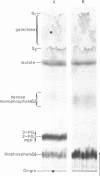
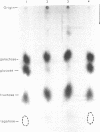
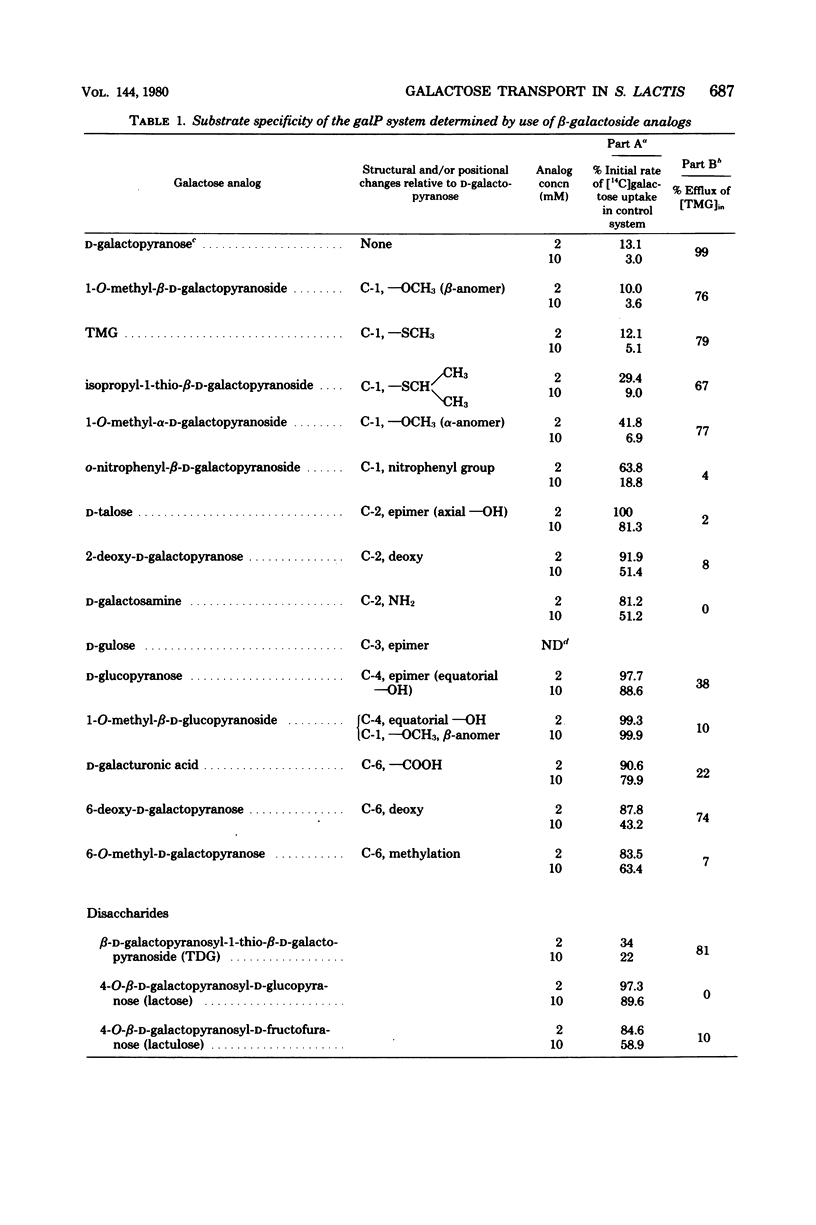
Abstract
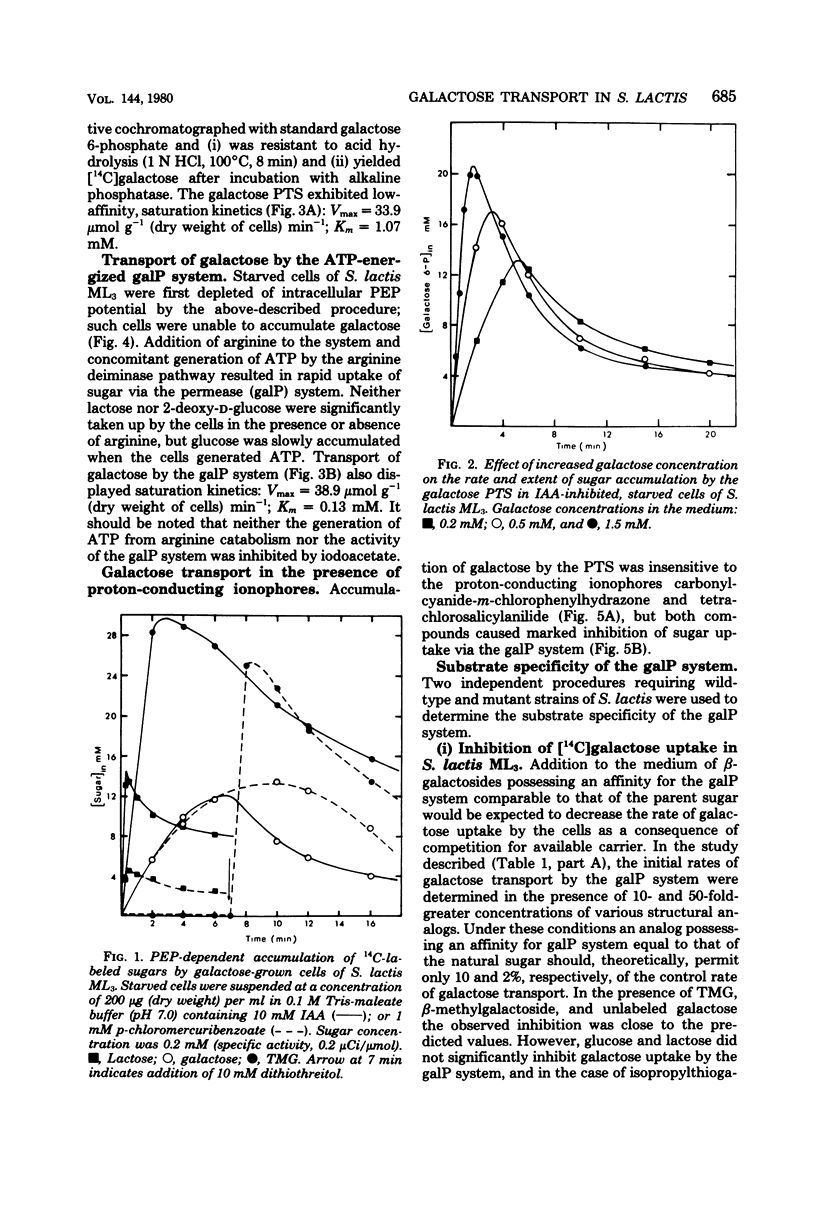
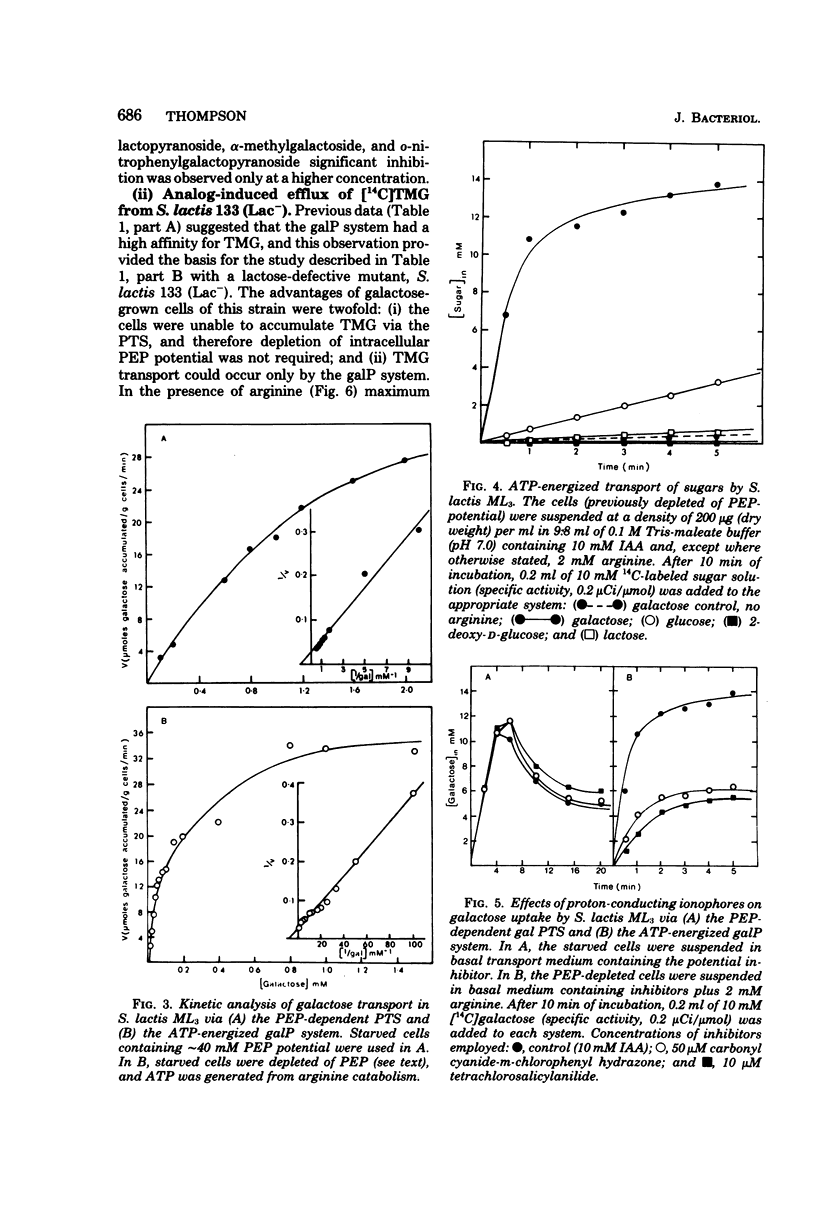
Galactose-grown cells of Streptococcus lactis ML3 have the capacity to transport the growth sugar by two separate systems: (i) the phosphoenolpyruvate-dependent phosphotransferase system and (ii) an adenosine 5'-triphosphate-energized permease system. Proton-conducting uncouplers (tetrachlorosalicylanilide and carbonyl cyanide-m-chlorophenyl hydrazone) inhibited galactose uptake by the permease system, but had no effect on phosphotransferase activity. Inhibition and efflux experiments conducted using beta-galactoside analogs showed that the galactose permease had a high affinity for galactose, methyl-beta-D-thiogalactopyranoside, and methyl-beta-D-galactopyranoside, but possessed little or no affinity for glucose and lactose. The spatial configurations of hydroxyl groups at C-2, C-4, and C-6 were structurally important in facilitating interaction between the carrier and the sugar analog. Iodoacetate had no inhibitory effect on accumulation of galactose, methyl-beta-D-thiogalactopyranoside, or lactose via the phosphotransferase system. However, after exposure of the cells to p-chloromercuribenzoate, phosphoenolpyruvate-dependent uptake of lactose and methyl-beta-D-thiogalactopyranoside were reduced by 75 and 100%, respectively, whereas galactose phosphotransferase activity remained unchanged. The independent kinetic analysis of each transport system was achieved by the selective generation of the appropriate energy source (adenosine 5'-triphosphate or phosphoenolpyruvate) in vivo. The maximum rates of galactose transport by the two systems were similar, but the permease system exhibited a 10-fold greater affinity for sugar than did the phosphotransferase system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L. Characterization of lactose-fermenting revertants from lactose-negative Streptococcus lactis C2 mutants. J Bacteriol. 1974 Sep;119(3):830–839. doi: 10.1128/jb.119.3.830-839.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demko G. M., Blanton S. J., Benoit R. E. Heterofermentative carbohydrate metabolism of lactose-impaired mutants of Streptococcus lactis. J Bacteriol. 1972 Dec;112(3):1335–1345. doi: 10.1128/jb.112.3.1335-1345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Plasmids in Streptococcus lactis: evidence that lactose metabolism and proteinase activity are plasmid linked. Appl Environ Microbiol. 1976 Jul;32(1):38–44. doi: 10.1128/aem.32.1.38-44.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORZENOVSKY M., WERKMAN C. H. Conversion of citrulline to ornithine by cell-free extracts of Streptococcus lactis. Arch Biochem Biophys. 1953 Sep;46(1):174–185. doi: 10.1016/0003-9861(53)90180-5. [DOI] [PubMed] [Google Scholar]
- KORZENOVSKY M., WERKMAN C. H. Stoicheiometry of the citrulline phosphorylase reaction of Streptococcus lactis. Biochem J. 1954 Jun;57(2):343–347. doi: 10.1042/bj0570343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Protonmotive force in fermenting Streptococcus lactis 7962 in relation to sugar accumulation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):879–886. doi: 10.1016/s0006-291x(74)80061-6. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. Genetics in the study of carbohydrate transport by bacteria. Sixth Griffith Memorial Lecture. J Gen Microbiol. 1976 Sep;96(1):1–16. doi: 10.1099/00221287-96-1-1. [DOI] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Molskness T., Sandine W. E., Elliker P. R. Carbohydrate metabolism in lactic streptococci: fate of galactose supplied in free or disaccharide form. Appl Microbiol. 1973 Dec;26(6):951–958. doi: 10.1128/am.26.6.951-958.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. A SECOND PERMEASE FOR METHYL-THIO-BETA-D-GALACTOSIDE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:591–593. doi: 10.1016/0304-4165(65)90029-2. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The formation of arginine dihydrolase by streptococci and some properties of the enzyme system. J Bacteriol. 1952 Oct;64(4):455–466. doi: 10.1128/jb.64.4.455-466.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Thomas T. D. Activator specificity of pyruvate kinase from lactic streptococci. J Bacteriol. 1976 Mar;125(3):1240–1242. doi: 10.1128/jb.125.3.1240-1242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Lactose metabolism in Streptococcus lactis: phosphorylation of galactose and glucose moieties in vivo. J Bacteriol. 1979 Dec;140(3):774–785. doi: 10.1128/jb.140.3.774-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Turner K. W., Thomas T. D. Catabolite inhibition and sequential metabolism of sugars by Streptococcus lactis. J Bacteriol. 1978 Mar;133(3):1163–1174. doi: 10.1128/jb.133.3.1163-1174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]