This study shows that the TDIF signal from the phloem plays a crucial role in the maintenance of vascular stem cells via two independent pathways: WOX4-independent inhibition of xylem commitment of vascular stem cells and WOX4-mediated enhancement of their proliferation.
Abstract
The indeterminate nature of plant growth and development depends on the stem cell system found in meristems. The Arabidopsis thaliana vascular meristem includes procambium and cambium. In these tissues, cell–cell signaling, mediated by a ligand-receptor pair made of the TDIF (for tracheary element differentiation inhibitory factor) peptide and the TDR/PXY (for TDIF RECEPTOR/ PHLOEM INTERCALATED WITH XYLEM) membrane protein kinase, promotes proliferation of procambial cells and suppresses their xylem differentiation. Here, we report that a WUSCHEL-related HOMEOBOX gene, WOX4, is a key target of the TDIF signaling pathway. WOX4 is expressed preferentially in the procambium and cambium, and its expression level was upregulated upon application of TDIF in a TDR-dependent manner. Genetic analyses showed that WOX4 is required for promoting the proliferation of procambial/cambial stem cells but not for repressing their commitment to xylem differentiation in response to the TDIF signal. Thus, at least two intracellular signaling pathways that diverge after TDIF recognition by TDR might regulate independently the behavior of vascular stem cells. Detailed observations in loss-of-function mutants revealed that TDIF-TDR-WOX4 signaling plays a crucial role in the maintenance of the vascular meristem organization during secondary growth.
INTRODUCTION
Meristems are plants’ stem cell tissues essential for lifelong growth and development (Stahl and Simon, 2005; Scheres 2007; Dinneny and Benfey, 2008). Vascular cells, like any other cell lineages, originate from the apical meristems in shoot and root tips (shoot and root apical meristems, SAMs and RAMs, respectively) during postembryonic development. Precise regulation of cell fates in the apical meristem establishes a well-organized radial structure for the vascular system, containing two conductive tissues: phloem and xylem. Between these tissues, procambial cells retain their meristematic activity even when they are outside of the apical meristem. These intervening procambial cells give rise to a lateral meristem, the vascular meristem/vascular cambium, which facilitates the radial growth of the vascular system (secondary growth). The intervening procambial/cambial cells continue to divide periclinally to increase their own cell lineages by producing xylem cells on one side and phloem cells on the other side, thus sustaining the indeterminate property of secondary growth (Evert, 2006).
Cell-to-cell communication is a fundamental mechanism for maintaining homeostasis of the meristem (Reddy 2008). Various signaling molecules, such as plant hormones and secretory peptides, have been implicated in the regulation of this process (De Smet et al., 2009; Wolters and Jürgens, 2009). Similarly, cellular proliferation and differentiation in the vascular meristem are regulated by hormonal signals (Uggla et al., 1996; Fukuda, 2004; Björklund et al., 2007; Matsumoto-Kitano et al., 2008; Nieminen et al., 2008; Nilsson et al., 2008; Elo et al., 2009; Love et al., 2009). Recent studies have uncovered the importance of an intercellular signaling system consisting of a small peptide TDIF and its receptor PXY/TDR in determining the fates of procambial cells, although it remains elusive whether the TDIF signal regulates directly the identity of stem cells (Ito et al., 2006; Fisher and Turner, 2007; Hirakawa et al., 2008; Whitford et al., 2008). TDIF is secreted from the phloem and its neighboring cells, and this signal is mediated by the TDR receptor kinase located on the plasma membrane of procambial cells. TDR-mediated TDIF signaling suppresses the differentiation of procambial cells into xylem cells and promotes their proliferation (Hirakawa et al., 2008). However, it is largely unknown whether these two functions of TDIF are a consequence of an intracellular signaling pathway or are regulated by two or more independent pathways.
The ligand-receptor pair of TDIF and TDR shares high similarity with that of the CLAVATA3 (CLV3) peptide and the CLV1 receptor kinase, which regulates stem cell homeostasis in the SAM (Clark et al., 1997; Fletcher et al., 1999; Ogawa et al., 2008). Mature TDIF peptide was isolated from Zinnia elegans and Arabidopsis thaliana as a 12–amino acid peptide containing two Hyp residues (Ito et al., 2006; Ohyama et al., 2008). TDIF is encoded by the conserved C-terminal domain of two members of the CLE (for CLAVATA3/EMBRYO SURROUNDING REGION-related) gene family, CLE41 and CLE44, in Arabidopsis (Cock and McCormick, 2001; Ito et al., 2006; Jun et al., 2008). TDR/PXY belongs to the Leu-rich repeat receptor-like kinase subclass XI that includes CLV1 (Shiu and Bleecker, 2001; Fisher and Turner, 2007; Hirakawa et al., 2008). The similarity of signaling components between the TDIF and CLV pathways suggests that CLV-like signaling also operates downstream of the TDIF-TDR pathway. WUSCHEL (WUS), a homeodomain transcription factor essential for stem cell maintenance in the SAM, is well established as a key target of CLV signaling. The CLV3 signal represses the expression of WUS, thereby restricting the stem cell population (Mayer et al., 1998; Brand et al., 2000; Schoof et al., 2000). Collectively, WUSCHEL-related HOMEOBOX (WOX) genes are the best candidates for the transcriptional target of the TDIF signal.
The Arabidopsis genome has 15 WOX family members, which are classified into three subgroups (Haecker et al., 2004). They influence diverse aspects of development, including establishment of the embryonic axis (Wu et al., 2007; Breuninger et al., 2008), stem cell maintenance and proliferation in the apical meristems (Mayer et al., 1998; Wu et al., 2005; Sarkar et al., 2007), leaf and flower development (Matsumoto and Okada, 2001; Vandenbussche et al., 2009), and ovule development (Park et al., 2005). At the cellular level, most of these genes seem to regulate proliferation, while each also affects cell identity in a specific developmental context. In other plant species, a number of different WOX genes are involved in specific developmental processes (Nardmann et al., 2004; Rebocho et al., 2008; Vandenbussche et al., 2009; Zhao et al., 2009). By contrast, the function of WOX genes in vascular development is poorly understood. Some WOX genes were shown to be expressed in the vasculature in Arabidopsis, poplar (Populus tremula), and tomato (Solanum lycopersicum), but their functions remain to be elucidated (Haecker et al., 2004; Schrader et al., 2004; Deveaux et al., 2008; Ji et al., 2010).
Here, we show that the expression of Arabidopsis WOX4 is upregulated very rapidly in response to TDIF application in a TDR-dependent manner. We provide genetic evidence that WOX4 mediates the TDIF signal’s promotion of proliferation of procambial/cambial cells but not its inhibition of their spontaneous differentiation into xylem. Further observations in the mature vascular tissues of these mutants show that TDR inhibits the xylem commitment of vascular stem cells and WOX4 promotes stem cell maintenance in plants with a tdr mutant background. Therefore, proliferation and commitment of vascular stem cells are separately regulated by at least two pathways that appear to diverge early in TDIF-TDR signaling.
RESULTS
TDIF Promotes WOX4 Expression
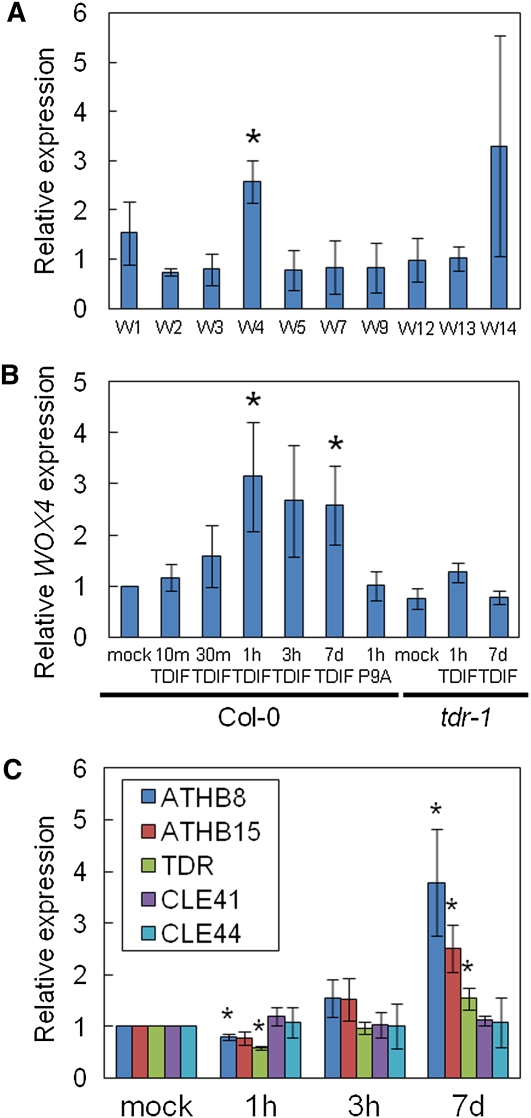
To identify the target gene(s) of TDIF signaling, we analyzed the expression profiles of all WOX genes by RT-PCR in Arabidopsis. Because the global expression profiles for most WOX genes were not found in the public databases, we first searched for genes expressed in seedlings containing vascular tissues. Total RNA was isolated from the aerial part of 7-d-old seedlings grown in liquid medium. Considerable amounts of cDNAs were detected for 10 genes (WOX1-5, 7, 9, and 12-14) in high-number PCR cycles (see Supplemental Figure 1 online). Accordingly, we further examined the effects of TDIF on the expression of these genes. The P9A peptide was used as a nonfunctional dodecapeptide in which a Pro residue at the ninth position of TDIF is substituted by an Ala residue (Ito et al., 2006). Arabidopsis seedlings were grown with 5 μM TDIF or P9A for 7 d, and the transcript levels of the seven WOX genes were measured using quantitative RT-PCR (qRT-PCR). TDIF treatment increased the transcript level of WOX4 (Figure 1A), which belongs to the WUS subclade among the WOX family (Breuninger et al., 2008). We detected no significant and reproducible change in the expression of any other tested WOX gene (Figure 1A). Although the level of WOX14 mRNA was high in the presence of TDIF, the expression of WOX14 may be much less or more limited to specific tissues than that of WOX4, judging from the RT-PCR experiment (see Supplemental Figure 1 online). These results suggest that WOX4 is the main WOX gene affected by the TDIF signal.
Figure 1.
Identification and Expression Analyses of TDIF Signaling Target Genes.
(A) Effects of TDIF treatment on relative expression levels of WOX genes in the top part of 7-d-old seedlings. For each WOX gene, the rate of expression level of TDIF treatment per P9A treatment as measured by qRT-PCR is shown. The asterisk indicates significant difference between TDIF and P9A treatment. Peptides were treated for 7 d.
(B) Relative WOX4 expression level measured by qRT-PCR in Col-0 and tdr-1 plants that were either mock treated or treated with TDIF or P9A for the indicated amounts of time. Enhancement of WOX4 expression compared with mock treatment was observed only in the samples treated with TDIF for 1 h or more. The values of expression level are normalized by both the mock treatment in Col-0 and TUA4.
(C) Relative expression levels of procambium marker genes (ATHB8, ATHB15, and TDR) and the TDIF genes (CLE41 and CLE44). Expression levels of the procambium marker genes but not the TDIF genes were significantly upregulated compared with mock treatment only when plants were treated with TDIF for 7 d. The values of expression level are normalized by both the mock treatment and TUA4.
Error bars indicate sd, n = 3; *Student’s t test significance at P < 0.05 for different means.
Expression Pattern and Subcellular Localization of WOX4
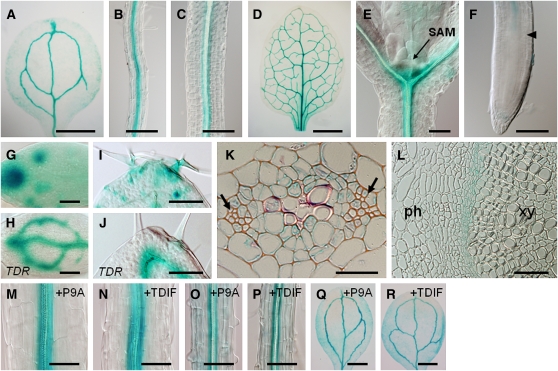
Because TDIF is perceived by TDR in procambial cells (Hirakawa et al., 2008), WOX4 should also be expressed in procambial cells if it acts downstream of the TDIF-TDR pathway. We examined the spatial pattern of WOX4 expression using the promoter-GUS (β-glucuronidase) reporter gene. Expression of WOX4pro:GUS was closely associated with the vasculature in the whole plant, including cotyledons, leaves, hypocotyls, and roots, but was not detected in the SAM or RAM (Figures 2A to 2F). In the root tip, the GUS signal in the vasculature was detected above the elongation zone (Figure 2F). This pattern resembles that of TDR (Fisher and Turner, 2007; Hirakawa et al., 2008). However, in the veins of young leaves, the expression of WOX4 was much weaker than that of TDR, and it was instead associated with trichomes and stomata (Figures 2G to 2J). In transverse sections of 7-d-old hypocotyls, WOX4pro:GUS was expressed mainly in the procambium, although weak staining was also detected in the phloem and pericycle (Figure 2K). In 4-week-old hypocotyls, GUS staining was detected specifically in the cambium (Figure 2L). This vascular-related expression of WOX4pro:GUS was not affected even when grown with 1 μM TDIF or P9A peptide (Figures 2M to 2R).
Figure 2.
Expression Patterns of WOX4.
WOX4 promoter activity was observed using WOX4pro:GUS plants.
(A) to (D) The 7-d-old cotyledon (A), root (B), and hypocotyl (C) and 14-d-old first leaf (D).
(E) and (F) In the SAM (E) and RAM (F), the GUS signal was below the detectable level.
(G) to (J) Comparison of WOX4pro:GUS ([G] and [I]) and TDRpro:GUS ([H] and [J]) expression in young leaves. Each pair of (G) and (I), and (H) and (J) shows nearly the same stages in leaf development.
(K) and (L) Procambium/cambium-specific WOX4pro:GUS staining in transverse sections of 7-d-old (K) and 4-week-old (L) hypocotyls. Weak staining was also observed in the phloem and pericycle cells in the 7-d-old hypocotyl.
(M) to (R) Treatment with 1 μM P9A ([M], [O], and [Q]) or TDIF ([N], [P], and [R]) did not change the pattern of GUS staining in the hypocotyl ([M] and [N]), root ([O] and [P]), and cotyledon ([Q] and [R]) of 7-d-old plants.
The arrow in (E) indicates the SAM. The arrowhead in (F) indicates the position of the cortex transition zone of the root meristem. Arrows in (K) indicate the phloem cells. In (L), ph and xy indicate phloem and xylem tissues, respectively. Bars = 500 μm in (A), (Q), and (R), 200 μm in (B) and (C), 1 mm in (D), 50 μm in (E) and (F), 10 μm in (G) and (H), 20 μm in (I) to (L), and 100 μm in (M) to (P).
Subcellular localization of WOX4-CFP (cyan fluorescent protein) and WUS-CFP fusion proteins was analyzed by transient expression assay in Nicotiana benthamiana leaf disks. Fluorescence for WOX4-CFP, as well as that for WUS-CFP, was detected specifically in the nucleus (see Supplemental Figure 2 online), consistent with WOX4’s putative role as a transcription factor.
Rapid Activation of WOX4 Expression by TDIF Signaling
To address how TDIF might regulate WOX4 expression, we used qRT-PCR to analyze the dynamics of the WOX4 transcript level after TDIF application. The WOX4 transcript level was upregulated by about threefold as rapidly as 1 h after TDIF application, and high transcript levels were also detected in plants treated with TDIF for 3 h or 7 d (Figure 1B). This rapid activation of WOX4 expression was not observed in the tdr-1 mutant (also called pxy-5) (Figure 1B), which lacks sensitivity to TDIF (Hirakawa et al., 2008). However, a similarly low level of WOX4 expression was observed in both wild-type and tdr-1 strains (Figure 1B), suggesting that basal WOX4 expression does not depend on TDIF-TDR signaling. By contrast, the transcript levels for other procambium marker genes, ARABIDOPSIS THALIANA HOMEOBOX GENE8(ATHB8), ATHB15, and TDR (Baima et al., 1995; Ohashi-Ito and Fukuda, 2003; Fisher and Turner, 2007; Hirakawa et al., 2008), were not significantly upregulated by brief treatment with TDIF, whereas they were upregulated in seedlings grown with TDIF for 7 d (Figure 1C). Expression levels of CLE41 and CLE44 were not affected significantly by TDIF application (Figure 1C). These results indicate that TDIF promotes the expression of WOX4 rapidly and specifically in a TDR-dependent manner.
WOX4 Is Not Required for the Suppression of Xylem Differentiation by TDIF
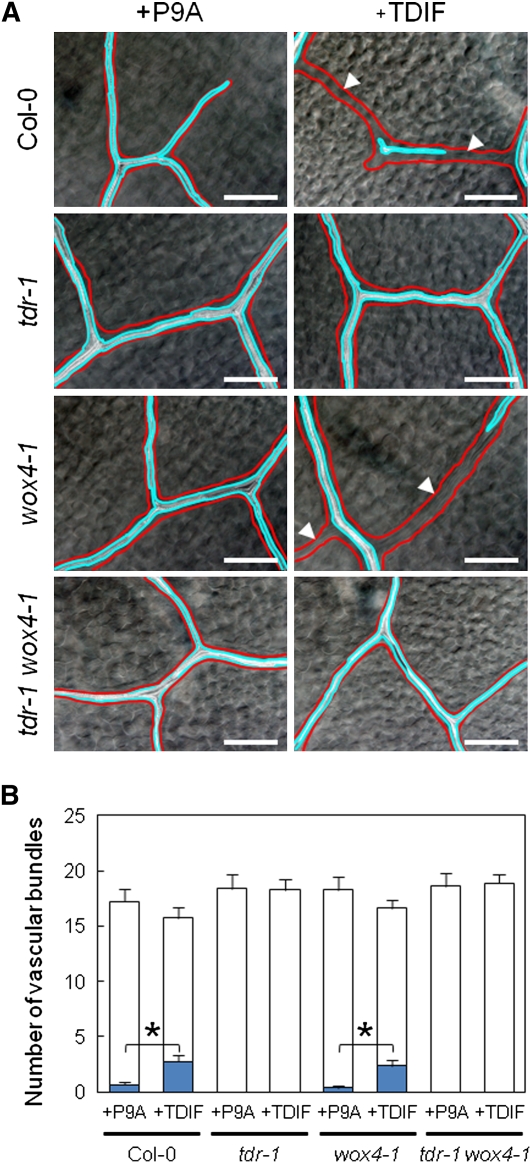
Our gene expression analysis suggested that WOX4 might act as a positive regulator downstream of TDIF-TDR signaling. To investigate the function of WOX4, we observed the phenotype of a mutant allele, wox4-1 (GABI462G01). This allele has a T-DNA insertion in the first exon of WOX4, and homozygous wox4-1seedlings lacked the normal WOX4 transcript (see Supplemental Figure 3 online), suggesting that it is a null allele. The mutant plants did not show visible defects in overall morphology (data not shown). Growing wild-type seeds in the presence of 1 μM TDIF leads to the production of discontinuous xylem strands in the higher-order veins of leaves in 10-d-old seedlings (Figure 3A), as described previously (Hirakawa et al., 2008). Similarly, TDIF caused the formation of discontinuous xylem strands in veins of wox4-1 mutants (Figure 3A). The frequency of discontinuous xylem strands in wox4-1 was almost the same as that in the wild type (Figure 3B). By contrast, TDIF did not cause the fragmentation of xylem strands in tdr-1 or tdr-1 wox4-1 double mutants (Figures 3A and 3B; Hirakawa et al., 2008). These results indicate that WOX4 does not contribute to the suppression of the xylem differentiation caused by TDIF-TDR signaling.
Figure 3.
TDIF-Sensitive Xylem Formation in wox4 Mutants.
(A) Effects of 1 μM TDIF on 10-d-old leaf veins of different strains. TDIF caused fragmentation of xylem strands (indicated by white arrowheads) in wox4-1 and in the wild type but did not in tdr-1 or the tdr-1 wox4-1double mutant. Veins are outlined in red, and xylem strands are outlined in blue.
(B) Quantification of the effects of TDIF. The blue and white boxes indicate the frequencies of the xylem-absent and xylem-present veins, respectively.
Bars = 100 μm in (A). Error bars indicate se, n = 8; *Student’s t test significance at P < 0.01 for different means of the frequency of xylem-absent veins in (B).
WOX4 Is Necessary for the Enhancement of Procambial Cell Proliferation by TDIF Signaling
Another function of TDIF is to promote the proliferation of procambial cells (Hirakawa et al., 2008). To evaluate the involvement of WOX4 in the procambial cell proliferation, we analyzed the phenotypes of procambial cell proliferation in the hypocotyl. In the lower part of the hypocotyl, the vascular cylinder is composed of two phloem poles, central xylem and intervening procambium, and is surrounded by a pericycle cell layer (see Supplemental Figure 4A online). This basic anatomy is conserved in the hypocotyl and the root (Dolan and Roberts, 1995; Gendreau et al., 1997). During secondary growth, procambial cells divide continually and the numbers of both phloem and xylem cells are increased by the differentiation of adjacent procambial cells. Cells in different vascular tissues can be distinguished by their characteristic histological shapes. Unlike procambial cells, phloem cells are small and clustered in the two phloem poles, whereas xylem vessel cells have thick secondary cell walls seen in transverse sections.
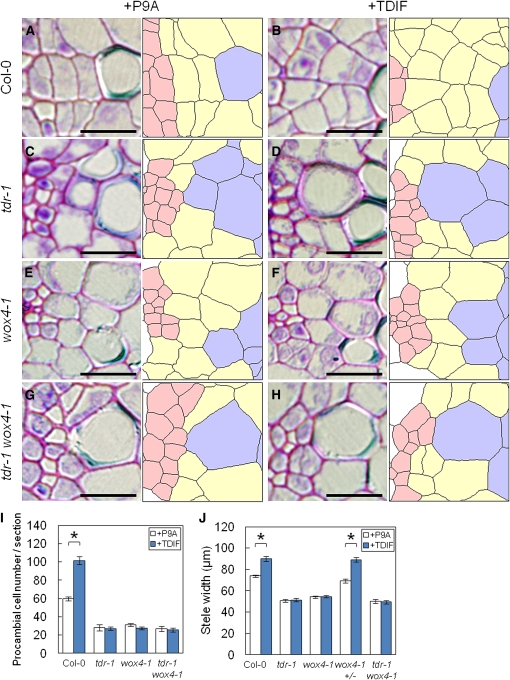
We first examined 5- and 7-d-old seedlings of wild-type, tdr-1, wox4-1, and tdr-1 wox4-1 strains grown in liquid medium. All of the 5-d-old seedlings grown with nonfunctional P9A peptide or without peptide (data not shown) had one or two intervening procambial cell layer(s) between the phloem and xylem tissues when seen in transverse section (see Supplemental Figure 4B online). This basic radial structure was not altered even when the seedlings were grown with 1 μM TDIF. In 7-d-old wild-type seedlings grown with P9A or without peptide (data not shown), intervening procambial cells divided periclinally into two or three cell layers (Figure 4A). This corresponds to the initiation of secondary growth in vascular cambium, as reported previously in 6-d-old seedlings grown on moist filter paper (Busse and Evert, 1999). In hypocotyls of 7-d-old tdr-1, wox4-1, and tdr-1wox4-1 seedlings, procambial cell division was suppressed, forming one or two cell layers (Figures 4C, 4E, and 4G). In whole sections, all mutant lines had about half as many procambial cells as did the wild-type plants (Figure 4I), suggesting that TDR and WOX4 might work on the same genetic pathway in promoting procambial cell proliferation.
Figure 4.
Differences and Similarities between TDR and WOX4 Functions in Determining Procambial Cell Behavior.
(A) to (H) Magnification of the procambium region in hypocotyls of seedlings grown for 7 d with 1 μM P9A ([A], [C], [E], and [G]) or TDIF ([B], [D], [F], and [H]). tdr ([C] and [D]) and tdr wox4-1 ([G] and [H]), but not the wild type ([A] and [B]) or wox4-1 ([E] and [F]), often had adjoining phloem and xylem cells. Panels at the right show the arrangement of vascular cells. Red, blue, and yellow areas show phloem, xylem, and procambial cells, respectively.
(I) and (J) Number of procambial cells per section (I) and stele width (J) in 7-d-old plants grown with P9A (white) or TDIF (blue). The ± indicates heterozygous mutants.
Bars = 10 μm in (A) to (H). Error bars indicate se, n = 9 to 12; *Student’s t test significance at P < 0.01 for different means in (I) and (J).
In half of the individual hypocotyl sections of tdr-1 and tdr-1wox4-1 plants grown with P9A or TDIF, xylem cells were formed adjacent to the phloem tissues (Figures 4C, 4D, 4G, and 4H, Table 1). This suggests that a deficiency in TDIF perception promotes xylem differentiation of phloem-adjoining procambial cells during postembryonic development, consistent with a previous report (Hirakawa et al., 2008). However, in wox4-1plants, at least one procambial cell layer existed between the phloem and xylem tissues (Figures 4E and 4F, Table 1). These results strongly suggest that a mutation in TDR causes both the suppression of procambial cell proliferation and the enhancement of xylem differentiation, whereas a mutation in WOX4 causes only the suppression of procambial cell proliferation.
Table 1.
Phenotypes of the Spatial Arrangement of Vascular Cells
| 5 d | 7 d | Genotype | Peptide | Adja | nb | Adj | n | ||||
| Col-0 | P9A | 0 | 15 | 0 | 22 | ||||||
| TDIF | 0 | 15 | 0 | 22 | |||||||
| tdr-1 | P9A | 0 | 15 | 12 | 21 | ||||||
| TDIF | 1 | 15 | 10 | 20 | |||||||
| wox4-1 | P9A | 0 | 15 | 1 | 21 | ||||||
| TDIF | 0 | 15 | 0 | 20 | |||||||
| tdr-1 wox4-1 | P9A | 1 | 15 | 10 | 21 | ||||||
| TDIF | 0 | 19 | 11 | 21 | |||||||
| cle41-1 | P9A | 0 | 17 | 5 | 24 | ||||||
| TDIF | 0 | 16 | 5 | 22 | |||||||
Frequency of phloem-associated xylem differentiation events were examined in 5- or 7-d-old hypocotyls grown in the presence of 1 μM P9A or TDIF.
Number of plants with adjoining phloem and xylem cells.
Total number of plants.
Exogenously supplied TDIF activated both periclinal and anticlinal division of procambial cells in wild-type hypocotyls (Figures 4B and 4I). By contrast, it did not promote the procambial cell proliferation in tdr-1, wox4-1, or tdr-1 wox4-1 hypocotyls (Figure 4I). These results support the idea that TDIF, TDR, and WOX4 act in the same genetic pathway in terms of the enhancement of the procambial cell proliferation. In wild-type seedlings, the enhancement of the procambial cell proliferation by TDIF resulted in an increase in stele width of the hypocotyl (Figure 4J; Hirakawa et al., 2008). Consistent with the mutant phenotypes in terms of procambial cell proliferation, the steles of tdr-1, wox4-1, and tdr-1 wox4-1 hypocotyls were thinner than that of the wild type and were not enlarged by TDIF (Figure 4J). Heterozygous wox4-1 mutants had nearly the same width of steles as the wild type when they were grown with P9A or TDIF (Figure 4J), indicating that wox4-1 is a recessive allele.
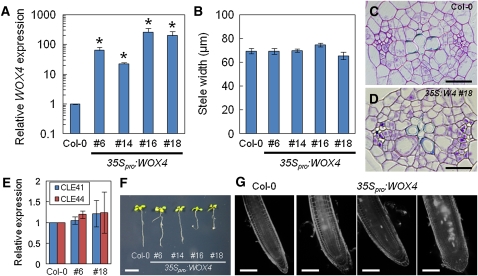
Overexpression Phenotypes of WOX4
To determine if WOX4 alone could promote procambial cell proliferation, we examined the gain-of-function phenotypes of WOX4. We observed four independent lines harboring 35Spro:WOX4. Expression levels of WOX4 in these lines were ~20- to 250-fold higher than that of the wild type (Figure 5A), but their steles were not thickened significantly (Figure 5B). In transverse sections, cellular arrangement in the stele did not differ between wild-type and 35Spro:WOX4 hypocotyls (Figures 5C and 5D). These results indicate that the overexpression of WOX4 does not mimic the TDIF-induced enhancement of radial growth in hypocotyls. Expression levels of CLE41 and CLE44 were not significantly affected in the 35Spro:WOX4 lines (Figure 5E). Root elongation was impaired in these transgenic plants, and this effect was especially severe in two lines in which WOX4 was highly expressed. These lines often exhibited abnormal gravitropism in their roots (Figure 5F). Correct tissue organization in the RAM was not maintained, and abnormal cell proliferation was observed in the columella and lateral root cap, which resulted in an increase of radial layers of the root (Figure 5G). The steles were often thickened in these lines compared with the wild type (Figure 5G). Therefore, ectopically expressed WOX4 seems to affect proliferation in the RAM but does not do so in the procambium of the hypocotyl, indicating that WOX4 expression alone is not sufficient for procambial cell proliferation.
Figure 5.
Effects of WOX4 Overexpression on Vascular Development and Root Growth.
(A) Relative expression level of WOX4 per TUA4 in the 35Spro:WOX4 lines measured by qRT-PCR. The wild-type expression level is set to 1.
(B) Stele width of 7-d-old hypocotyls. No significant differences were found in the four different 35Spro:WOX4 lines when compared with the wild type (Col-0).
(C) and (D) Transverse sections of 7-d-old hypocotyls. Cellular arrangement in the stele was similar between the wild type (C) and 35Spro:WOX4 (D).
(E) Relative expression of TDIF genes (CLE41 and CLE44) per TUA4 in 35Spro:WOX4 lines (#6 and #18). The wild-type expression level is set to 1.
(F) Root growth of 7-d-old plants.
(G) Tissue anatomy in the RAM of 7-d-old plants.
Bars = 20 μm in (C) and (D), 1 cm in (F), and 100 μm in (G). Error bars indicate sd (n = 3, *Student’s t test significance at P < 0.05 for different means compared with the wild-type expression levels) in (A) and (E) and se (n = 9 to 10, *Student’s t test significance at P < 0.01 for different means compared with the wild type) in (B).
CLE41 Acts in Endogenous TDIF Signaling
To investigate further the in planta function of TDIF signaling, we analyzed an ethyl methanesulfonate–mutagenized allele of CLE41 obtained through the Arabidopsis Targeting-Induced Local Lesions in Genomes (TILLING) project (Till et al., 2003). This allele, named cle41-1, had a point mutation in the coding sequence of CLE41, causing a translational termination before the CLE domain, suggesting that it is a null allele.
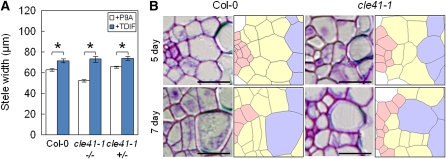
Homozygous cle41-1 plants formed a thinner stele in the hypocotyl than did wild-type plants (Figure 6A), as observed in the tdr and wox4 mutants. In contrast with these mutants, the cle41-1 plants were sensitive to TDIF: their steles were enlarged by TDIF treatment (Figure 6A). Heterozygous cle41-1 mutants showed almost the same phenotype as the wild type, suggesting that this allele is recessive (Figure 6A). There were fewer procambial cells in the sections of cle41-1 hypocotyls (26.4 ± 1.8, n = 10) than in the wild type (47.7 ± 2.1, n = 10). In addition, xylem vessel cells were sometimes formed adjacent to the phloem cells in 7-d-old but not 5-d-old seedlings, as observed in the tdr mutants (Figure 6B). The frequency of this phenotype in cle41-1 was only half that in the tdr mutants (Table 1). These results indicate that CLE41 regulates both proliferation and xylem differentiation of procambial cells in a similar fashion to TDR. Therefore, the phloem-derived CLE41 peptide must act as an endogenous form of TDIF.
Figure 6.
Phenotypes of cle41 Mutants.
(A) Stele widths of the 7-d-old hypocotyls treated with P9A (white) or TDIF (blue).
(B) Magnification of the procambial region in 5-d-old (top) and 7-d-old (bottom) hypocotyls. Only the 7-d-old cle41-1 mutants had adjoining phloem and xylem cells.
Scale bars = 10 μm in (B). Error bars indicate se, n = 9 to 10; *Student’s t test significance at P < 0.01 for different means in (A).
TDIF Signaling Influences Stem Cell Behavior in the Vascular Meristem
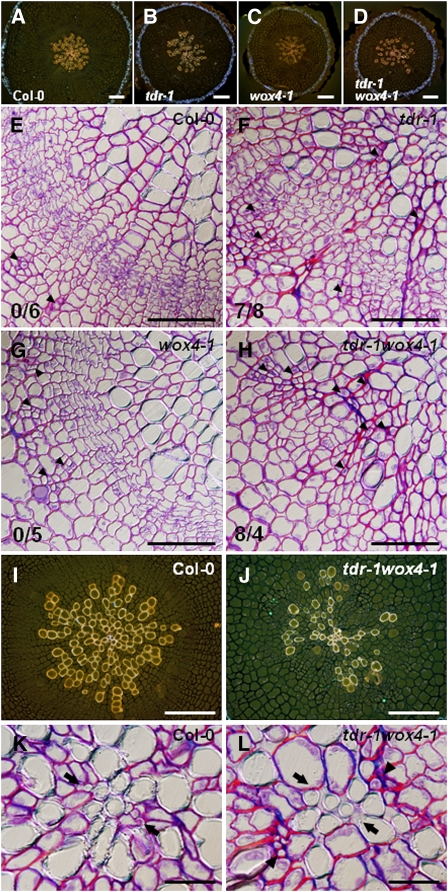
An important function of stem cells is the long-term production of descendants. To examine the involvement of TDIF signaling in this function, we observed secondary growth in the aforementioned mutants. In the reproductive phase of Arabidopsis development, hypocotyls and roots form radial structures consisting of concentrically arranged tissues of periderm, phloem, vascular cambium, and xylem (Chaffey et al., 2002). Grown under continuous light, hypocotyls of all 4-week-old plants tested showed this pattern, although secondary xylem did not mature fully. At this stage, hypocotyls of tdr-1, wox4-1, and tdr-1 wox4-1 plants were thinner than those of the wild type (Figures 7A to 7D), in agreement with the idea that cellular proliferation in their procambium/cambium tissues was reduced. In the wild type and wox4-1, there was a continuous and active cambial layer (Figures 7E and 7G). By contrast, in hypocotyls of tdr-1 and tdr-1 wox4-1, the continuous ring of cambium was often interrupted, making cavities in the circular outline of the xylem tissue (Figures 7F and 7H). At the bottom of the cavities, sieve elements often were adjacent to xylem cells (Figures 7F and 7H; see Supplemental Figure 5C online) The number of cavities per section was higher in tdr-1 wox4-1 than in tdr-1 (Figures 7E to 7H). Furthermore the cavities were wider in tdr-1 wox4-1 plants than in tdr-1 (see Supplemental Figures 5A and 5B online). These results suggest that WOX4 also acts independently of TDIF signaling to promote proliferation. In the most severe phenotype of tdr-1 wox4-1, the cavities reached very close to the center of the xylem tissue (Figure 7J). The cellular organization at the center of the vascular cylinder resembled that in young hypocotyls (Figures 7K and 7L; see Supplemental Figures 4A and 5C online). These observations suggest that xylem differentiation of intervening procambial cells caused by a defect in TDR results in the termination of the vascular meristem and secondary growth. Hence, TDIF signaling appears to regulate the stem cell maintenance by inhibiting their spontaneous differentiation into xylem. With regard to the enlarged cavity phenotype of tdr-1 wox4-1 compared with tdr-1 (Figures 7F and 7H; see Supplemental Figure 6A online), we suggest that WOX4 promotes the maintenance of stem cells in a tdr mutant background.
Figure 7.
Exhaustion of Vascular Stem Cells Caused Premature Termination of Secondary Growth.
(A) to (D) Overall radial structure of the 4-week-old hypocotyls.
(E) to (H) Magnifications of the cambial region located between the secondary phloem and xylem tissues. In contrast with the wild-type (E) and wox4-1 (G) plants, tdr (F) and tdr wox4 (H) plants had cavities in the outline of xylem tissues and the continuous cambium ring was disrupted. Some sieve elements (white arrowheads) were found at the bottom of the cavities ([F] and [H]). Numerals at left bottom show the number of cavities per the number of plants tested in (E) to (H).
(I) to (L) Central regions of the vascular cylinder in the wild type ([I] and [K]) and the most severely affected tdr-1 wox4-1 plant ([J] and [L]). In (K) and (L), the most central region of the vascular cylinder was magnified. Black arrows and arrowheads in (L) indicate the axis of primary xylem and primary phloem cells respectively. Stained by safranin-O and aniline blue, cells in xylem, phloem, and phellem (cork) show orange, green, and blue fluorescence, respectively, in (A) to (D), (I), and (J).
Bars = 100 μm in (A) to (D), (I), and (J), 50 μm in (E) to (H), and 20 μm in (K) and (L).
In cavities formed in tdr-1 and tdr-1 wox4-1 mutants, procambial/cambial cells are exhausted to form xylem cells adjacent to phloem cells, and new cell proliferation occurs outside of the phloem cells (Figures 7F, 7H, and 7J; see Supplemental Figure 5C online). These proliferating cells are likely to be derived from the pericycle, considering its proliferative activity (Dolan and Roberts, 1995; Gendreau et al., 1997) and relative position in the radial tissue anatomy (Figure 7L; see Supplemental Figure 4A online).
DISCUSSION
Indeterminate growth in plants depends on stem cell populations capable of long-term cell production. Stem cells are maintained in a specific local environment termed the stem cell niche, which is characterized by positional cues produced by niche cells. In this study, we revealed that the phloem-originated secretory peptide TDIF/CLE41 acts as one such positional cue for stem cell maintenance in the vascular meristem. The TDR/PXY membrane receptor mediates this signal, regulating both cellular proliferation and differentiation. We showed WOX4 to be a downstream component of this signaling pathway, which seems to regulate proliferation but not xylem differentiation in the vascular stem cells, showing independency of the two functions for this stem cell maintenance signal.
Our previous study showed that exogenously supplied TDIF suppressed xylem differentiation of procambial cells and promoted their proliferation (Hirakawa et al., 2008). In this study, we found that CLE41, which is expressed specifically in phloem cells (Hirakawa et al., 2008), acts as a gene for endogenous TDIF. In addition, our finding that the phenotype of the cle41 mutant is slightly weaker than that of the tdr mutant suggests that TDIF derived from other genes might also function in this process. This is consistent with the fact that CLE44, preferentially expressed in phloem and nearby, also encodes TDIF. The fact that phloem expresses the stem cell maintenance signal suggests that it may act as a stem cell niche tissue for the vascular meristem.
Gene expression and genetic analyses in this study showed WOX4 to be a transcriptional target of the TDR-dependent TDIF signaling pathway. Preferential promoter activity of WOX4 was observed in the procambium and cambium in a similar manner to that of TDR, supporting the functional overlap of these genes. The rapid response of WOX4 to the TDR-dependent TDIF signal suggests the functional importance of WOX4 activation in TDIF signaling. In the SAM, activation of CLV3 expression results in the reduction of the WUS expression within 3 h (Müller et al., 2006). Because the CLV3 was activated by an alcohol induction system in that study, the actual response of WUS to the CLV3 peptide signal must occur in less than three hours. WOX4 activation is induced 1 h after TDIF application. This similarity in the timing of induction suggests that similar signaling components regulate WOX gene expression after the perception of the CLE ligand in both TDIF-TDR and CLV3-CLV1 pathways, although the directions of their effects on the target gene expression level are opposite. In addition, it was revealed recently that a signaling module composed of CLE40-ACR4-WOX5 affects the size and position of the niche in the distal root meristem (Stahl et al., 2009). In the SAM and RAM, WUS and WOX5 are expressed in niche cells and function non-cell-autonomously. By contrast, WOX4 is expressed in the vascular meristem, which contains stem cells, and acts in the same tissue. Therefore, the CLE-WOX signaling pathway might be adopted generally to mediate cell-to-cell communication in plant meristems, but the expression and function of WOX genes may differ between the vascular meristem and apical meristems.
Genetic analysis in this study showed that WOX4 is required to promote cell proliferation, mediating a part of the function of the TDIF signal. However, because WOX4 overexpression itself does not affect proliferation, it would require additional components to mediate fully the effect of TDIF on proliferation. On the other hand, a WOX4-independent signaling pathway(s) must mediate the TDIF signal’s regulation of xylem differentiation. Our observations in mature plants revealed that the TDIF-TDR-WOX4 signaling functions in the secondary growth of vascular tissues. Vascular tissues are developed through primary growth from the procambium and by secondary growth from the cambium. Continued secondary growth of vascular tissues was not maintained in tdr mutants, showing that the inhibition of xylem differentiation by TDIF signaling is a prerequisite for maintaining the vascular meristem. WOX4 deficiency promotes the disruption of the vascular meristem in a tdr mutant background. Thus, the regulation of proliferation is also important for maintenance of the vascular meristem. These findings strongly support the idea that the TDIF signaling pathway plays a crucial role in the maintenance of vascular stem cells, which have the capacity of continuous generation of descendant vascular cells.
The similarity of secondary vascular tissues in the herbaceous plant Arabidopsis to that in woody plants, such as poplar, has been shown by anatomical studies on these tissues (Chaffey et al., 2002). Indeed, it is reported that Populus tremula × tremuloides orthologs for WOX4 and TDR, Ptt-HB3 and Ptt-RLK3, are both expressed in the vascular cambium, and their expression patterns within the cambial zone have similar patterns (Schrader et al., 2004). This fact supports the idea that the TDIF-TDR-WOX4 signaling pathway functions generally in secondary growth of the vascular meristem.
In conclusion, the TDIF signal from phloem plays a crucial role in the maintenance of vascular stem cells by two independent pathways: WOX4-independent inhibition of xylem commitment of vascular stem cells and WOX4-mediated enhancement of their proliferation (see Supplemental Figure 6B online).
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana seeds were from the Columbia-0 (Col-0) accession except for those with cle41-1, which were obtained from an ethyl methanesulfonate–mutagenized er-105 (Col-0 background) population through the TILLING project (Till et al., 2003) and were received from the ABRC (stock ID, CS92206). This allele had a point mutation of C217T in the coding sequence of CLE41, making a stop codon (CGA to TGA). Seeds of tdr-1/pxy-5 were received from the ABRC stock center (stock ID, SALK_002910; Fisher and Turner, 2007; Hirakawa et al., 2008). Seeds of wox4-1/GABI462G01 (Rosso et al., 2003) were received from the Nottingham Arabidopsis Stock Centre (NASC; stock ID, N444329). All mutants were backcrossed into Col-0 plants one to three times. Genotyping primers for wox4-1 were used in the combination of LP-RP and BP-RP (LP, 5′-GCTCGTAGGTCAATGTCAATCTC-3′; RP, 5′-CCTATCTGTTCTTGAGTCGGG-3′; and BP/GABI_o8409, 5′-ATATTGACCATCATACTCATTGC-3′). For the production of the 35Spro:WOX4 construct, the coding sequence of WOX4 was amplified from genomic DNA (the primer set, 5′-CACCATGAAGGTTCATGAGTTTTCGAATGG-3′ and 5′-TCATCTCCCTTCAGGATGGAG-3′), cloned into pENTR vector (Invitrogen), and subcloned into the pH35GS vector using the Gateway cloning system (Invitrogen). Transformation of Col-0 plants was performed with the floral dip method (Clough and Bent, 1998). For peptide treatment experiments, seeds were germinated and grown in half Murashige and Skoog liquid medium at 22°C under continuous light with 110 rpm shaking, as described previously (Hirakawa et al., 2008). Dodecapeptides of TDIF (HEVHypSGHypNPISN) and P9A (HEVHypSGHypNAISN) were synthesized with a purity of >95% (Operon) and added to the liquid medium at a final concentration of 1 or 5 μM. None was added to the medium in mock treatments. For observing plants in the reproductive phase, they were grown under continuous light at 22°C for 28 d.
Whole-Mount Observation of Vasculature
Leaves and hypocotyls of seedlings fixed in a 1:3 mixture of acetic acid and ethanol were mounted in clearing solution (chloral hydrate-glycerol-water, 8:1:2) and then observed under a light microscope (BX51; Olympus). Quantification of the inhibitory effects of TDIF on xylem differentiation in leaf veins (Figure 3B) was performed as described (Hirakawa et al., 2008). Briefly, the presence or absence of the xylem vessels was examined at the position where high-ordered veins cross arbitrary transverse lines that partition a leaf blade into eight portions.
Histology
Sectioning was performed as described with minor modifications (Hirakawa et al., 2008). Samples were fixed in FAA solution (ethanol:water:acetic acid:formalin = 50:35:5:10), dehydrated in a graded ethanol series, and embedded in Technovit 7100 resin (Kulzer) according to the manufacturer’s instructions. Sections of 1 μm (for young hypocotyls) or 2 μm (for mature hypocotyls) were cut using a microtome (Leica RM2165) and stained with 0.02% toluidine blue-O solution. For GUS-stained sections, 2-μm sections were cut and counterstained briefly with 0.02% safranin-O. For visualization of the xylem and phloem cells, sections were stained briefly with 0.02% safranin-O and 0.005% aniline blue solution (Fisher Scientific) and then mounted in water. Sections were observed under a light microscope equipped with the U-MWU2 mirror unit (Olympus).
qRT-PCR
Total RNA was isolated from the upper part of seedlings using RNeasy plant mini kits and RNase-free DNase sets (Qiagen). Reverse transcription reactions were performed using SuperScript III first-strand synthesis system (Invitrogen) according to the manufacturer’s instructions. Primer sets for the RT-PCR and qRT-PCR (Figure 1) are described in Supplemental Methods online. qRT-PCR was performed using the LightCycler real-time PCR system (Roche) with the TaqMan method (Roche). Three independent samples were used, and the PCR reaction was performed twice for each sample. The expression levels of specific genes were normalized to that of the control gene TUA4, and the means of relative expression for each sample were examined using Student’s t test (significance at P < 0.05).
Promoter-GUS Assay
The promoter activity of genes was analyzed using the GUS activity as reporter. The 2-kb upstream sequence of the ATG translation start of WOX4 was amplified from genomic DNA (the primer set, 5′-CACCGGCAAGTGTAGTGGAGGAGG-3′ and 5′-TGCTATATGTTAAAACTAGCAAATGC-3′), cloned into pENTR vector (Invitrogen), and subcloned into the pMDC167 vector to produce a promoter-GUS construct using the Gateway cloning system (Invitrogen). Transformation of Col-0 plants was performed with the floral dip method (Clough and Bent, 1998). The TDRpro:GUS line (Hirakawa et al., 2008) was a gift from Taku Demura. For GUS staining, plants were fixed in 90% acetone overnight, washed twice with 100 mM phosphate buffer, pH 7.4, and stained for 1 h with GUS solution (100 mM phosphate buffer, pH 7.4, 0.5 mg/mL 5-bromo-4-chloro-3-indolyl-β-glucuronic acid, and 2.5 mM FeCN). GUS-stained samples were rinsed in 70% ethanol, mounted in the clearing solution, and then observed using light microscopy.
Accession Numbers
Sequence data from this study can be found in the Arabidopsis Genome Initiative data library under the following accession numbers: At4g32880 (ATHB8), At1g52150 (ATHB15), At3g24770 (CLE41), At4g13195 (CLE44), At5g61480 (PXY/TDR), At1g04820 (TUA4), At2g17950 (WUS), At3g18010 (WOX1), At5g59340 (WOX2), At2g28610 (PRS/WOX3), At1g46480 (WOX4), At3g11260 (WOX5), At2g01500 (WOX6), At5g05770 (WOX7), At5g45980 (WOX8), At2g33880 (WOX9), At1g20710 (WOX10), At3g03660 (WOX11), At5g17810 (WOX12), At4g35550 (WOX13), and At1g20700 (WOX14).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Expression of WOX Family Genes in Arabidopsis Seedlings.
Supplemental Figure 2. Subcellular Localization of WOX4.
Supplemental Figure 3. Gene Structure of WOX4.
Supplemental Figure 4. TDIF Signaling Does Not Affect the Radial Structure of Primary Vascular Tissues.
Supplemental Figure 5. Cavity Formation in tdr Mutants.
Supplemental Figure 6.Schematic Diagrams of Mutant Phenotypes and a Model for Stem Cell Maintenance in the Vascular Meristem.
Supplemental Methods.
Supplementary Material
Acknowledgments
We thank Nam-Hai Chua and Kyoko Ohashi-Ito for providing the pER8 vector and a derivative. We thank the ABRC, NASC, GABI-Kat, and Taku Demura for providing seed materials. We thank the members of the Fukuda laboratory for technical support and valuable discussions. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (19060009) to H.F., from the Japan Society for the Promotion of Science (20247003 to H.F. and JSPS Research Fellowships for Young Scientists to Y.H.), and from Bio-oriented Technology Research Advancement Institution to H.F.
References
- Baima S., Nobili F., Sessa G., Lucchetti S., Ruberti I., Morelli G. (1995). The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121: 4171–4182 [DOI] [PubMed] [Google Scholar]
- Björklund S., Antti H., Uddestrand I., Moritz T., Sundberg B. (2007). Cross-talk between gibberellin and auxin in development of Populus wood: Gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J. 52: 499–511 [DOI] [PubMed] [Google Scholar]
- Brand U., Fletcher J.C., Hobe M., Meyerowitz E.M., Simon R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619 [DOI] [PubMed] [Google Scholar]
- Breuninger H., Rikirsch E., Hermann M., Ueda M., Laux T. (2008). Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev. Cell 14: 867–876 [DOI] [PubMed] [Google Scholar]
- Busse J.S., Evert R.F. (1999). Vascular differentiation and transition in the seedling of Arabidopsis thaliana (Brassicaceae). Int. J. Plant Sci. 160: 241–251 [Google Scholar]
- Chaffey N., Cholewa E., Regan S., Sundberg B. (2002). Secondary xylem development in Arabidopsis: A model for wood formation. Physiol. Plant. 114: 594–600 [DOI] [PubMed] [Google Scholar]
- Clark S.E., Williams R.W., Meyerowitz E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575–585 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cock J.M., McCormick S. (2001). A large family of genes that share homology with CLAVATA3. Plant Physiol. 126: 939–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I., Voss U., Jürgens G., Beeckman T. (2009). Receptor-like kinases shape the plant. Nat. Cell Biol. 11: 1166–1173 [DOI] [PubMed] [Google Scholar]
- Deveaux Y., Toffano-Nioche C., Claisse G., Thareau V., Morin H., Laufs P., Moreau H., Kreis M., Lecharny A. (2008). Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 8: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny J.R., Benfey P.N. (2008). Plant stem cell niches: standing the test of time. Cell 132: 553–557 [DOI] [PubMed] [Google Scholar]
- Dolan L., Roberts K. (1995). Secondary thickening in roots of Arabidopsis thaliana: Anatomy and cell surface changes. New Phytol. 131: 121–128 [DOI] [PubMed] [Google Scholar]
- Elo A., Immanen J., Nieminen K., Helariutta Y. (2009). Stem cell function during plant vascular development. Semin. Cell Dev. Biol. 20: 1097–1106 [DOI] [PubMed] [Google Scholar]
- Evert R.F. (2006). Esau's Plant Anatomy, Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed (Hoboken, NJ: John Wiley & Sons; ). [Google Scholar]
- Fisher K., Turner S. (2007). PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr. Biol. 17: 1061–1066 [DOI] [PubMed] [Google Scholar]
- Fletcher J.C., Brand U., Running M.P., Simon R., Meyerowitz E.M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Fukuda H. (2004). Signals that control plant vascular cell differentiation. Nat. Rev. Mol. Cell Biol. 5: 379–391 [DOI] [PubMed] [Google Scholar]
- Gendreau E., Traas J., Desnos T., Grandjean O., Caboche M., Höfte H. (1997). Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker A., Gross-Hardt R., Geiges B., Sarkar A., Breuninger H., Herrmann M., Laux T. (2004). Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668 [DOI] [PubMed] [Google Scholar]
- Hirakawa Y., Shinohara H., Kondo Y., Inoue A., Nakanomyo I., Ogawa M., Sawa S., Ohashi-Ito K., Matsubayashi Y., Fukuda H. (2008). Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. USA 105: 15208–15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Nakanomyo I., Motose H., Iwamoto K., Sawa S., Dohmae N., Fukuda H. (2006). Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845 [DOI] [PubMed] [Google Scholar]
- Ji J., Strable J., Shimizu R., Koenig D., Sinha N., Scanlon M.J. (2010). WOX4 promotes procambial development. Plant Physiol. 152: 1346–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.H., Fiume E., Fletcher J.C. (2008). The CLE family of plant polypeptide signaling molecules. Cell. Mol. Life Sci. 65: 743–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J., Björklund S., Vahala J., Hertzberg M., Kangasjärvi J., Sundberg B. (2009). Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc. Natl. Acad. Sci. USA 106: 5984–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N., Okada K. (2001). A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev. 15: 3355–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Kitano M., Kusumoto T., Tarkowski P., Kinoshita-Tsujimura K., Václavíková K., Miyawaki K., Kakimoto T. (2008). Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA 105: 20027–20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K.F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- Müller R., Borghi L., Kwiatkowska D., Laufs P., Simon R. (2006). Dynamic and compensatory responses of Arabidopsis shoot and floral meristems to CLV3 signaling. Plant Cell 18: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J., Ji J., Werr W., Scanlon M.J. (2004). The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 131: 2827–2839 [DOI] [PubMed] [Google Scholar]
- Nieminen K., et al. (2008). Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 105: 20032–20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Karlberg A., Antti H., Lopez-Vernaza M., Mellerowicz E., Perrot-Rechenmann C., Sandberg G., Bhalerao R.P. (2008). Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Shinohara H., Sakagami Y., Matsubayashi Y. (2008). Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319: 294. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K., Fukuda H. (2003). HD-zip III homeobox genes that include a novel member, ZeHB-13 (Zinnia)/ATHB-15 (Arabidopsis), are involved in procambium and xylem cell differentiation. Plant Cell Physiol. 44: 1350–1358 [DOI] [PubMed] [Google Scholar]
- Ohyama K., Ogawa M., Matsubayashi Y. (2008). Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. Plant J. 55: 152–160 [DOI] [PubMed] [Google Scholar]
- Park S.O., Zheng Z., Oppenheimer D.G., Hauser B.A. (2005). The PRETTY FEW SEEDS2 gene encodes an Arabidopsis homeodomain protein that regulates ovule development. Development 132: 841–849 [DOI] [PubMed] [Google Scholar]
- Rebocho A.B., Bliek M., Kusters E., Castel R., Procissi A., Roobeek I., Souer E., Koes R. (2008). Role of EVERGREEN in the development of the cymose petunia inflorescence. Dev. Cell 15: 437–447 [DOI] [PubMed] [Google Scholar]
- Reddy G.V. (2008). Live-imaging stem-cell homeostasis in the Arabidopsis shoot apex. Curr. Opin. Plant Biol. 11: 88–93 [DOI] [PubMed] [Google Scholar]
- Rosso M.G., Li Y., Strizhov N., Reiss B., Dekker K., Weisshaar B. (2003). An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 53: 247–259 [DOI] [PubMed] [Google Scholar]
- Sarkar A.K., Luijten M., Miyashima S., Lenhard M., Hashimoto T., Nakajima K., Scheres B., Heidstra R., Laux T. (2007). Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814 [DOI] [PubMed] [Google Scholar]
- Scheres B. (2007). Stem-cell niches: nursery rhymes across kingdoms. Nat. Rev. Mol. Cell Biol. 8: 345–354 [DOI] [PubMed] [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K.F., Jürgens G., Laux T. (2000). The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644 [DOI] [PubMed] [Google Scholar]
- Schrader J., Nilsson J., Mellerowicz E., Berglund A., Nilsson P., Hertzberg M., Sandberg G. (2004). A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 16: 2278–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. (2001). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 98: 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl Y., Simon R. (2005). Plant stem cell niches. Int. J. Dev. Biol. 49: 479–489 [DOI] [PubMed] [Google Scholar]
- Stahl Y., Wink R.H., Ingram G.C., Simon R. (2009). A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr. Biol. 19: 909–914 [DOI] [PubMed] [Google Scholar]
- Till B.J., et al. (2003). Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res. 13: 524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C., Moritz T., Sandberg G., Sundberg B. (1996). Auxin as a positional signal in pattern formation in plants. Proc. Natl. Acad. Sci. USA 93: 9282–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M., Horstman A., Zethof J., Koes R., Rijpkema A.S., Gerats T. (2009). Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petuniaand Arabidopsis. Plant Cell 21: 2269–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford R., Fernandez A., De Groodt R., Ortega E., Hilson P. (2008). Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc. Natl. Acad. Sci. USA 105: 18625–18630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H., Jürgens G. (2009). Survival of the flexible: hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 10: 305–317 [DOI] [PubMed] [Google Scholar]
- Wu X., Chory J., Weigel D. (2007). Combinations of WOX activities regulate tissue proliferation during Arabidopsis embryonic development. Dev. Biol. 309: 306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Dabi T., Weigel D. (2005). Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr. Biol. 15: 436–440 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Hu Y., Dai M., Huang L., Zhou D.X. (2009). The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21: 736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.