Abstract
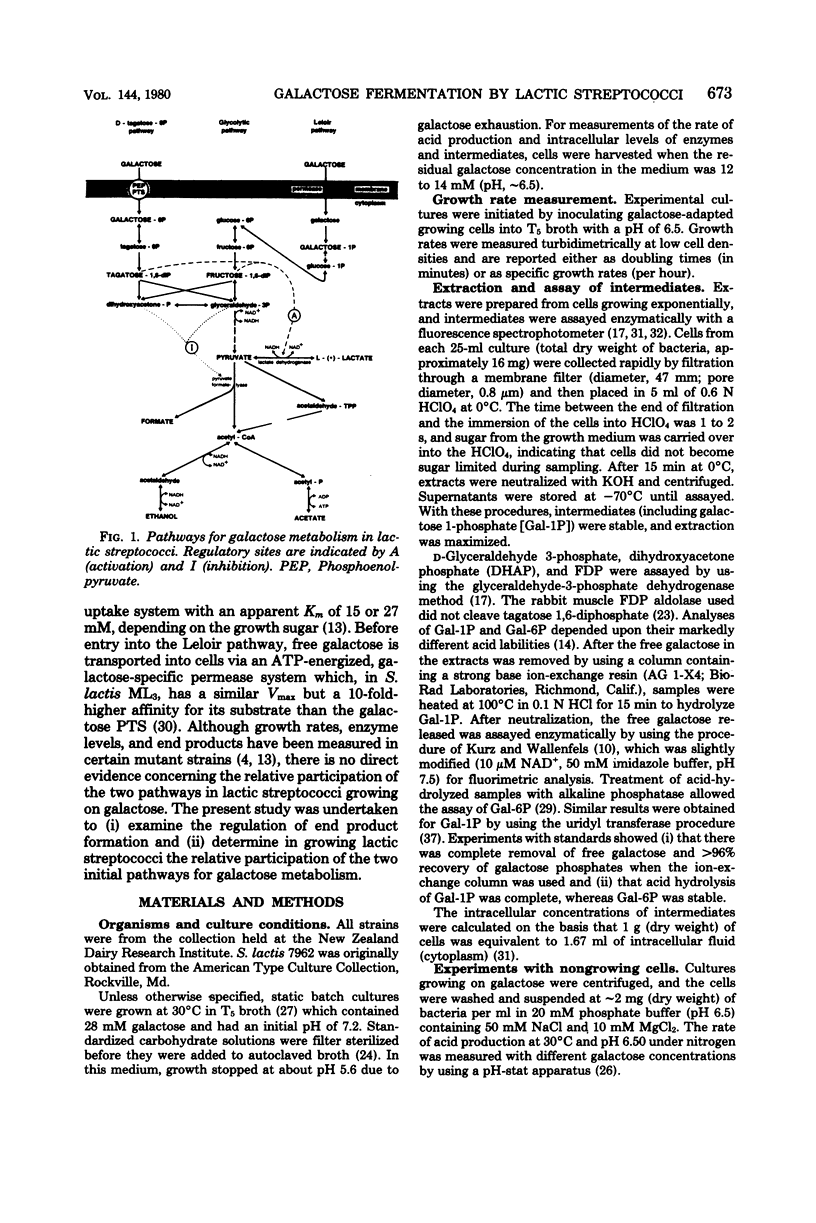
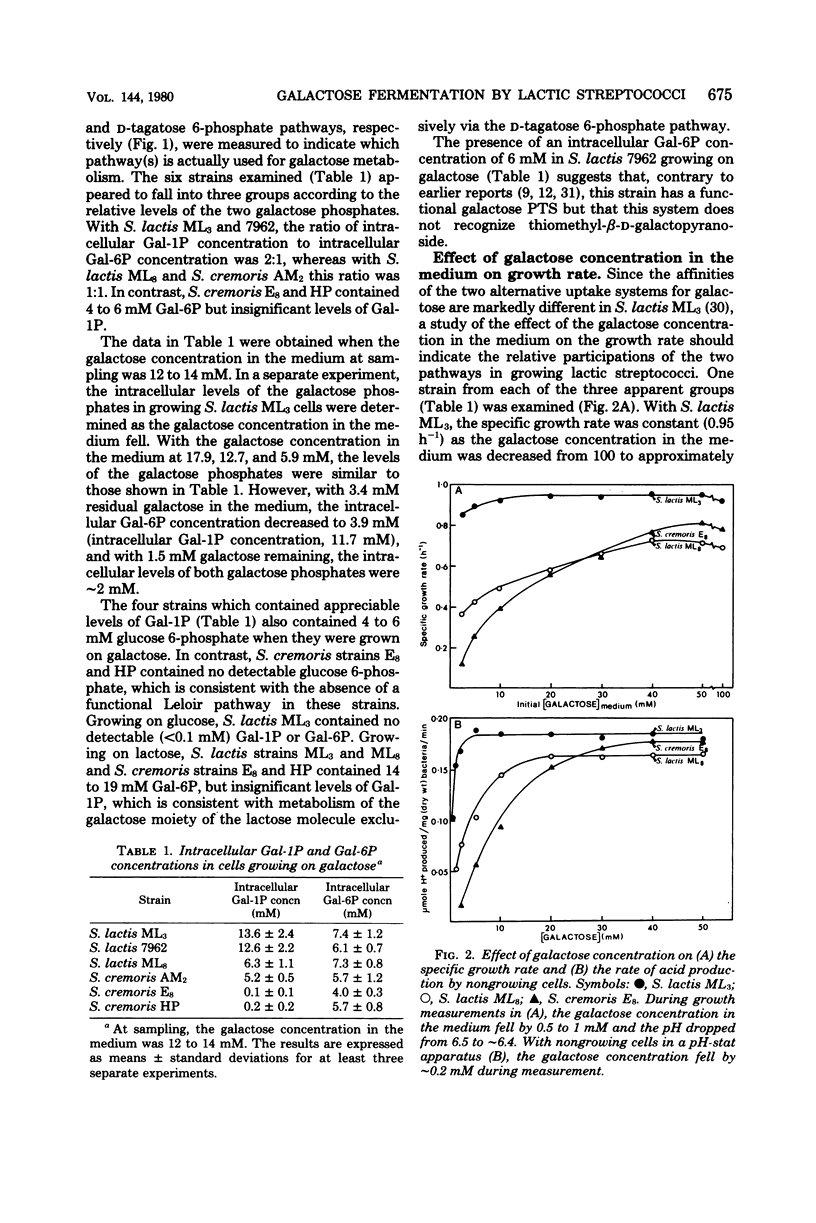
All of the lactic streptococci examined except Streptococcus lactis ML8 fermented galactose to lactate, formate, acetate, and ethanol. The levels of pyruvate-formate lyase and lactate dehydrogenase were elevated and reduced, respectively, in galactose-grown cells compared with glucose- or lactose-grown cells. Reduced intracellular levels of both the lactate dehydrogenase activator (fructose, 1,6-diphosphate) and pyruvate-formate lyase inhibitors (triose phosphates) appeared to be the main factors involved in the diversion of lactate to the other products. S. lactis ML8 produced only lactate from galactose, apparently due to the maintenance of high intracellular levels of fructose 1,6-diphosphate and triose phosphates. The growth rates of all 10 Streptococcus cremoris strains examined decreased markedly with galactose concentrations below about 30 mM. This effect appeared to be correlated with uptake predominantly by the low-affinity galactose phosphotransferase system and initial metabolism via the D-tagatose 6-phosphate pathway. In contrast, with four of the five S. lactis strains examined, galactose uptake and initial metabolism involved more extensive use of the high-affinity galactose permease and Leloir pathway. With these strains the relative flux of galactose through the alternate pathways would depend on the exogenous galactose concentration.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews K. J., Lin E. C. Selective advantages of various bacterial carbohydrate transport mechanisms. Fed Proc. 1976 Aug;35(10):2185–2189. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demko G. M., Blanton S. J., Benoit R. E. Heterofermentative carbohydrate metabolism of lactose-impaired mutants of Streptococcus lactis. J Bacteriol. 1972 Dec;112(3):1335–1345. doi: 10.1128/jb.112.3.1335-1345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUYAMA T. T., O'KANE D. J. Galactose metabolism. I. Pathway of carbon in fermentation by Streptococcus faecalis. J Bacteriol. 1962 Oct;84:793–796. doi: 10.1128/jb.84.4.793-796.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark D. G., Paolella P., Wood N. P. The pyruvate formate-lyase system of Streptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. J Biol Chem. 1969 Jul 10;244(13):3605–3612. [PubMed] [Google Scholar]
- STEELE R. H., WHITE A. G., PIERCE W. A., Jr The fermentation of galactose by Streptococcus pyogenes. J Bacteriol. 1954 Jan;67(1):86–89. doi: 10.1128/jb.67.1.86-89.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Synthesis of protein and ribonucleic acid by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):363–369. doi: 10.1099/00221287-58-3-363. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Tagatose-1, 6-diphosphate activation of lactate dehydrogenase from Streptococcus cremoris. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1035–1042. doi: 10.1016/0006-291x(75)90673-7. [DOI] [PubMed] [Google Scholar]
- Thompson J. Galactose transport systems in Streptococcus lactis. J Bacteriol. 1980 Nov;144(2):683–691. doi: 10.1128/jb.144.2.683-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Lactose metabolism in Streptococcus lactis: phosphorylation of galactose and glucose moieties in vivo. J Bacteriol. 1979 Dec;140(3):774–785. doi: 10.1128/jb.140.3.774-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Turner K. W., Thomas T. D. Catabolite inhibition and sequential metabolism of sugars by Streptococcus lactis. J Bacteriol. 1978 Mar;133(3):1163–1174. doi: 10.1128/jb.133.3.1163-1174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD N. P., O'KANE D. J. FORMATE-PYRUVATE EXCHANGE REACTION IN STREPTOCOCCUS FAECALIS. I. FACTOR REQUIREMENT FOR INTACT CELLS. J Bacteriol. 1964 Jan;87:97–103. doi: 10.1128/jb.87.1.97-103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


