’ peripheral blood mononuclear cells (PBMC) 21.
We now report that MHC II NSCLC vaccines prime and boost T cells that cross-react with the three dominant hisiologic subtypes of NSCLC, and that multiple MHC II alleles present related tumor antigens. These findings suggest that NSCLC adenocarcinoma, squamous cell carcinoma, and large cell carcinoma share common tumor antigens that are presented by multiple MHC II alleles. Therefore, it is likely that MHC II NSCLC vaccines will have a broad spectrum of activity in NSCLC, regardless of histology or clinical setting.
Materials and Methods
Cultured cells
The human NSCLC lines H358 (bronchioalveolar adenocarcinoma), HTB-177 (large cell carcinoma; hereafter called H177), the mammary carcinoma MCF10CA1 (hereafter called MCF10), the uveal melanoma OMM2.3, and HLA-DR4+ normal lung fibroblasts were obtained and maintained as described 21. The NSCLC lines H292 and H182 (squamous cell carcinomas) and H183 (large cell carcinoma) were obtained from the American Type Culture Collection and cultured in tumor medium (RPMI supplemented with 10% heat inactivated fetal calf serum, 1.5g/L sodium bicarbonate, 10mM HEPES, 1.0mM sodium pyruvate, 1% penicillin/streptomycin (Biosource, Rockville, MD), 2mM Glutamax (BRL/Life Sciences, Grand Island, NY) and 1% gentamycin). H358 and H177 cells transfected with DR1, DR4, DR7, DR1501, and CD80 (H358/DR7/CD80, H358/DR4/CD80, H358/DR1/CD80, H358/DR1501/CD80, H177/DR7/CD80) were generated and maintained as previously described 21. All protocols and cell lines for human studies were approved by the Institutional Review Boards of the participating institutions.
Antibodies and immunofluorescence
mAbs W6/32 (pan HLA-A, B, C) and L243 (pan HLA-DR) were prepared as described 21. CD80-PE and mouse FITC-IgG2a and PE-IgG2a isotype controls were from BD PharMingen (San Jose, CA). Goat-anti-mouse IgG-FITC was from ICN (Costa Mesa, CA). Immunofluorescence and flow cytometry were performed as described 21.
PBMC, HLA typing, and HLA nomenclature
Healthy donors’ and lung cancer patients’ PBMC were bled by venipunture and the resulting cells purified by Ficoll gradient and stored in liquid nitrogen as described 21. PBMCs that were >80% viable after thawing were used for the experiments. PBMC, lung cancer cell lines, and normal lung fibroblasts were HLA-typed and analyzed using MicroSSP ™ HLA Class I and II ABDR DNA typing trays and analysis software (One Lambda, Inc., Canoga Park, CA) according to the manufacturer’s instructions. HLA genotypes of tumor cell lines, fibroblasts, and PBMC from healthy donors and from lung cancer patients are shown in Table 1. HLA genotypes are referred to by their short hand form (e.g. HLA-DRB1*0701 is HLA-DR7).
Table 1.
HLA haplotypes and characteristics of the cell lines and patients’ and healthy donors’ PBMC used in these studies.
| Cells | HLA-A | HLA-B | HLA-DR | Disease Stage |
Histologic Subtype |
|---|---|---|---|---|---|
| H 292 | A2, A74 | B58, B15 | DR15, DR11 | Squamous cell carcinoma | |
| H182 | All, A* | B15, B35 | DR4, DR11 | Squamous cell carcinoma | |
| H183 | A*, A* | B51, B35 | DR1, DR4 | Large cell carcinoma | |
| H522 | A2, A* | B55, B44 | DR4, DR15 | Adenocarcinoma | |
| H838 | A68, A68 | B18, B35 | DR1, DR1 | Adenocarcinoma | |
| H358 | A*03 | B35 | DR1, DR- | Adenocarcinoma | |
| H177 | A24, A68 | B35, B51 | DR1, DR4 | Large cell carcinoma | |
| Lung fibroblasts | DR1, DR4 | ATCC CCL-202 | |||
| Healthy donor | A33, A36 | B-, B44 | DR1, DR7 | ||
| BC123104(HD1) | |||||
| Healthy donor | All, A29 | B44, B51 | DR4, DR7 | ||
| BC070605 & | |||||
| BC100504(HD2) | |||||
| Healthy donor | A23, A68 | B44, B * | DR7, DR15 | ||
| BC011405(HD3) | |||||
| Healthy donor | A3, A24 | B55, B62 | DR4,- | ||
| BC013103(HD4) | |||||
| Patient#1 | A29, A32 | B44, B60 | DR4,DR7 | IV | Adenocarcinoma |
| Patient#3 | Al | B8, B57 | DR7, DR15 | IIIB | Adenocarcinoma |
| Patient#4 | Al, A2 | B15, B60 | DR1, DR8 | IV | Squamous cell carcinoma |
| Patient#5 | A3 | B7, B- | DR15 | IB | Adenocarcinoma |
| Patient#6 | A2, A24 | B13, B51 | DR4, DR7 | IV | Adenocarcinoma & Squamous carcinoma |
| Patient#9 | A2 | B44, B62 | DR4, DR16 | IV | Adenocarcinoma |
| Patient#10 | A-, All | B7, B44 | DR7, DR15 | IV | Adenocarcinoma |
DNA constructs and Nucleofection transfection
To make the pLNCX2/DR1501 construct, the HLA-DRB1*1501 gene was cloned into TOPO 2.1 vector (Clonetech, Mountain View, CA) and transformed into TOP10 chemically competent E.coli (Invitrogen, Carlsbad, CA). The fidelity of the DRB1*1501 insert was confirmed by sequencing using M13 universal primers. The DRB1*1501 gene was excised from the TOPO vector with BamH1 and Not1 and ligated into the pLNCX2/DRA0101/IRES vector 30 which had been digested with BamH1 and Not1. Human lung cancer lines H292, H183, H177 and H182 were stably transfected with pLHCX/CD80 30 and/or pLNCX2/DR4 31, pLNCX2/DR1, and/or pLNCX2/DR7 30, and/or pLNCX2/DR1501 constructs by Nucleofector ™ technology according to the manufacturer's instructions (Amaxa Biosystems) and as described previously 21. Transfected cells were grown for 3–4 days in complete culture medium supplemented with 200µg/ml hygromycin (CD80 transfectants; Calbiochem, San Diego, CA) or 400µg/ml G418 (MHC II transfectants; Sigma, St. Louis, MO). Stable transfectants were obtained by multiple rounds of drug treatment followed by magnetic bead sorting using L243 and CD80 primary mAbs and goat-anti-mouse microbeads (Miltenyi Biotec) as described 30, and were grown in the same culture medium as their parental cells. The stable expression of CD80 and MHC II was confirmed by flow cytometry.
CD4+ T cell depletion
PBMC were depleted for CD4+ T cells using magnetic beads, LD columns, and QuadroMACS separation system according to the manufacturer’s instructions (Miltenyi Biotech) as described 21. Depleted populations contained <2% CD4+ cells as measured by flow cytometry. CD4+ T cells were positively purified using CD4 magnetic beads according to the manufacturer’s instructions (Miltenyi Biotech). Briefly, PBMC were washed twice with degassed cold MACS buffer and ~107 cells were incubated with anti-human CD4 microbeads for 15 min at 4°C. The resulting cells were resuspended and passed through an LS column. Cells released from the column were 87–90% CD4+. Prior to depletion or positive purification, 40–50% of PBMC were CD4+ T cells.
T cell activation
Thawed PBMC were in vitro activated with MHC II vaccines as described 21, 29. Briefly, 2.5×106 PBMC were primed for three days with 2.5×105 irradiated (50Gys) vaccine cells in 2ml T cell medium (Iscove’s modified Dulbecco’s medium, 5% human AB serum (Gemini Bio-Products, Woodland, CA), 1% penicillin, 1% streptomycin, 2mM glutamax, 1.0mM sodium pyruvate, 5×10−5 β-mercaptoethanol, 10mM HEPES, gentamycin) in 24-well plates. Non-adherent cells were then harvested, washed and re-plated with human recombinant IL-15 (Peprotech) (20ng/ml) in 24-well plates at 1×106/2ml T cell medium. Five days later, non-adherent cells were harvested, washed, re-plated in 24-well plates at 1×106 cells/2ml of T cell medium for 24 hrs. The primed cells were then boosted by co-culturing in flat bottom 96 well plates with live stimulator cells at a ratio of 1:2 (2.5×104 vaccine cells: 5×104 primed PBMC/200 µl/well). T cell activation was quantified by measuring IFNγ release by ELISA 29. Primed PBMC cultured in the absence of boosting vaccine cells were included as negative controls in all experiments and consistently released less IFNγ than boosting with HLA-DR negative parental cells.
MDSC
PBMC were stained with mAbs to CD33 and CD11b and analyzed by flow cytometry. CD33+CD11b+ double positive cells were considered MDSC and had suppressive activity as previously described 21.
Statistical analyses
Statistical significance for triplicate values within individual experiments was determined by ANOVA. Figures 2–5 show individual experiments. With the exception of experiments using PBMC from NSCLC patients #3, #6, and #9, which were limited by availability of material, T cell activation experiments included triplicate samples, were repeated a minimum of 2–5 times, and the pooled results were analyzed by Wilcoxon rank test. In all cases, Wilcoxon p values were equal to or less than ANOVA p values.
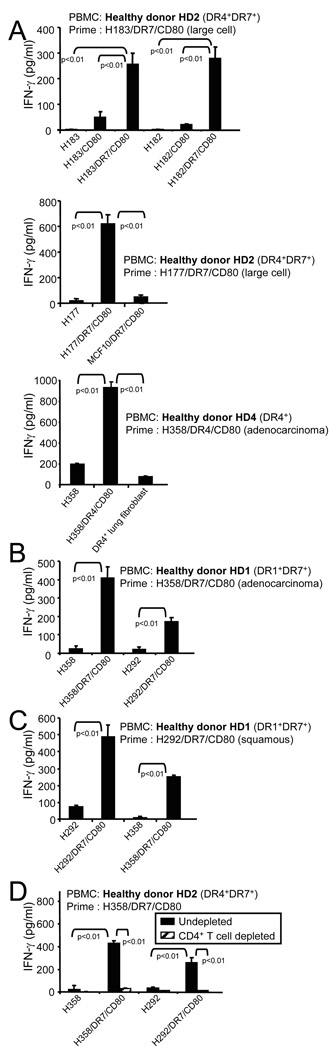
Fig. 2.
MHC II vaccines made from one histologic subtype of NSCLC activate healthy donor HLA-DR-restricted tumor-specific CD4+ T cells that cross-react with multiple subtypes of NSCLC and do not react with HLA-DR-matched normal lung tissue or non NSCLC tumor cells. HLA-DR4+ or HLA-DR4+DR7+ PBMC from healthy donors were primed with HLA-DR-matched (A) large cell (H183/DR7/CD80 or H177/DR7/CD80) or adenocarcinoma H358/DR4/CD8) vaccine, (B) adenocarcinoma H358/DR4/CD80 vaccine, or (C) squamous H292/DR7/CD80 vaccine, and boosted with the indicated MHC II NSCLC vaccines, non-transfected parental cells, DR4+ normal lung fibroblasts, or DR7+CD80+ breast cancer cells (MCF10/DR7/CD80). T cell activation was measured by IFNγ secretion. (D) Healthy donor HLA-DR4+DR7+ PBMC were primed with adenocarcinoma H358/DR7/CD80 vaccine and boosted as in panels A-C, except the PBMC were either not depleted or depleted for CD4+ T cells before priming. Depleted populations contained <2% CD4+ T cells. Individual representative experiments are shown with p values determined by ANOVA. Pooled data from multiple experiments were analyzed by Wilcoxon rank test which gave p values equal to or less than ANOVA analysis of individual experiments
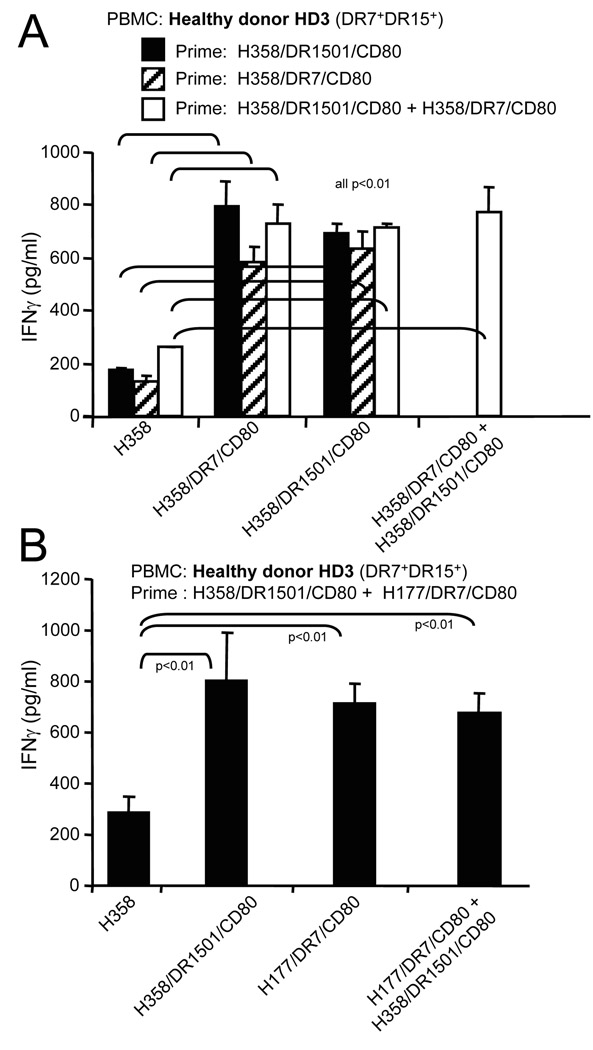
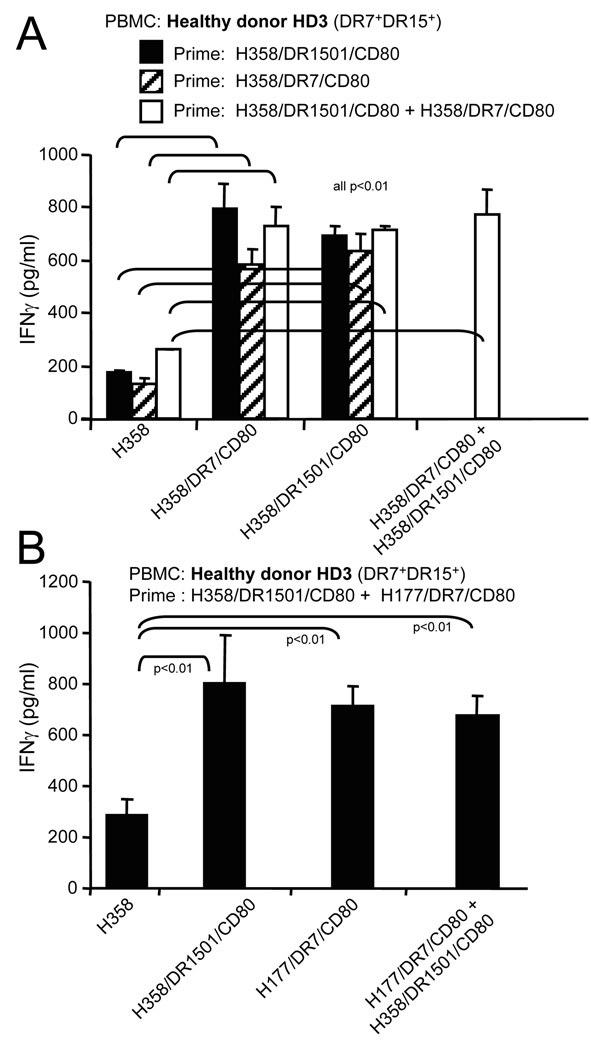
Fig. 5.
Priming and boosting with a single MHC II lung cancer vaccine gives maximum T cell activation. Healthy donor HLA-DR7+DR1501+ PBMC were primed and/or boosted with one or a combination of two NSCLC MHC II vaccines. T cell activation was measured by analyzing IFNγ secretion. Data for both panels are from one of two independent experiments
Results
Lung cancer cells transfected with HLA-DR7 or HLA-DR1501 and/or CD80 stably express HLA-DR and CD80
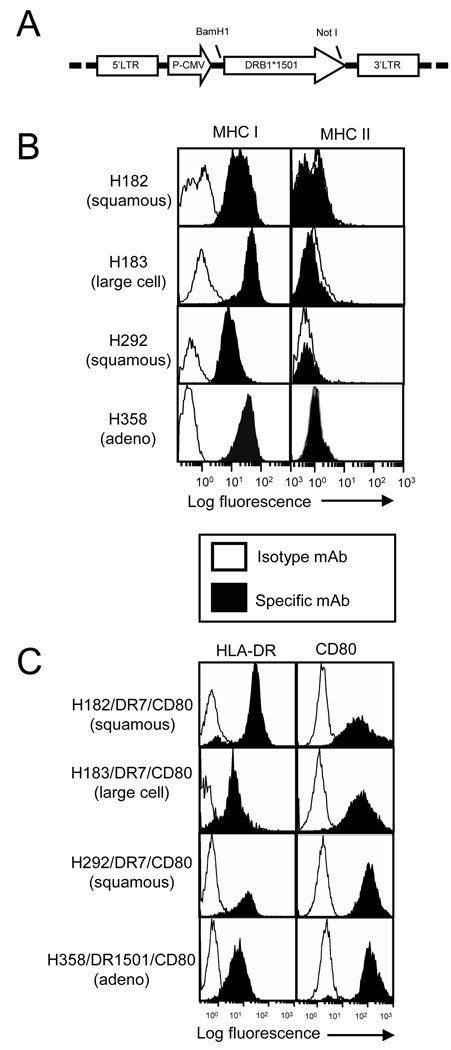
To generate the DR1501 retroviral vector, the DRB1501 gene was PCR amplified and excised from the pLHCX/DR1501 plasmid using BamH1 and Not1 restriction enzymes and ligated into BamHI and Not1 digested pLNCX2/DRα/IRES (Fig 1A). Because the expression of MHC II and Ii is coordinately regulated 32 and Ii expression blocks vaccine efficacy 22, we tested established adenocarcinoma, squamous cell carcinoma, and large cell carcinoma cell lines for constitutive expression of MHC I and MHC II (Fig 1B). Those cells that express MHC I and do not constitutively express MHC II were transfected with plasmids encoding HLA-DR1, HLA-DR7, HLA-DR4 31, or HLA-DR1501, and/or the costimulatory molecule CD80. The transfected cells stably expressed MHC II and CD80 as assessed by flow cytometry (Fig 1C and 21).
Fig. 1.
Lung cancer cells transfected with HLA-DR7, HLA-DR1501 and/or CD80 stably express HLA-DR and CD80. A. Retroviral construct for pLNCX2/DR1501 B. Lung cancer cell lines (H182, H183, H292, and H358) were stained with MHC I (W6/32), MHC II (mAb L243), or isotype control mAbs, and analyzed by flow cytometry C. NSCLC cells were transfected with pLNCX2/DR7, pLNCX2/DR1501, and/or pLHCX2/CD80 plasmids using Nucleofection technology (Amaxa), stained for MHC class II (HLA-DR, mAb L243) and CD80, and analyzed by flow cytometry. Profiles are for cell transfectants that have been in culture for > 6–9 months. Data in panels B and C are representative of 2–3 independent experiments.
MHC II vaccines made from one subtype of NSCLC activate NSCLC-specific CD4+ cells from healthy donors’ PBMC that cross-react with other subtypes of NSCLC
Adenocarcinoma, squamous cell carcinoma, and large cell carcinoma NSCLCs are derived from a common lung progenitor cell and therefore may share common tumor antigens. To determine if vaccine cells generated from one NSCLC subtype activate T cells that cross-react with other NSCLC subtypes, we used MHC II lung cancer vaccines made from, large cell carcinomas (H177, H183), adenocarcinoma (H358), and squamous cell carcinoma (H292, H182). Healthy donor HLA-DR4+DR7+ PBMC were primed with the large cell vaccine H183/DR7/CD80 or H177/DR7/CD80. Following priming, non-adherent cells were expanded in IL-15 and then boosted with parental untransfected cells (H177 or H183), the priming cells H183/DR7/CD80 or H177/DR7/CD80), DR negative CD80+ lung cancer cells (183/CD80 or 182/CD80), a squamous carcinoma vaccine (H182/DR7/CD80 or H292/DR7/CD80), a breast cancer vaccine (MCF10/DR7/CD80), or HLA-DR4+ normal lung fibroblasts. T cell activation was measured by IFN-γ production, a hallmark cytokine of activated type 1 CD4+ T cells. As previously shown 21, CD80 expression was required on the boosting cells since NSCLC cells without CD80 did not stimulate high levels of IFNγ release (fig. 2A, top panel). Activated T cells were MHC II-restricted and lung cancer specific since there was no reactivity in the absence of HLA-DR (fig. 2A all panels) or against HLA-DR7+CD80+ breast cancer cells (fig. 2A, middle panel). There was also no reactivity against non-malignant lung fibroblasts even when the HLA-DR4+ PBMC were primed with an HLA-DR4+ vaccine and tested on HLA-DR4+ normal lung fibroblasts (fig. 2A,bottom panel).
Healthy donor HLA-DR4+DR7+ PBMC primed with the large cell vaccine H183/DR7/CD80 reacted not only with the priming cells, but also with H182/DR7/CD80 squamous cell carcinoma cell (fig. 2A,top panel). Similarly, healthy donor HLA-DR1+DR7+ PBMC primed with an HLA-DR7 adenocarcinoma vaccine (H358/DR7/CD80) (Fig 2B) or a squamous cell carcinoma vaccine (H292/DR7/CD80) (Fig 2C) showed similar cross-reactivity against squamous and adenocarcinoma cells, respectively. Healthy donor HLA-DR4+DR7+ PBMC primed with another squamous carcinoma vaccine (H182/DR7/CD80) showed similar cross-reactivity (data not shown). Cross-reactivity was also observed when healthy donor PBMC were primed with the H358/DR7/CD80 adenocarcinoma vaccine, the H292/DR7/CD80 squamous cell vaccine, or the H177/DR7/CD80 large cell vaccine , and boosted with the H177/DR7/CD80 large cell, H358/DR7/CD80 adenocarcinoma, or H292/DR7/CD80 squamous cell vaccine (data not shown), demonstrating that each histologic subtype vaccine induces reactivity against all three subtypes.
To ascertain that the MHC II vaccines activate CD4+ T cells, healthy donor DR4+DR7+ PBMC were depleted for CD4+ T cells prior to priming and boosting with adenocarcinoma H358/DR7/CD80 vaccine cells (Fig 2D). Depletion of CD4+ T cells eliminated IFN-γ release. Therefore, MHC II lung cancer vaccines prepared from a single subtype of NSCLC activate CD4+ T cells that react with all subtypes of NSCLC and do not react with normal lung fibroblasts or with non-NSCLC tumor cells.
Cross-reactivity of activated T cells is not impaired by myeloid-derived suppressor cells
Cancer patients accumulate high levels of immune suppressive cells such as MDSC which are potent inhibitors of both innate and adaptive immunity 33, 34. To determine if MDSC interfere with vaccine-induced cross-reactivity, we tested PBMC from NSCLC patients with varying levels of MDSC in their blood. Blood leukocytes were characterized as MDSC if they were CD33+CD11b+ and suppressed anti-CD3 activation of T cells 21. HLA-DR4+DR7+ PBMC from NSCLC patient #1 with an adenocarcinoma (14% of PBMC were MDSC) were primed with a large cell carcinoma (H177/DR7/CD80) vaccine and boosted with the priming vaccine, or with an adenocarcinoma (H358/DR7/CD80) vaccine. H177/DR7/CD80-primed T cells reacted equally well with the priming and adenocarcinoma vaccines (Fig 3A). HLA-DR7+DR15+ PBMC from NSCLC patient #3 (25% of PBMC were MDSC) were primed with an adenocarcinoma vaccine (H358/DR7/CD80) and boosted with the adenocarcinoma or with a large cell carcinoma (H177/DR7/CD80) vaccine (Fig 3B). T cells primed with the H358/DR7/CD80 vaccine showed similar reactivity with large cell carcinoma vaccine cells as with the priming vaccine cells. HLA-DR4+DR7+ PBMC from NSCLC patient #6 (adenocarcinoma and squamous cell carcinoma; 53% of PBMC were MDSC) primed with an adenocarcinoma vaccine (H358/DR7/CD80) were also equally reactive with the priming cells as with large cell carcinoma vaccine cells (H177/DR7/CD80) (Fig 3C). PBMC from adenocarcinoma patients #9 and #10 (HLA-DR4+DR7+ and HLA-DR7+DR15+, respectively) were also activated by vaccines despite the presence of 32% and 28% MDSC, respectively (figs. 3D). PBMC from HLA-DR4+ patient #9 primed with the HLA-DR4+ vaccine had no activity against HLA-DR4+ normal lung fibroblasts, further confirming that the NSCLC vaccines activate tumor-reactive T cells that do not react with normal lung tissue. Therefore, the presence of varying levels of MDSC in lung cancer patients’ PBMC does not impair the cross-reactivity induced by MHC II NSCLC vaccines.
Fig. 3.
Myeloid-derived suppressor cells do not block the activation of CD4+ T cells. PBMC from (A) HLA-DR4+DR7+ patient #1, (B) HLA-DR7+DR15+ patient #3, (C) HLA-DR4+DR7+ patient #6, or (D) HLA-DR4+DR16+ patient #9 and HLA-DR7+DR15+ patient #10 were primed and boosted with the indicated cells. T cell activation was analyzed by measuring IFNγ secretion. Patients #1, #3, #6, #9, and #10 had 14%, 25%, 53%, 32.4%, and 28% CD33+CD11b+ MDSC, respectively. Statistical analysis was performed as for figure 2; however, the limited amount of patient material precluded sufficient repeats for Wilcoxon rank testing for patients #3, #6, and #9. Therefore, B, C, and the top panel of D are one experiment done in triplicate, and A and the bottom panel of D are one of two independent experiments
MHC II NSCLC vaccines activate CD4+ T cells restricted to both HLA-DR alleles of the responding donor
Optimal CD4+ T cell activation would involve the generation of tumor-reactive T cells that are MHC restricted to both HLA-DR alleles of the host. To determine if the MHC II NSCLC vaccines do so, healthy donor HLA-DR7+15+ PBMC were primed with H358/DR1501/CD80 and boosted with H358/DR7/CD80 or H358/DR1501/CD80 vaccine (fig. 4A, top panel). Significant reactivity was boosted by both vaccines indicating that the vaccines present multiple tumor antigen epitopes that can be presented by at least two HLA-DR alleles. Similar results were obtained when healthy donor HLA-DR1+DR7+ PBMC were primed with H358/DR7/CD80 and boosted with H358/DR7/CD80 or H358/DR1/CD80 (fig. 4A, bottom panel).
Fig. 4. MHC II NSCLC vaccines activate CD4+ T cells restricted to both HLA-DR alleles of the PBMC donor.
(A) Healthy donor HLA-DR7+DR15+ PBMC (top panel) or HLA-DR1+DR7+ (bottom panel) PBMC were primed with H358/DR1501/CD80 (top panel) or H358/DR7/CD80 vaccine cells (bottom panel) and boosted with the indicated cells. Data for each panel are representative of 2–3 independent experiments. (B) Healthy donor HLA-DR4+DR7+ PBMC were untreated or depleted for CD4+ T cells (top panel; <2% of depleted cells were CD4+) or CD4+ T cells were positively purified from healthy donor HLA-DR4+DR7+ PBMC (bottom panel;>87% of cells were CD4+) prior to priming with H358/DR7/CD80 vaccine cells and boosting with the indicated cells. Statistical analysis by ANOVA and Wilcoxon rank test are as per figure 2.
The reactivity to tumor peptides presented by HLA-DR alleles that are not expressed by the priming vaccine is unexpected and could be mediated by NK cells rather than by CD4+ T cells. To confirm that CD4+ T cells are the effecter cells in these responses, healthy donor HLA-DR4+DR7+ PBMC were either untreated or depleted for CD4+ T cells prior to priming with H358/DR7/CD80 vaccine cells, and subsequently boosted with H358/DR7/CD80 or H358/DR4/CD80 (fig. 4B, upper panel). Depletion of CD4+ T cells eliminated IFNγ production demonstrating that the activated effector cells were exclusively CD4+ T cells. To further confirm that the activated IFNγ-secreting cells were CD4+ T cells, CD4+ T cells were isolated from HD2 PBMC (HLA-DR4+DR7+) using negative selection prior to priming with H358/DR7/CD80 vaccine cells, and then boosted with H358/DR7/CD80 or H358/DR4/CD80 vaccine (fig. 4B, lower panel). Purified CD4+ T cells (fig. 4B, lower panel) and PBMC (fig. 4B, upper panel) produced similar levels of IFNγ in response to the boosts, further demonstrating that CD4+ T cells are the cells activated by the vaccines. Therefore, MHC II vaccines activate tumor-specific CD4+ T cells that cross-react with multiple HLA-DR alleles. This cross-reactivity is likely due to PBMC uptake and presentation of tumor cell peptides from vaccine cells since the original priming vaccine expresses only one HLA-DR allele.
Priming and boosting of T cells with a single MHC II vaccine is sufficient to obtain maximum T cell activation
Figures 2–4 indicate that the NSCLC vaccines share antigens that are presented by multiple HLA-DR alleles. If each vaccine also contains unique antigens, then T cell activation may be increased by priming with a mixture of MHC II NSCLC vaccines. To test this possibility, healthy donor DR7+DR15+ PBMC were primed with adenocarcinoma H358/DR1501/CD80, H358/DR7/CD80, or H358/DR1501/CD80 plus H358/DR7/CD80 vaccine cells, and boosted with H358/DR1501/CD80, H358/DR7/CD80, or H358/DR1501/CD80 plus H358/DR7/CD80. Priming with the mixture of two vaccines did not significantly increase T cell activation compared to priming with a single vaccine (Fig 5A). Similarly, priming and boosting with a mixture of two vaccines prepared from different NSCLC subtypes (adenocarcinoma plus large cell carcinoma) did not significantly increase T cell activation as compared to activation with a single vaccine (Fig 5B). Therefore, combining vaccines does not improve T cell activation, indicating that a single MHC II NSCLC vaccine is sufficient for maximal T cell activation.
Discussion
NSCLC accounts for approximately 75–80% of all lung cancer cases and represents a heterogeneous group of cancers consisting mainly of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma 2. In addition, many tumors are poorly differentiated and not readily classificable. Immunotherapy with vaccines is a promising strategy for the treatment of NSCLC; however, it has been unclear if individuals will require vaccines customized for their histologic subtype of NSCLC and their specific HLA-DR genotype. In this report we show that MHC II vaccines derived from a single type of NSCLC activate tumor-reactive CD4+ T cells that cross-react with the major subtypes of NSCLC, demonstrating that adenocarcinoma, squamous cell carcinoma, and large cell carcinoma share tumor antigens. Furthermore, MHC II NSCLC vaccines expressing a single HLA-DR allele activate CD4+ T cells MHC-restricted to additional alleles, demonstrating that the vaccines facilitate cross-priming and that NSCLC contain shared tumor antigens presented by multiple HLA-DR alleles. Presentation of the same tumor antigen (peptide) by different HLA-DR alleles is not uncommon, since similar results were shown for HER2/neu peptides 35. Thus, potentially, a patient with NSCLC could be treated with an HLA-DR-matched vaccine irrespective of histological subtype, and the resulting activated CD4+ T cells would be tumor specific, non-reactive with normal lung tissue, and MHC-restricted to both of the patient’s HLA-DR alleles.
MHC II vaccines were designed to activate tumor-reactive CD4+ T cells that facilitate the optimal development of tumoricidal CD8+ T cells and promote long-term immune memory 36, which should be advantageous if patients are at risk of progressive disease due to the outgrowth of latent tumor cells. CD4+ T cells mediate their effects by interacting with CD8+ T cells and/or dendritic cells 37–39, and once activated do not need to interact with tumor cells. Therefore, NSCLC MHC II vaccines have the potential to facilitate CD8+ T cell-mediated anti-tumor immunity regardless of whether patients’ tumor cells express MHC II molecules.
The MHC II vaccine strategy is based on the hypothesis that in the absence of the MHC class II-associated accessory molecule invariant chain (Ii), vaccine-encoded MHC II molecules present a repertoire of novel endogenously synthesized tumor peptides that is not presented by Ii+ professional antigen presenting cells (APC). In Ii+ APC the peptide binding cleft of newly synthesized MHC II molecules is occupied by Ii. In contrast, in Ii− MHC II vaccine cells, the peptide binding cleft is accessible, so that endogenous peptides have the potential to bind. The concept that the absence of Ii favors the presentation of an alternate repertoire of peptides is supported by studies demonstrating that Ii+ and Ii− APC activate different T cell repertoires 22, 25. The absence of Ii enables MHC II molecules to bind peptides that are not bound by traditional APC, so that the enhanced immunogenicity of the vaccines is likely due to the presentation of novel endogenous peptides which have not previously been encountered by the host. Since activated CD4+ T cells facilitate anti-tumor immunity by interacting with CD8+ T cells and/or dendritic cells, Ii expression by the recipients’ tumor cells is irrelevant.
Cell-based cancer vaccines are attractive immunotherapy agents because they have the potential to present a broad repertoire of tumor peptides 36. Nemunaitis et al reported a phaseI/II trial in NSCLC patients using GM-CSF-transduced autologous tumor cells. Three out of 33 patients treated with the vaccine had complete responses, and median survival time was significantly extended for patients whose vaccines secreted greater quantities of GM-CSF 9, 40. Two of these three patients (and the ones with durable benefit) had bronchioloalveolar carcinoma, a subtype of adenocarcinoma, indicating that the benefit of that strategy is histologically restricted. Although these results are encouraging, allogeneic vaccines have also been developed, because of the expense, time, and sophistication needed to prepare autologous vaccines. Podack and colleagues conducted a clinical trial of 19 NSCLC patients with advanced disease using an allogeneic cell-based vaccine consisting of NSCLC cells transduced with CD80 and MHC I genes 12, 41. One patient experienced a partial response, five patients had stable disease, the median survival of all patients was more than 18 months, and all except one patient had measurable CD8+ T cell responses. MHC II vaccines similarly express CD80 and activate CD8+ T cells 42; however, they have the additional benefit of activating CD4+ T cells and therefore may be more effective therapeutic agents as well as facilitate immune memory.
A major short-coming of cell-based vaccines is the perceived requirement for customizing the vaccine to individual patients. As demonstrated here, vaccines and patients should be HLA-DR-matched for one HLA-DR allele; however, MHC II NSCLC vaccines activate CD4+ T cells that cross-react with multiple histologic subtypes of NSCLC, so neither autologous nor subtype-matched vaccines are needed. In addition, previous studies have established that MHC II vaccines activate non-MHC I-restricted cytotoxic tumor-specific CD8+ T cells 42, so MHC I matching of patient and vaccine is not necessary Therefore, MHC II NSCLC vaccines require minimal patient customization and may be useful therapeutic agents for a variety of NSCLC patients.
Acknowledgments
We thank Ms. Virginia Clements for her excellent technical assistance, Dr. Dean Mann for providing the PBMC, Adam Mueller for generating the HLA-DR1501 retroviral construct, and Ejiofor Ezekwe for transfecting H358/CD80 cells with the DR1501 construct.
Grant Support: These studies were supported by grants from the National Institutes of Health (R01CA84232 and R01CA115880).
Abbreviations
- APC
antigen presenting cell
- HD
healthy donor
- MHC II
major histocompatibility complex class II
- MDSC
myeloid-derived suppressor cells
- NSCLC
non-small cell lung cancer
- PBMC
peripheral blood mononuclear cells
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Wright SR, Boag AH, Valdimarsson G, Hipfner DR, Campling BG, Cole SP, Deeley RG. Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res. 1998;4:2279–2289. [PubMed] [Google Scholar]
- 3.Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Moller M, Eriksen JA, Gaudernack G. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 6.Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA, Matsuo M, Selvakumar A, Dupont B, Chen YT, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172:3289–3296. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 7.Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007 doi: 10.1016/j.lungcan.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulieres D, Cormier Y, Ellis P, Price A, Sawhney R, Davis M, Mansi J, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 9.Nemunaitis J. GVAX (GMCSF gene modified tumor vaccine) in advanced stage non small cell lung cancer. J Control Release. 2003;91:225–231. doi: 10.1016/s0168-3659(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 10.Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, Jablons D, Aimi J, Lin A, Hege K. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 11.Ramos TC, Vinageras EN, Ferrer MC, Verdecia BG, Rupale IL, Perez LM, Marinello GG, Rodriguez RP, Davila AL. Treatment of NSCLC patients with an EGF-based cancer vaccine: report of a Phase I trial. Cancer Biol Ther. 2006;5:145–149. doi: 10.4161/cbt.5.2.2334. [DOI] [PubMed] [Google Scholar]
- 12.Raez LE, Cassileth PA, Schlesselman JJ, Sridhar K, Padmanabhan S, Fisher EZ, Baldie PA, Podack ER. Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:2800–2807. doi: 10.1200/JCO.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 13.O'Mahony D, Kummar S, Gutierrez ME. Non-small-cell lung cancer vaccine therapy: a concise review. J Clin Oncol. 2005;23:9022–9028. doi: 10.1200/JCO.2005.02.3101. [DOI] [PubMed] [Google Scholar]
- 14.Nemunaitis J. A review of vaccine clinical trials for non-small cell lung cancer. Expert Opin Biol Ther. 2007;7:89–102. doi: 10.1517/14712598.7.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Raez LE, Rosenblatt JD, Podack ER. Present and future of lung cancer vaccines. Expert Opin Emerg Drugs. 2006;11:445–459. doi: 10.1517/14728214.11.3.445. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Hao S, Li F, Ye Z, Yang J, Xiang J. CD4+ Th1 cells promote CD8+ Tc1 cell survival, memory response, tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007;120:148–159. doi: 10.1111/j.1365-2567.2006.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 19.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 20.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients' CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol Immunother. 2008;57:389–398. doi: 10.1007/s00262-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busch R, Cloutier I, Sekaly RP, Hammerling GJ. Invariant chain protects class II histocompatibility antigens from binding intact polypeptides in the endoplasmic reticulum. Embo J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 24.Pieters J. MHC class II-restricted antigen processing and presentation. Adv Immunol. 2000;75:159–208. doi: 10.1016/s0065-2776(00)75004-8. [DOI] [PubMed] [Google Scholar]
- 25.Muntasell A, Carrascal M, Alvarez I, Serradell L, van Veelen P, Verreck FA, Koning F, Abian J, Jaraquemada D. Dissection of the HLA-DR4 peptide repertoire in endocrine epithelial cells: strong influence of invariant chain and HLA-DM expression on the nature of ligands. J Immunol. 2004;173:1085–1093. doi: 10.4049/jimmunol.173.2.1085. [DOI] [PubMed] [Google Scholar]
- 26.Baskar S, Glimcher L, Nabavi N, Jones RT, Ostrand-Rosenberg S. Major histocompatibility complex class II+B7-1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor-bearing mice. J Exp Med. 1995;181:619–629. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulaski BA, Clements VK, Pipeling MR, Ostrand-Rosenberg S. Immunotherapy with vaccines combining MHC class II/CD80+ tumor cells with interleukin-12 reduces established metastatic disease and stimulates immune effectors and monokine induced by interferon gamma. Cancer Immunol Immunother. 2000;49:34–45. doi: 10.1007/s002620050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 29.Bosch JJ, Thompson JA, Srivastava MK, Iheagwara UK, Murray TG, Lotem M, Ksander BR, Ostrand-Rosenberg S. MHC class II-transduced tumor cells originating in the immune-privileged eye prime and boost CD4(+) T lymphocytes that cross-react with primary and metastatic uveal melanoma cells. Cancer Res. 2007;67:4499–4506. doi: 10.1158/0008-5472.CAN-06-3770. [DOI] [PubMed] [Google Scholar]
- 30.Dissanayake SK, Thompson JA, Bosch JJ, Clements VK, Chen PW, Ksander BR, Ostrand-Rosenberg S. Activation of tumor-specific CD4(+) T lymphocytes by major histocompatibility complex class II tumor cell vaccines: a novel cell-based immunotherapy. Cancer Res. 2004;64:1867–1874. doi: 10.1158/0008-5472.can-03-2634. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JA, Dissanayake SK, Ksander BR, Knutson KL, Disis ML, Ostrand-Rosenberg S. Tumor cells transduced with the MHC class II Transactivator and CD80 activate tumor-specific CD4+ T cells whether or not they are silenced for invariant chain. Cancer Res. 2006;66:1147–1154. doi: 10.1158/0008-5472.CAN-05-2289. [DOI] [PubMed] [Google Scholar]
- 32.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 33.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation, cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009 doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, Schiffman K, Disis ML. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9:5559–5565. [PubMed] [Google Scholar]
- 36.Ostrand-Rosenberg S, Pulaski BA, Clements VK, Qi L, Pipeling MR, Hanyok LA. Cell-based vaccines for the stimulation of immunity to metastatic cancers. Immunol Rev. 1999;170:101–114. doi: 10.1111/j.1600-065x.1999.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 37.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 40.Nemunaitis J, Sterman D, Jablons D, Smith JW, 2nd, Fox B, Maples P, Hamilton S, Borellini F, Lin A, Morali S, Hege K. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 41.Raez LE, Cassileth PA, Schlesselman JJ, Padmanabhan S, Fisher EZ, Baldie PA, Sridhar K, Podack ER. Induction of CD8 T-cell-Ifn-gamma response and positive clinical outcome after immunization with gene-modified allogeneic tumor cells in advanced non-small-cell lung carcinoma. Cancer Gene Ther. 2003;10:850–858. doi: 10.1038/sj.cgt.7700641. [DOI] [PubMed] [Google Scholar]
- 42.Bosch JJ, Iheagwara UK, Reid S, Srivastava MK, Wolf J, Lotem M, Ksander BR, Ostrand-Rosenberg S. Uveal melanoma cell-based vaccines express MHC II molecules that traffic via the endocytic and secretory pathways and activate CD8(+) cytotoxic, tumor-specific T cells. Cancer Immunol Immunother. 59:103–112. doi: 10.1007/s00262-009-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]