This article identifies two previously unknown flavonol glycosyltransferases of grapevines and compares them in terms of sugar donor specificity. These enzymes are considered paralogous, and a scenario for evolution of new sugar donor specificity of glycosyltransferases is proposed based on the results of phylogenetic, biochemical, and molecular modeling studies of these enzymes.
Abstract
We identified two glycosyltransferases that contribute to the structural diversification of flavonol glycosides in grapevine (Vitis vinifera): glycosyltransferase 5 (Vv GT5) and Vv GT6. Biochemical analyses showed that Vv GT5 is a UDP-glucuronic acid:flavonol-3-O-glucuronosyltransferase (GAT), and Vv GT6 is a bifunctional UDP-glucose/UDP-galactose:flavonol-3-O-glucosyltransferase/galactosyltransferase. The Vv GT5 and Vv GT6 genes have very high sequence similarity (91%) and are located in tandem on chromosome 11, suggesting that one of these genes arose from the other by gene duplication. Both of these enzymes were expressed in accordance with flavonol synthase gene expression and flavonoid distribution patterns in this plant, corroborating their significance in flavonol glycoside biosynthesis. The determinant of the specificity of Vv GT5 for UDP-glucuronic acid was found to be Arg-140, which corresponded to none of the determinants previously identified for other plant GATs in primary structures, providing another example of convergent evolution of plant GAT. We also analyzed the determinants of the sugar donor specificity of Vv GT6. Gln-373 and Pro-19 were found to play important roles in the bifunctional specificity of the enzyme. The results presented here suggest that the sugar donor specificities of these Vv GTs could be determined by a limited number of amino acid substitutions in the primary structures of protein duplicates, illustrating the plasticity of plant glycosyltransferases in acquiring new sugar donor specificities.
INTRODUCTION
Grapevine (Vitis vinifera) (Figure 1A) has been one of the most important fruit crops from the beginning of human civilization (McGovern et al., 1997). Dietary intake of grapevine-based products results in diverse biological effects that are beneficial to human health, in part due to polyphenols, such as flavonoids (e.g., flavonols, proanthocyanidins, and anthocyanins) (Renaud and de Lorgeril, 1992; Corder et al., 2001, 2006; Baur et al., 2006).
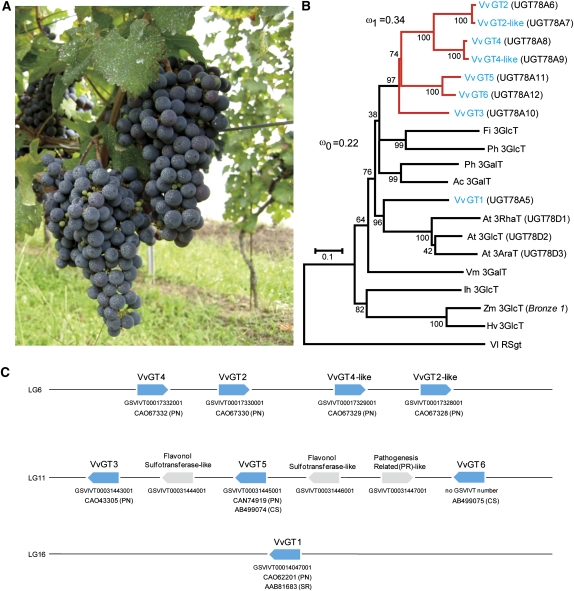
Figure 1.
Phytogenetics of V. vinifera Flavonoid-3-O-Glycosyltransferases and the Related UGTs.
(A) Grapes (cv Cabernet Sauvignon) at a vineyard in the TOMI NO OKA Winery, Yamanashi Prefecture, Japan.
(B) An unrooted phylogenetic tree was constructed as described in Methods. The alignment used for this analysis is available as Supplemental Data Set 1 online. The percentages of replicate trees, in which the associated taxa clustered together in the bootstrap test (1000 replicates), are shown. Bar = 0.1 amino acid substitutions per site. Resveratrol glucosyltransferase (Vl RSgt) was used as an outgroup sequence (Hall and De Luca, 2007). The branches shown in red and black indicate the foreground and background lineages, respectively, of the UGT gene clusters on the basis of a branch-specific model with the dN/dS ratios of ω1 = 0.34 and ω0 = 0.22, as suggested by the maximum likelihood analysis (Yang, 2007). Vv, Vitis vinifera; Vl, Vitis labrusca; Ph, Petunia hybrida; Fi, Forsythia intermedia; At, Arabidopsis thaliana; Ac, Aralia cordata; Vm, Vigna mungo; Ih, Iris hollandica; Zm, Zea mays; Hv, Hordeum vulgare. Ih, Zm, and Hv are monocots.
(C) Graphical map of Vv GT genes (shown in blue) in the grapevine genome. Accession numbers of Vv GTs are shown below each gene. PN, Pinot Noir; CS, Cabernet Sauvignon; SR, Shiraz.
Flavonols, such as kaempferol, quercetin, and isorhamnetin, are an important class of bioactive flavonoids, which are most abundant as their 3-O-glycosides (i.e., glucoside, glucuronoside, galactoside, and rutinoside) in the berries, seeds, and leaves of grapevine (Hmamouchi et al., 1996; Castillo-Munoz et al., 2009). In this plant, biosynthesis of flavonol glycosides primarily serves to protect the plant from excess UV light (Price et al., 1995; Matus et al., 2009). Flavonol aglycons are biosynthesized from dihydroflavonols by the action of flavonol synthase (Vv FLS), a 2-oxoglutarate-dependent dioxygenase, which is temporally regulated by an R2R3-Myb transcription factor, Vv MYBF1 (Fujita et al., 2006; Czemmel et al., 2009). Subsequently, glycosylation of flavonols enhances their water solubility, such that high concentrations of flavonols can accumulate in plant cells.
From a food chemistry perspective, flavonol glycosides define the color of wine grapes and products by acting as copigments (Yoshitama et al., 1992; Boulton, 2001; Schwarz et al., 2005). Thus, they are important phytochemicals for viticulture and enology. Moreover, they also have important biomedical activities. The activities that are unique to flavonol glycosides and are not associated with the aglycon forms include the following: the cytoprotective effects of quercetin 3-O-glucoside (isoquercitrin) by induction of cholesterol biosynthesis (Soundararajan et al., 2008), antiatherosclerotic activity of quercetin glucuronides that are specifically incorporated by macrophages (Kawai et al., 2008), and antidepressant effects of quercetin 3-O-glucuronide (miquelianin), quercetin 3-O-glucoside, and quercetin 3-O-galactoside (hyperoside) (Butterweck et al., 2000; Jürgenliemk et al., 2003). Importantly, the bioactivities of these compounds are greatly modulated by their glycon structures (Jürgenliemk et al., 2003; Soundararajan et al., 2008).
Glycosylation of flavonoids in plant cells usually takes place in a regio-(or position-)specific manner after the completion of aglycon biosynthesis (Heller and Forkmann, 1994), providing a basis for the structural diversification of these important metabolites. Glycosylation is catalyzed by glycosyltransferases (UGTs), which generally catalyze transfer of the glycosyl group from nucleoside diphosphate–activated sugars (e.g., UDP-sugars) to acceptor molecules (Campbell et al., 1997; Coutinho et al., 2003; Bowles et al., 2005; Wang, 2009). The UGTs that are involved in plant secondary metabolism, including flavonoid biosynthesis, have been assigned to family 1 of UGTs (Vogt and Jones, 2000; Bowles et al., 2005). These UGTs are characterized by a unique, well-conserved sequence of ~45–amino acid residues (called a PSPG box) (Vogt and Jones, 2000) and a catalytic mechanism that inverts the anomeric configuration of a transferred sugar (Campbell et al., 1997; Wang, 2009). Phylogenetic analyses of plant family 1 UGTs show several phylogenetic clusters, which appear to be characterized by the regiospecificity of their glycosyl transfer to flavonoids (Vogt and Jones, 2000; Sawada et al., 2005; Noguchi et al., 2007). Moreover, the differentiation of the sugar donor specificity of UGTs appears to have occurred in a lineage-specific manner after establishment of general regiospecificity for the glycosyl acceptor (Yonekura-Sakakibara et al., 2007; Noguchi et al., 2009a; Ono et al., 2010).
The grapevine genome contains as many as 240 UGT genes (The French-Italian Public Consortium for Grapevine Genome Characterization, 2007), and of these, the only grapevine UGT that has been functionally characterized is Vv GT1, which catalyzes the 3-O-specific glucosylation of anthocyanidin to produce anthocyanidin 3-O-glucoside and is involved in the coloration of grape skin (Ford et al., 1998). This study aimed at identifying the UGTs that are responsible for the observed structural diversity of flavonol glycosides in grapevine. To that end, the genome of grapevine cv Pinot Noir (PN42004 genotype) was screened in silico for genes that are related to Vv GT1 and therefore potentially encode flavonoid-3-O-glycosyltransferases. Seven such Vv GT1–related genes were identified, two of which were heterologously expressed in Escherichia coli cells as catalytically active proteins. These enzymes, termed Vv GT5 and Vv GT6, have very similar primary structures and are encoded in tandem on the same chromosome, strongly suggesting that one evolved from the other through gene duplication. Biochemical analyses of these enzymes revealed that Vv GT5 is a UDP-glucuronic acid:flavonol-3-O-glucuronosyltransferase, whereas Vv GT6 is a bifunctional UDP-glucose/UDP-galactose:flavonol-3-O-glucosyltransferase/galactosyltransferase. Detailed analyses of these Vv GTs led us to propose a scenario for evolution of a new sugar donor specificity of UGT after gene duplication, illustrating the plasticity of UGTs that enhance the chemical diversity of plant secondary metabolites.
RESULTS
Screening of V. vinifera Flavonoid-3-O-Glycosyltransferase Genes
In an effort to the identify genes that encode flavonol-3-O-glycosyltransferases in grapevine, the cv Pinot Noir (PN42004 genotype) genome sequence (The French-Italian Public Consortium for Grapevine Genome Characterization, 2007) was screened in silico using the Vv GT1 nucleotide sequence. We identified seven UGT-related genes with appreciable sequence identity to Vv GT1: Vv GT2, Vv GT2-like, Vv GT3, Vv GT4, Vv GT4-like, Vv GT5, and Vv GT6 (Table 1; see Supplemental Table 1 online). Each of these Vv GT genes was predicted to have a single intron. Phylogenetic analysis revealed that each of these genes is related to the GT1 family phylogenetic cluster (cluster I) of flavonoid 3-O-glycosyltransferases, as expected (Vogt and Jones, 2000; Sawada et al., 2005; Noguchi et al., 2007). Moreover, these genes formed a subcluster that was distinct from the Vv GT1–related subcluster (Figure 1B). Comparison of these sequences with the physical map of the PN42004 genotype allowed us to clarify their location on the genome, as summarized in Figure 1C. Vv GT2, Vv GT4, as well as a set of copies of Vv GT2 and Vv GT4 (termed Vv GT2-like and Vv GT4-like with nucleotide sequences that are 99.3 and 98.2% identical to those of the Vv GT2 and Vv GT4 genes, respectively; see Supplemental Table 1 online) are located in the same orientation on chromosome 6. However, the Vv GT4-like gene was incomplete in length and should therefore be considered a pseudogene. Vv GT3, Vv GT5, and Vv GT6 were located in the same orientation on chromosome 11, where particularly high sequence identity (91%) was noted between Vv GT5 and Vv GT6 (see Supplemental Table 1 online). These three Vv GT genes alternate with two putative flavonol sulfotransferase genes (Figure 1C). The committee responsible for UDP-glucuronosyltransferase nomenclature (http://somflinders.edu.au/FUSA/ClinPharm/UGT/) assigned these Vv GTs as follows: Vv GT1, UGT78A5; Vv GT2, UGT78A6; Vv GT2-like, UGT78A7; Vv GT3, UGT78A10; Vv GT4, UGT78A8; Vv GT4-like, UGT78A9; Vv GT5, UGT78A11; and Vv GT6, UGT78A12.
Table 1.
Sequence Identities of Vv GT Proteins
| Sequence Identity (%) to |
|||||||||
| Protein | Length (aa)a | Vv GT1 | Vv GT2 | Vv GT2-Like | Vv GT3 | Vv GT4 | Vv GT4-Like | Vv GT5 | Vv GT6 |
| Vv GT1 | 452b | 100 | 51.3 | 51.3 | 56.3 | 55.9 | 56.5 | 53.8 | 55.0 |
| Vv GT2 | 445b | 100.0 | 98.2 | 60.1 | 73.3 | 72.3 | 57.6 | 58.7 | |
| Vv GT2-like | 445b | 100.0 | 59.7 | 73.3 | 71.9 | 57.6 | 58.7 | ||
| Vv GT3 | 531b | 100.0 | 62.6 | 68.2 | 64.0 | 64.1 | |||
| Vv GT4 | 455b | 100.0 | 98.3 | 61.6 | 62.3 | ||||
| Vv GT4-like | 295c | 100.0 | 64.1 | 63.9 | |||||
| Vv GT5 | 459b | 100.0 | 88.2 | ||||||
| Vv GT6 | 458b | 100.0 | |||||||
Amino acids.
Full length.
N-terminally truncated.
Biochemical Characterization of Vv GT5 and Vv GT6
Among the Vv GT genes identified, Vv GT5 and Vv GT6 were heterologously expressed in E. coli as soluble and catalytically active proteins. The specificity and catalytic properties of these recombinant proteins were analyzed using a wide variety of phenolics as glycosyl acceptors and UDP-glucose (UDP-Glc), UDP-glucuronic acid (UDP-GA), UDP-galactose (UDP-Gal), and UDP-rhamnose as sugar donors.
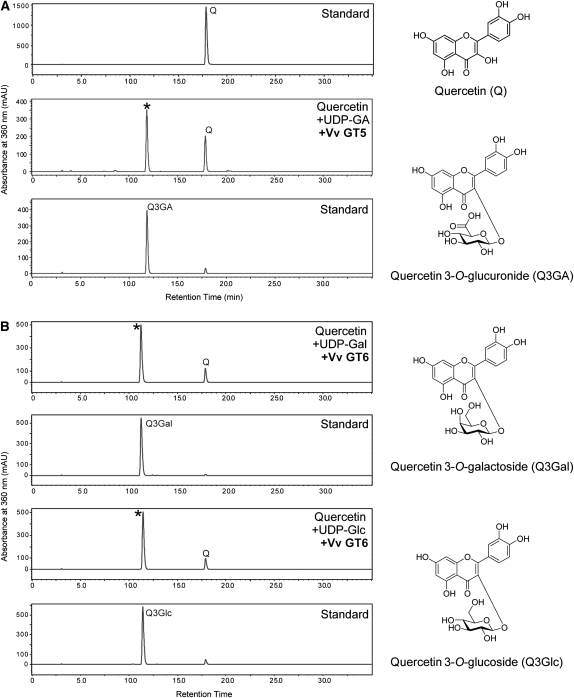
Vv GT5 displayed a strong glucuronosyl transfer activity from UDP-GA to flavonols (kaempferol, quercetin, and isorhamnetin) (Figure 2). The relative activities for flavonols (100 μM), examined using 100 μM UDP-GA as the sugar donor, were as follows: quercetin, 100%; kaempferol, 51.5%; and isorhamnetin, 5.6%. The following 14 phenolic compounds were inert as glucuronosyl acceptors: pelargonidin, cyanidin, and delphinidin (anthocyanidins), apigenin and luteolin (flavones), (+)-catechin (a flavan-3-ol), genistein (an isoflavone), naringenin (a flavanone), ferulic acid, caffeic acid, and salicylic acid (aromatic carboxylic acids), capsaicin (a capsaicinoid), trans-resveratrol (a stilbene), and esculetin (a coumarin). Moreover, UDP-Glc, UDP-Gal, and UDP-rhamnose were inert as sugar donors. Thus, Vv GT5 was highly specific for both flavonols and UDP-GA. Vv GT5–catalyzed transfer of glucuronic acid to quercetin produced a single transfer product, which was clearly identified as quercetin 3-O-glucuronide based on its cochromatography with an authentic sample and absorption spectral characteristics (λmax, 356 nm; see Supplemental Table 2 online for HPLC and spectrophotometric identification of regioisomers of quercetin monoglucuronides). Therefore, this enzyme was defined as a UDP-GA:flavonol-3-O-glucuronosyltransferase (termed hereafter flavonol-3GAT). The optimal pH and temperature for Vv GT5–catalyzed glucuronosyl transfer to quercetin were 9.1 and 45°C, respectively.
Figure 2.
Enzymatic Analyses of Recombinant Vv GT5 and Vv GT6 Proteins for Glycosylation of Quercetin.
(A) HPLC chart for standard quercetin (top), enzyme reaction with quercetin, UDP-GA, and Vv GT5 (middle), and standard quercetin 3-O-glucuronide (bottom).
(B) HPLC chart for the enzyme reaction with quercetin, UDP-Gal, and Vv GT6 (top), standard quercetin 3-O-galactoside (second), the enzyme reaction with quercetin, UDP-Glc, and Vv GT6 (third), and standard quercetin 3-O-glucoside (bottom). Asterisk indicates product peak for each enzyme reaction.
The enzymatic reaction followed Michaelis-Menten kinetics, and the steady state kinetic parameters were determined at pH 9.1 and 30°C as summarized in Table 2. The enzyme displayed high kcat values for quercetin and kaempferol, which were 10- to 100-fold higher than the values reported for many other UGTs at optimal pH (e.g., Noguchi et al., 2007).
Table 2.
Kinetic Parameters of Vv GT5 and Its R140W Mutanta
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (s−1 μM−1) |
| Wild-Type Vv GT5 (GAT Activity) | |||
| Quercetinb | 7.16 ± 0.37 | 5.60 ± 1.76 | 1.280 |
| Quercetinc | 1.63 ± 0.26 | 54.7 ± 17.4 | 0.030 |
| Kaempferolb | 6.05 ± 0.05 | 10.8 ± 0.4 | 0.560 |
| Isorhamnetinb | 0.413 ± 0.003 | 7.92 ± 0.32 | 0.052 |
| UDP-GAb,d | 17.7 ± 2.9 | 0.405 | |
| Vv GT5-R140W (GAT Activity)b | |||
| Quercetinb | 0.00016 ± 0.00002 | 29.0 ± 15.5 | 5.52 × 10−6 |
| UDP-GAe | 0.0015 × 10−6 | ||
| Vv GT5-R140W (GlcT activity)b | |||
| Quercetinb | 2.94 ± 0.11 | 9.69 ± 1.50 | 0.303 |
| Kaempferolb | 1.04 ± 0.01 | 1.96 ± 0.17 | 0.531 |
| Isorhamnetinb | 0.148 ± 0.004 | 5.85 ± 0.86 | 0.025 |
| UDP-Glcd | 77.2 ± 8.4 | 0.038 | |
| Vv GT5-R140W (GalT activity)b | |||
| Quercetinb | 0.429 ± 0.020 | 10.6 ± 1.5 | 0.041 |
| Kaempferolb | 0.395 ± 0.005 | 3.02 ± 0.19 | 0.131 |
| Isorhamnetinb | 0.0243 ± 0.0007 | 11.9 ± 0.9 | 0.002 |
| UDP-Gald | 225 ± 21 | 0.002 | |
Only flavonols were accepted as sugar acceptor substrates.
Kinetic parameters were determined at pH 9.1 with the appropriate UDP-sugar (1.0 mM), as described in Methods.
Kinetic parameters were determined at pH 7.0, as described in Methods.
100 μM quercetin was used as the sugar acceptor.
Km value was too large to be determined precisely.
Vv GT6 catalyzed glucosyl transfer from UDP-Glc to flavonols. Remarkably, this enzyme also displayed a strong galactosyl transfer activity (126% of glucosyl transfer activity) from UDP-Gal to flavonols (Figure 2). UDP-GA and UDP-rhamnose were inert as sugar donors. The relative activities for flavonols (100 μM) with UDP-Glc (100 μM) were as follows: quercetin, 100%; kaempferol, 84.6%; and, isorhamnetin, 61.2%. The relative activities for flavonols (100 μM) with UDP-Gal (100 μM) were as follows: quercetin, 100%; kaempferol, 110%; and, isorhamnetin, 26.9%. Other phenolics (see above) were all inert as glycosyl acceptors, indicating that the enzyme is also highly specific for flavonols. Vv GT6–catalyzed transfer of glucose to quercetin produced a single transfer product, which was identified as quercetin 3-O-glucoside based on its coelution with an authentic sample and NMR spectra. The optimal pH and temperature for Vv GT6–catalyzed glucosyl transfer to quercetin was 8.0 and 35°C, respectively.
Both the glucosyl and galactosyl transfer reactions catalyzed by Vv GT6 followed Michaelis-Menten kinetics when the flavonol concentration was <150 μM; substrate inhibition was observed when high concentrations (higher than 150 μM) of flavonol were used. Apparent steady state kinetic parameters were determined at pH 7.4 and 30°C, as summarized in Table 3 (for GlcT activity and GalT activity). Based on the kcat/Km values, the best substrates for the GlcT and GalT activities of Vv GT6 were kaempferol (0.115 s−1·μM−1) and quercetin (0.304 s−1·μM−1), respectively. When Vv GT6 activity was assayed in the presence of both UDP-Glc and UDP-Gal (1 mM each), both quercetin 3-O-glucoside and quercetin 3-O-galactoside were produced in an approximate molar ratio of 1:1 (see Supplemental Figure 1 online). These results show that this enzyme is bifunctional, catalyzing both glucosyl and galactosyl transfer to flavonols, and can be defined as a UDP-Glc/UDP-Gal:flavonol 3-O-glucosyltransferase/galactosyltransferase (termed hereafter flavonol-3GlcT/GalT).
Table 3.
Kinetic Parameters of Vv GT6a
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (s−1 μM−1) |
| GlcT Activity | |||
| Quercetin | 0.760 ± 0.023 | 9.24 ± 1.20 | 0.082 |
| Kaempferol | 0.473 ± 0.013 | 4.11 ± 0.47 | 0.115 |
| Isorhamnetin | 0.273 ± 0.017 | 7.43 ± 1.62 | 0.037 |
| UDP-Glcb | 28.5 ± 3.9 | 0.027 | |
| GalT Activity | |||
| Quercetin | 1.09 ± 0.001 | 3.59 ± 0.01 | 0.304 |
| Kaempferol | 0.725 ± 0.044 | 10.4 ± 1.9 | 0.070 |
| Isorhamnetin | 0.707 ± 0.019 | 5.04 ± 0.49 | 0.140 |
| UDP-Galb | 37.2 ± 2.2 | 0.029 | |
Kinetic parameters were determined at pH 7.4 with the appropriate UDP-sugar (1.0 mM), as described in Methods. Only flavonols were accepted as sugar acceptor substrates.
100 μM quercetin was used as the sugar acceptor.
Quantitative RT-PCR
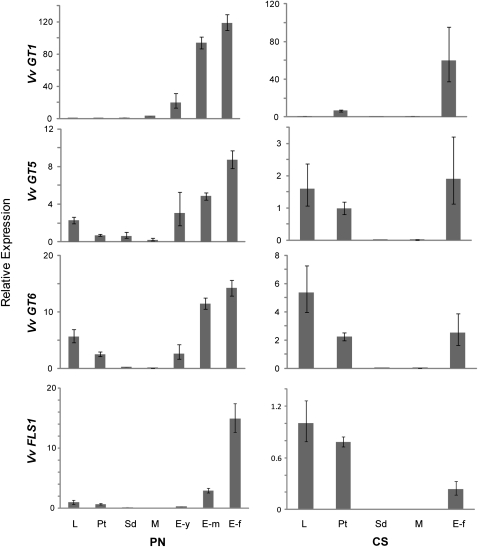
To evaluate the functional significance of Vv GT5 and Vv GT6 in the biosynthesis of flavonol glycosides in the grapevine plant, transcription levels of Vv GT5 and Vv GT6 in an array of organs and tissues of cv Pinot Noir, including the leaves, stems, and developing berries, were quantified by quantitative RT-PCR using total RNA. The results were compared with those of Vv GT1 (Ford et al., 1998), which is responsible for anthocyanin biosynthesis in grapevine berry skin and Vv FLS1 (also termed Vv FLS4), which is responsible for flavonol biosynthesis (Fujita et al., 2006; Czemmel et al., 2009) (Figure 3, left panels). The Vv GT5 transcript was abundant in berry skin and increased at the onset of pigmentation (veraison), with the highest level of transcription detected in the fully pigmented skin (exocarp) of mature berries (Figure 3, second panel). An appreciable amount of transcript was also found in leaves and petioles, and only small amounts of transcript were found in seeds and berry flesh (mesocarp). The pattern of Vv GT6 expression was similar to that of Vv GT5 expression (Figure 3, third panel). Moreover, the expression profiles of both Vv GT5 and Vv GT6 were similar to that of Vv FLS1. By contrast, Vv GT1 expression was highly specific for berry skin, and the highest transcription level was found in fully pigmented berries (Figure 3, first panel). Organ/tissue specificities of Vv GT cDNA expression in another grapevine cultivar (cv Cabernet Sauvignon) were also analyzed (Figure 3, right panels). In Cabernet Sauvignon, considerable amounts of Vv GT5, Vv GT6, and Vv FLS1 transcript were found in both leaves and petioles (Figure 3, second through fourth panels) in addition to berry skin. By contrast, Vv GT1 expression was highly specific for berry skin (Figure 3, first panel), as was the case for cv Pinot Noir.
Figure 3.
Gene Expression Analysis of V. vinifera Flavonoid-3-O-Glycosyltransferases.
Expression analysis was performed on each organ using quantitative RT-PCR (n = 3). These graphs show the expression of Vv GT1, Vv GT5, Vv GT6, and Vv FLS1 relative to that of the reference gene, V. vinifera UBIQUITIN2. Means ± se are shown. PN, Pinot Noir; CS, Cabernet Sauvignon; L, leaf; Pt, petiole; Sd, seed; M, mesocarp (fruit flesh); E-y, exocarp (fruit skin)-young, -m (medium), and -f (fully mature).
Flavonoid Analysis
To validate further the functional significance of Vv GT5 and Vv GT6 in the biosyntheses of both the 3-O-glucuronide and the 3-O-glucoside/galactoside of quercetin, the contents of these quercetin 3-O-glycosides in grapevine leaves and berries (cv Pinot Noir, Cabernet Sauvignon, and Shiraz) were analyzed by liquid chromatography–mass spectrometry (LC-MS) and HPLC (Table 4). It must be noted that, for the 3-O-glucoside and the 3-O-galactoside of quercetin, only the sum of both stereoisomers was determined because our analytical systems could not separate these isomers in crude samples. Both isomers are present in the leaves and berry skins (exocarp) of grapevine and in red wines (Hmamouchi et al., 1996; Downey et al., 2007; Castillo-Munoz et al., 2009).
Table 4.
Analysis of Quercetin 3-O-Glycosides in the Leaves and the Berry Skins (Exocarps) of Grapevines
| Cultivar Organ/Tissue | Quercetin 3-O-glucoside + 3-O-galactoside (mg/g fresh weight) | Quercetin 3-O-glucuronide (mg/g fresh weight) |
| Pinot Noir | ||
| Leaves | 3.242 | 9.724 |
| Exocarp | 0.418 | 0.367 |
| Cabernet Sauvignon | ||
| Leaves | 0.045 | 0.255 |
| Exocarp | 0.178 | 0.422 |
| Shiraz | ||
| Exocarp | 0.057 | 0.219 |
| Dried fruits | 0.029 | 0.030 |
Contents of quercetin 3-O-glycosides in grapevine leaves and berry skins were determined by LC-MS and HPLC. For experimental details, see Methods.
Results of this study showed that the leaves and berries of these cultivars each contained quercetin glycosides. In particular, very large amounts of quercetin 3-O-glucuronide and quercetin 3-O-glucoside/galactoside were found in leaves of cv Pinot Noir (9.7 and 3.2 mg/g fresh weight, respectively). Berry skins of both varieties contained appreciable amounts (a total of 600 to 780 μg/g fresh weight) of these quercetin 3-O-glycosides. Moreover, the quercetin glycosides were also observed in dried fruits. These results, along with the biochemical properties and enzyme expression profiles, corroborate the involvement of Vv GT5 and Vv GT6 in the biosyntheses of quercetin 3-O-glucuronide, 3-O-glucoside, and 3-O-galactoside.
Identification of a Residue That Critically Determines the Sugar Donor Specificity of Vv GT5
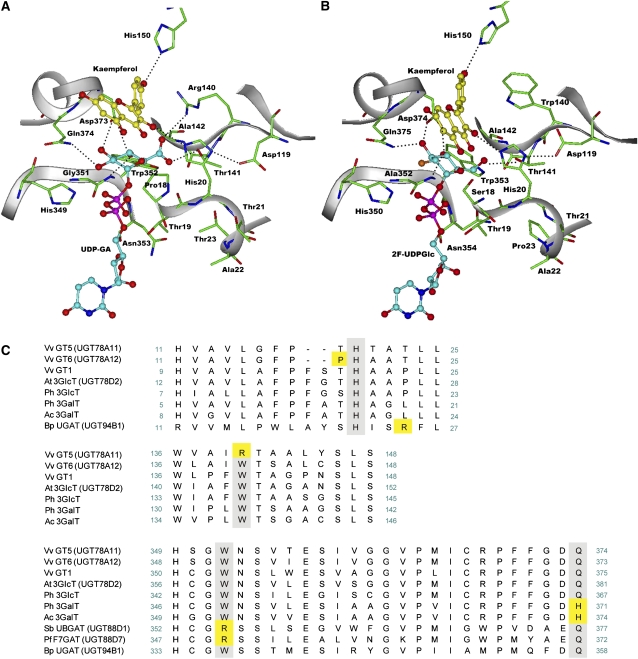
Previous studies of two GATs, Bp UGAT (UGT94B1) of the red daisy flower (Bellis perennis) (Sawada et al., 2005; Osmani et al., 2008) and Pf F7GAT (UGT88D7) of Perilla frutescens (Noguchi et al., 2009a), showed that Arg-25 in Bp UGAT and Arg-350 in Pf F7GAT play an important role in recognition of UDP-GA. These results suggest plasticity of the mechanisms that alter the sugar donor specificity of UGTs. To examine the molecular basis for the specificity of Vv GT5 for UDP-GA, a three-dimensional structural model of Vv GT5 docked with UDP-GA and a flavonol (kaempferol) was constructed using the crystal structure of Vv GT1 as a template (Figure 4A) (Offen et al., 2006). The structural model predicted an interaction between the guanidinium group of Arg-140 of Vv GT5 and the carboxyl group of the glucuronic acid moiety of bound UDP-GA. A sequence comparison showed that Arg-140 of Vv GT5 is replaced by a Trp residue in each UGT examined, which, for example, corresponds to Trp-140 in Vv GT1 and Vv GT6 (Figure 4C). In the reported crystal structure of the ternary complex of Vv GT1 (Figure 4B), Trp-140 does not point toward the bound UDP-2-deoxy-2-fluoroglucose. These observations imply the importance of Arg-140 for recognition of UDP-GA in the Vv GT5–catalyzed glucuronosyl transfer. To validate this possibility, Arg-140 was replaced with a Trp residue, and the catalytic properties of the resultant mutant, R140W, were examined. Unlike the wild-type Vv GT5, the R140W mutant displayed strong glucosyl and galactosyl transfer activities, but no detectable glucuronosyl transfer activity (i.e., the relative activity was <0.01% of the glucosyl transfer activity). These results indicate that the enzyme was converted from a GAT to a bifunctional GlcT/GalT as a result of the single R140W substitution. Kinetic analyses revealed that both the kcat of glucuronosyl transfer and the affinity for UDP-GA were greatly reduced by this substitution (Table 2), while the Km for the glucuronosyl acceptor (quercetin) was only slightly affected. When quercetin was used as a glycosyl acceptor, the kcat values for the glucosyl and galactosyl transfer activities of the mutant were 41 and 6% of the wild-type glucuronosyl transfer activity, respectively (Table 2). The Km values for quercetin in the mutant (9.69 μM for glucosyl and 10.6 μM for galactosyl transfer) were somewhat larger than the Km for wild-type glucuronosyl transfer activity (5.60 μM). The Km values of the mutant for UDP-Glc (77.2 μM) and UDP-Gal (225 μM) were greater than the Km of the wild type for UDP-GA (17.7 μM). Overall, the specificity constants (i.e., the kcat/Km values) for quercetin in glucosyl and galactosyl transfer activities of the mutant were 24 and 3%, respectively, of the specificity constant for wild-type glucuronosyl transfer activity. The specificity constants (with quercetin) for UDP-Glc and UDP-Gal of the mutant were 9.4 and 0.5% of the value for UDP-GA of the wild type. It is noteworthy that, although the wild type showed the highest activity toward quercetin, the R140W mutant showed the highest activity toward kaempferol, with a specificity constant of glucosyl transfer activity (0.531 s−1·μM−1) that was comparable to that of wild-type glucuronosyl transfer activity for the same substrate (0.560 s−1·μM−1).
Figure 4.
Homology Docking and Multiple Alignment of Vv GTs.
(A) A structural model of Vv GT5 docked with UDP-GA and a flavonol (kaempferol).
(B) The crystal structure of Vv GT1 complexed with UDP-2-deoxy-2-fluoroglucose (2F-UDPGlc) and a flavonol (kaempferol).
(C) Multiple alignment of UGTs from V. vinifera and other plants (Arabidopsis thaliana, Petunia hybrida, B. perennis, P. frutescens, Scutellaria baicalensis, and A. cordata). The multiple alignment was performed using a ClustalW program packaged in MACVECTOR 7.2.2 software (Accelrys) (Thompson et al., 1994). The amino acids that are involved in sugar donor specificity are highlighted in yellow.
Analysis of the Specificity Determinant for Sugar Donors in Vv GT6
Because of the successful conversion of Vv GT5 from a GAT to a GlcT/GalT by an R140W substitution (see above) and the close evolutionary relationship predicted between Vv GT5 and Vv GT6, we examined whether the sugar donor specificity of Vv GT6 is converted to that of GAT upon W140R substitution. Thus, we prepared a W140R mutant of Vv GT6 and examined its catalytic activity. When quercetin was used as a glycosyl acceptor, the mutant showed no GlcT, GalT, or GAT activity, indicating that the mutant could not use UDP-Glc, UDP-Gal, or UDP-GA.
Vv GT6 is a native bifunctional enzyme that displays GlcT and GalT activity at comparable levels. Because most cluster I UGTs that have been functionally characterized are monofunctional (in most cases GlcTs), it is of interest to identify the structural characteristics that make Vv GT6 bifunctional. We explored characteristics that are unique to flavonoid GlcTs and those unique to flavonoid GalTs by means of sequence comparison as well as molecular modeling of a variety of flavonoid GlcTs and GalTs. Previous site-directed mutagenesis studies using a flavonoid GlcT and a flavonoid GalT showed that an amino acid residue located at the C-terminal position of the PSPG box sequence (Gln and His, respectively; see Figure 4C) critically determines the sugar donor specificity of these enzymes: Gln at that position is important for the maximal catalytic efficiency of GlcT activity, whereas His is required for GalT activity (Kubo et al., 2004); Vv GT6 has a Gln reside (Gln-373) at this position. Moreover, position 19 of Vv GT6 is uniquely occupied by a Pro residue, whereas the corresponding position in known flavonoid GlcTs and GalTs is occupied by Thr or Ser (Figure 4C). Molecular modeling of a Michaelis complex of Vv GT6 predicted that Pro-19 is in close proximity to the bound sugar moiety and potentially determines the sugar donor specificity of the enzyme.
To examine the effects of these amino acid substitutions on the GlcT/GalT bifunctionality of Vv GT6, we prepared single mutants of Vv GT6 (i.e., Q373H and P19T). The Q373H substitution essentially abolished the GlcT activity of this enzyme (relative activity was 1.4% of wild-type activity). Upon P19T substitution, the ratio of the GlcT and GalT activities of the enzyme (GlcT:GalT = 100:79) was reversed from that of the wild type (GlcT:GalT = 80:100).
To analyze further the effects of the Q373H and P19T substitutions on the preference of Vv GT6 for sugar donors, detailed kinetic studies of these mutants were performed (Table 5). The Q373H substitution of Vv GT6 resulted in a 5.4-fold enhancement of kcat for GalT activity, with a large (29-fold) decrease in the kcat for GlcT activity. This amino acid substitution also increased the Km values for UDP-Glc (5.4-fold), UDP-Gal (11.9-fold), and quercetin (7-fold with UDP-Gal and 2.4-fold with UDP-Glc). As a result, the specificity constant for the galactosyl transfer activity of the mutant (0.23 s−1·μM−1) was comparable to that of the wild-type enzyme (0.30 s−1·μM−1), while the glucosyl transfer activity of the mutant was severely impaired (0.0012 s−1·μM−1 versus 0.083 s−1·μM−1). Thus, bifunctional Vv GT6 was converted into a monofunctional GaT by a single Q373H substitution.
Table 5.
Kinetic Parameters of Vv GT6-Q373H, Vv GT6-P19T, and Vv GT5-R140W-T19P as Analyzed with Quercetin as a Sugar Acceptor
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (s−1 μM−1) |
| Vv GT6-Q373H (GlcT activity) | |||
| Quercetina | 0.026 ± 0.002 | 22.6 ± 14.9 | 0.0012 |
| UDP-Glcb | 157 ± 34 | 0.00017 | |
| Vv GT6-Q373H (GalT activity) | |||
| Quercetina | 5.89 ± 0.74 | 25.1 ± 11.9 | 0.23 |
| UDP-Galb | 439 ± 55 | 0.0134 | |
| Vv GT6-P19T (GlcT activity) | |||
| Quercetina | 0.24 ± 0.02 | 34.9 ± 7.9 | 0.0069 |
| UDP-Glcb | 62.3 ± 9.5 | 0.0038 | |
| Vv GT6-P19T (GalT activity) | |||
| Quercetina | 0.19 ± 0.02 | 21.5 ± 7.3 | 0.0088 |
| UDP-Galb | 118 ± 7.3 | 0.0016 | |
| Vv GT5-R140W-T19P (GlcT activity) | |||
| Quercetina | 1.40 ± 0.03 | 120 ± 59 | 0.012 |
| UDP-Glcb | 251 ± 58 | 0.0056 | |
| Vv GT5-R140W-T19P (GalT activity) | |||
| Quercetina | 0.66 ± 0.06 | 47.3 ± 12 | 0.014 |
| UDP-Galb | 337 ± 21 | 0.0019 | |
Kinetic parameters were determined at pH 7.4 with the appropriate UDP-sugar (1.0 mM), as described in Methods.
100 μM quercetin was used as the sugar acceptor.
The P19T substitution of Vv GT6 resulted in 3.2- and 5.7-fold decreases in the kcat values for GlcT and GalT activity, respectively, with slight increases (2.2- and 3.2-fold) in the Km values for UDP-Glc and UDP-Gal, respectively. Unlike wild-type enzyme, the GlcT activity of the mutant was higher than its GalT activity, judging from the kcat values (i.e., the kcat,GlcT/kcat,GalT value of P19T was 1.26; the ratio for the wild-type enzyme was 0.70) and kcat/Km values [i.e., (kcat/Km)UDP-Glc/(kcat/Km)UDP-Gal of P19T was 2.38; the value of the wild-type enzyme was 0.93; (kcat/Km)GlcT/(kcat/Km)GalT for quercetin for P19T was 0.78 and the value for the wild-type enzyme was 0.27] (Table 6). For comparison, the Vv GT5-R140W-T19P mutant, which mimicked Vv GT6, was prepared, and kcat and kcat/Km values of GlcT and GalT activities of the mutant were compared with those of Vv GT5-R140W (Table 5). The results showed that kcat,GlcT/kcat,GalT and (kcat/Km)UDP-Glc/(kcat/Km)UDP-Gal values of Vv GT5-R140W-T19P (2.12 and 2.95, respectively; Table 6) were lower than the values of Vv GT5-R140W (6.85 and 19.0, respectively), indicating relative enhancement of GalT activity for Vv GT5-R140W following the T19P substitution.
Table 6.
Ratios of Kinetic Parameters of GlcT and GalT Activities
| Enzyme | kcat,GlcT/kcat,GalT | Km,GlcT/Km,GalT | (kcat/Km)GlcT/(kcat/Km)GalT |
| Vv GT6-Q373Ha | 0.0044 | 0.90 (for quercetin) | 0.005 (for quercetin) |
| 0.36 (for UDP-sugar) | 0.013 (for UDP-sugar) | ||
| Vv GT6-P19Ta | 1.26 | 1.62 (for quercetin) | 0.78 (for quercetin) |
| 0.53 (for UDP-sugar) | 2.38 (for UDP-sugar) | ||
| Wild-type Vv GT6b | 0.70 | 2.57 (for quercetin) | 0.27 (for quercetin) |
| 0.77 (for UDP-sugar) | 0.93 (for UDP-sugar) | ||
| Vv GT5-R140Wc | 6.85 | 0.91 (for quercetin) | 7.39 (for quercetin) |
| 0.34 (for UDP-sugar) | 19 (for UDP-sugar) | ||
| Vv GT5-R140W-T19Pa | 2.12 | 2.53 (for quercetin) | 0.86 (for quercetin) |
| 0.74 (for UDP-sugar) | 2.95 (for UDP-sugar) |
Values for the Vv GT6-Q373H, Vv GT6-P19T, and Vv GT5-R140W-T19P mutants were calculated using the data presented in Table 5.
Values for the wild-type enzyme were calculated using the data presented in Table 3.
Values for the Vv GT5-R140W mutant were calculated using the data presented in Table 2.
DISCUSSION
Functional Significance of Vv GT5 and Vv GT6 in Biosynthesis and Structural Diversification of Flavonol Glycosides in Grapevines
In grapevines, the structural diversity of secondary metabolites is, in part, illustrated by the occurrence of a variety of flavonol 3-O-glycosides, which include 3-O-glucuronide, 3-O-glucoside, 3-O-galactoside, and 3-O-rutinoside of quercetin, implying that a variety of UGTs with different sugar donor specificities enhance the structural diversity of flavonols. This study successfully identified two such UGTs (Vv GT5 and Vv GT6), where Vv GT5 is a flavonol-3GAT and Vv GT6 is a UGT that exhibits comparable 3GlcT and 3GalT activity.
A previous study showed relatively high flavonol GlcT activities in the leaves and berries of cv Shiraz as well as in white grapevine lacking anthocyanin (cv Muscat of Alexandria), with activity ratios of flavonol GlcT:anthocyanidin GlcT ranging from 1:1.1 (for cv Muscat of Alexandria) and 1:24 (for cv Shiraz) (Ford et al., 1998). A recombinant Vv GT1 displayed flavonol GlcT activity, which was significantly lower than its activity toward anthocyanins (activity ratio of flavonol GlcT:anthocyanin GlcT = 1:48) and therefore does not account for the flavonol GlcT activity observed in planta (Ford et al., 1998). Because Vv GT6 is highly specific for flavonols and is expressed in the leaves and exocarp of berries, whereas Vv GT1 is preferentially expressed in exocarp (Figure 3), Vv GT6 is responsible for flavonol 3-O-GlcT activity in planta, while Vv GT1 is an UDP-glucose:anthocyanidin 3-O-GlcT (Ford et al., 1998). Thus, the results of this study show that anthocyanidins and flavonols are glycosylated at the same position (3-hydroxy group) by different, but structurally related, UGT enzymes.
The Vv GT5 and Vv GT6 cDNAs were consistently expressed in both the leaves and pigmented berry skins of different cultivars, where quercetin 3-O-glucuronide, quercetin 3-O-glucoside, and quercetin 3-O-galactoside accumulate. The spatial and temporal patterns of Vv GT5 and Vv GT6 cDNA expression were very similar and resembled those of Vv FLS1. These results, along with the fact that Vv GT5 and Vv GT6 genes are located in proximity to one another on the same chromosome, strongly suggest that expression of these two UGT genes is coordinately controlled by common transcription factors in grapevines, which probably include the flavonol-specific MYB transcription factor Vv MYBF1 (Czemmel et al., 2009).
Based on the absence of any appreciable signal sequences for translocation to organelles or secretion in primary structures, it is likely that both Vv GT5 and Vv GT6 are cytosolic enzymes. In this context, the optimal pH for Vv GT5 activity (pH 9.1) was appreciably higher than probable cytosolic pHs (7.0 to 7.5), with a high specificity constant (e.g., 1.28 μM−1·s−1 for quercetin). It is noteworthy, in this regard, that a UGT of Concord grape berries (Vitis labrusca), Vl RSgt, also optimally catalyzes the glucosylation of both flavonoids and trans-resveratrol at pH 9.0, but at pH 6.0, it glucosylates exclusively hydroxybenzoic and hydroxycinnamic acids (Hall and De Luca, 2007). However, Vv GT5 showed no glycosyl transfer activity toward these aromatic carboxylic acids at pH 6.0. The kcat/Km value of Vv GT5 at pH 7.0 (0.029 μM−1·s−1 for quercetin) was ~2% of the value at pH 9.1, which was comparable to the values reported for many other UGTs at their optimum pH. For example, the kcat/Km of a UGT of Glycine max (Gm IF7GlcT, UGT88E3) for genistein is 0.006 μM−1·s−1 (Noguchi et al., 2007). Hence, the specificity constant of Vv GT5 should be sufficiently high in the cytosol to fulfill its biosynthetic role.
Specificity Determinant of Vv GT5 for UDP-GA
The results of biochemical and molecular modeling studies of Vv GT5 and its mutant showed that Arg-140 critically determines the ability of Vv GT5 to use UGP-GA as the sugar donor. This conclusion corroborates the recent hypothesis that an Arg residue proximal to the carboxylate of UDP-GA is the biochemical basis of GATs of both plant and animal origin (Noguchi et al., 2009a). Arg-140 does not correspond to Arg-25 of Bp UGAT (UGT94B1) (Osmani et al., 2008) or Arg-350 of Pf F7GAT (UGT88D7) (Noguchi et al., 2009a) in primary structure, providing another example of convergent evolution of plant GATs. This illustrates the positional plasticity of the crucial Arg residue in acquiring specificity for UDP-GA in UGT catalysis (see Supplemental Figure 2 online).
It is important to note that, from the perspective of kinetics, the change in Vv GT5 sugar donor specificity observed after the R140W substitution arose from changes in both kcat and Km (Table 2). The observed diminution of the kcat value of Vv GT5 GAT activity upon R140W substitution corresponded to destabilization of the transition state of glucuronosyl transfer by 27.1 kJ·mol−1 (see Supplemental Results 1 online). Thus, the predicted Arg-140–mediated salt bridge is also of mechanistic significance in specific stabilization of the transition state of GAT catalysis, by which both rate acceleration and specificity of GAT catalysis are attained. This role of Arg-140 is consistent with a generally accepted theory of enzymatic catalysis, which is called the “transition state fitting theory” (Pauling, 1948; Copeland, 2000; see Supplemental Results 1 online for details). Moreover, in the model of the Vv GT5-R140W docked with ligands (see Supplemental Figure 3 online), due to the absence of Arg-140, the carboxylate of UDP-GA points to His-20, which corresponds to the catalytically important His-20 of Vv GT1 (Offen et al., 2006). This interaction takes His-20 away from the 3-OH group of flavonol substrates and likely impairs the role of His-20. This may also account for the absence of GAT activity in the Vv GT5-R140W mutant.
Molecular modeling also suggested that Arg-140 plays another role in the determination of the sugar donor specificity of Vv GT5, which may account for the emergence of strong GlcT/GalT activities as a result of a single R140W substitution. In the modeled structures of Vv GT5 and of the Michaelis Vv GT5-UDP-Glc complex, it is possible to create a salt bridge between Arg-140 and Asp-119. This Asp residue, which corresponds to Asp-119 of Vv GT1, might play an important role in UGT catalysis (Offen et al., 2006), providing the possibility that the free Vv GT5 enzyme might have latent UGT activity due to an interaction between Asp-119 and Arg-140. Upon UDP-GA binding to the enzyme, the electrostatic interaction of Arg-140 likely shifts from an interaction with Asp-119 to one with the carboxylate group of bound UDP-GA (Figure 4A), thereby permitting Asp-119 to participate in UGT catalysis. However, binding of UDP-Glc (or UDP-Gal) keeps Asp-119 bound to the salt bridge with Arg-140; hence, the enzyme remains latent in terms of GlcT/GalT activity. Therefore, it has been proposed that Arg-140 is important not only because of its role in UDP-GA recognition, but also because it provides a mechanism that keeps GlcT/GalT inactive.
Specificity Determinants of Vv GT6 for Sugar Donors
Judging from the occurrence of Gln at the C-terminal position of the PSPG box sequence (Mato et al., 1998; Miller et al., 1999; Kubo et al., 2004), the bifunctional Vv GT6 could be considered a variant of GlcT with an enhanced GalT activity. Replacement of Gln-373 with His consistently resulted in loss of Vv GT6 activity toward UDP-Glc, without substantial loss of GalT activity, giving rise to a monofunctional GalT (Table 5). By molecular modeling, the effect of the Q373H substitution could be explained in terms of a slight shift in the binding of the sugar donor molecule in the enzyme upon this substitution (see Supplemental Figure 4 online). It is important to note, however, that Vv GT1 also displayed low GalT activity, which was ~8% of its GlcT activity (in terms of kcat/Km value); and unlike the cases of Vv GT6 and other enzymes (Kubo et al., 2004), a Q375H substitution in Vv GT1 does not improve its GalT activity (Offen et al., 2006). Thus, the effect of the Gln/His substitution at that position on sugar donor specificity differs among UGTs. This observation could be explained by the fact that specificity for neutral sugar donors (such as UDP-Glc and UDP-Gal) is not established by a single amino acid residue, but is attained by a combination of multiple amino acid residues, which vary among the different UGTs.
We also showed that Pro-19 of Vv GT6 (Figure 4C) plays a unique role in enhancing the relative GalT activity of this enzyme. The GlcT activity of the P19T mutant of Vv GT6 exceeded its GalT activity (in terms of both kcat and kcat/Km; Table 5), in contrast with the wild-type enzyme, for which GalT activity exceeded GlcT activity (Table 3). In this context, Vv GT5-R140W, which has a primary structure that is very similar to that of Vv GT6, also showed both GlcT and GalT activity (Table 2); however, the GlcT activity of Vv GT5-R140W was higher than its GalT activity (e.g., kcat,GlcT/kcat,GalT = 6.85; Table 6), probably due to the absence of a Pro residue at position 19 (Figure 4C). Consistently, T19P substitution of Vv GT5-R140W resulted in a relative enhancement of its GalT activity (Table 6). Because Pro-19 is adjacent to His-20, which is the proposed catalytic residue of Vv GT1, and is also predicted to be in close proximity to the bound sugar moiety in the modeled structures (for example, see Supplemental Figure 4 online), substitution of Pro-19 with Thr (or another residue) likely affects the preference of this enzyme for sugar donors.
Evolution of Sugar Donor Specificity
Vv GT5 and Vv GT6 constitute a set of very similar genes, which are located in tandem on chromosome 11 (Figure 1). Thus, these genes are likely paralogs, where one gene results from the gene duplication of the other. This is consistent with a theory that explains the evolution of enzyme function (Ober, 2005; Des Marais and Rausher, 2008; Matsuno et al., 2009), which states that a set of enzymes with similar (but different) catalytic functions can be generated by gene duplication. As implicated from the genomic map and the phylogenetic tree (Figure 1), gene duplication of cluster I UGTs appears to have occurred in the grapevine genome, resulting in a small subcluster of functionally differentiated UGTs. The observed structural variations in the primary structures of grapevine Vv GTs (Table 1; see Supplemental Table 1 online) suggest that these genes probably have experienced neofunctionalization (or nonfunctionalization) after gene duplication. Maximum likelihood analysis (Yang, 2007) of the cluster I UGTs (Figure 1) suggested that, in the V. vinifera genome, differentiation of Vv GT genes has more recently occurred in a more positive manner than that of other cluster I enzymes due to either positive Darwinian selection or relaxation of functional constraints (see Supplemental Results 2 online for details of the analysis).
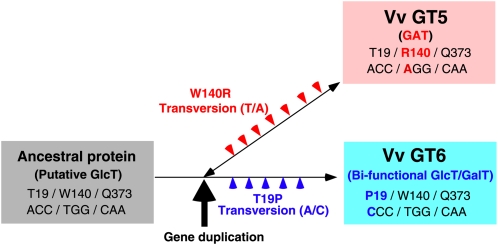
Maximum parsimony analysis (see Supplemental Figure 5 online) indicates that Vv GT5 and Vv GT6 might have been derived from a common ancestral GlcT gene and differentiated via subsequent mutations (Figure 5). The ancestral Trp140 (TGG) residue was replaced by Arg (AGG) in Vv GT5, whereas the ancestral Thr19 (ACC) residue was replaced by Pro (CCC) in Vv GT6 via single nonsynonymous substitutions (see Supplemental Figure 5 online). It is important to note, however, that acquisition of an Arg residue proximal to the carboxylate of UDP-GA is necessary, but not sufficient, for GAT function because the W140R mutant of Vv GT6 was devoid of GAT activity, as in the case of our recent mutational studies of Pf F7GlcT (UGT88A7) and Gm IF7GlcT (UGT88E3) (Noguchi et al., 2009a). It also was noteworthy that the GalT activity of the Vv GT5-R140W mutant was much lower than its GlcT activity and the fact that the T19P mutation of the Vv GT5-R140W mutant enhanced its GalT activity mimicked the evolutionary process of Vv GT6 for acquiring bifunctional specificity (Figure 5). The bifunctionality of Vv GT6 can be regarded as a promiscuous state with respect to sugar donor specificity, as indicated by previous directed evolution studies (Aharoni et al., 2005; Bloom and Arnold, 2009), which show that enzymes tend to go through a promiscuous state prior to neofunctionalization.
Figure 5.
Proposed Gene Duplication Evolutionary Process for a GAT Gene and a Bifunctional GlcT/GalT Gene from a Common Putative GlcT Gene in V. vinifera.
Horizontal arrow and double-headed arrow represent the evolutionary history of the Vv GT genes, with the red and blue arrowheads showing mutational events that occurred only in ancestral Vv GT5 and Vv GT6 genes, respectively. The results of this study suggest that a common ancestral gene of Vv GT5 and Vv GT6 encoded a putative GlcT (gray box), in which positions 19, 140, and 373 were occupied by Thr, Trp, and Gln, respectively. After gene duplication, which is shown by the thick vertical arrow, mutational events occurred in the respective ancestral genes. Among these mutations, a mutation resulting in W140R substitution in the ancestral Vv GT5 gene destined it to evolve into a GAT (pink box), while the mutation resulting in a T19P substitution in the ancestral Vv GT6 gene led to its evolution into a bifunctional GlcT/GalT (light-blue box). The results of mutational studies show that Vv GT5 could be converted into a GlcT/GalT by a R140W mutation, suggesting the reversibility of the evolutionary process in terms of sugar donor specificity, as shown by the double-headed arrow. Our results show that Vv GT6 could no longer be converted into GAT by a W140R mutation.
Finally, although flavonol glycosides in grapevine and grapevine products should be of biological, nutritional, and food chemical significance, how their glycon structures modulate the functional properties and bioactivities of these secondary metabolites remains to be thoroughly examined. Characterization of Vv GT5 and Vv GT6 now make it possible to produce efficiently flavonol 3-O-glucuronides and 3-O-galactosides, in addition to flavonol 3-O-glucosides, in large amounts, facilitating examination of unanswered questions in future studies.
METHODS
Plant Materials
Plant samples of Vitis vinifera cv Cabernet Sauvignon and cv Pinot Noir were obtained at a vineyard in the TOMI NO OKA Winery, Yamanashi, Japan, and those of V. vinifera cv Shiraz were kindly provided by N. Goto-Yamamoto, National Research Institute of Brewing, Hiroshima, Japan.
Chemicals
Quercetin, kaempferol, isorhamnetin, quercetin 3-O-glucoside, quercetin 3-O-galactoside, quercetin 3-O-glucuronide, kaempferol 3-O-glucoside, and isorhamnetin 3-O-glucoside were available from TransMIT Flavonoidforschung. UDP-Glc, UDP-Gal, and UDP-GA were products of Sigma-Aldrich. UDP-rhamnose was a generous gift from Keiko Yonekura-Sakakibara, RIKEN, Yokohama, Japan. Pelargonidin, cyanidin, delphinidin, apigenin, luteolin, (+)-catechin, genistein, naringenin, ferulic acid, caffeic acid, salicylic acid, capsaicin, trans-resveratrol, and esculetin were obtained as described previously (Sawada et al., 2005; Noguchi et al., 2007, 2008, 2009b).
Screening of V. vinifera Flavonoid-3-O-Glycosyltransferase Genes
The V. vinifera cv Pinot Noir (PN42004 genotype) genome database (http://genomics.research.iasma.it/iasma/) provided by Instituto Agrario Samn Michele all’Adige was screened using standard BLAST algorithms (Altschul et al., 1990) and the Vv GT1 sequence (Ford et al., 1998).
Total RNA was prepared from leaves (0.1 g) of V. vinifera cv Cabernet Sauvignon using commercially available kits (FruitMate RNA purification kit and Fast Pure RN kit from TaKaRa Bio). cDNA synthesis was performed using the Superscript first-strand synthesis system for RT-PCR (Invitrogen) starting with total RNA (1 μg) according to the manufacturer’s guidelines. Using the cDNA library as a template, Vv GT5 cDNA was amplified by PCR with ExTaq polymerase (TaKaRa Bio) using the primers 5′-CACCCATATGACTACCACCGCCAGCTCCAT-3′ and 5′-AGATCTCTACTTATTGGTGTCCAAAGGTA-3′. The thermal cycling conditions were 94°C for 3 min followed by 35 cycles of PCR (one cycle consisted of 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min). The PCR product, 1.4 kb in length, was gel purified and cloned into pENTER-Directional-TOPO vector (Invitrogen). The DNA insert was sequenced by the primer walking method using synthetic primers with an Applied Biosystems model 3100 sequencer to confirm the nucleotide sequence of Vv GT5. The Vv GT6 cDNA was obtained essentially as described above except that the PCR primers used were 5′-CACCCATATGACTGCCACCGCGAGCTCCATG-3′ and 5′-AGATCTCTACTTATTGGTACTCAAATGTAACT-3′.
Heterologous Expression and Purification of the Expressed Products
The pENTR-Directional-TOPO vector containing Vv GT5 cDNA was digested with BglII, followed by partial digestion with NdeI to obtain the full-length Vv GT5 cDNA. The Vv GT5 cDNA was ligated into a pET15b vector (Novagen) that had previously been digested with NdeI and BglII to obtain the plasmid pET15b-Vv GT5, which encoded an N-terminal in-frame fusion of Vv GT5 with a His6 tag. Escherichia coli BL21 (DE3) cells were transformed with the resulting plasmid. The Vv GT6 cDNA was excised from the recombinant pENTER-Directional-TOPO vector, as described above. The Vv GT6 cDNA was ligated into a pColdI vector (TaKaRa) that had previously been digested with NdeI and BglII to obtain the plasmid pColdI-Vv GT6, which encoded an N-terminal in-frame fusion of Vv GT6 with a His6 tag. E. coli BL21 cells were transformed with the resulting plasmid.
Heterologous expression of Vv GT5 cDNA was performed as follows. After the transformants were precultured at 37°C for 16 h in Luria-Bertani broth containing 50 μg/mL ampicillin, the culture was used to inoculate the same medium (1000 mL). After cultivating the cells at 37°C until the optical turbidity at 600 nm (OD600) of the culture reached 0.5, isopropyl 1-β-d-thiogalactoside was added to the medium at a final concentration of 0.5 mM, followed by cultivation at 18°C for 20 h. The cells were then harvested by centrifugation (10 min, 5000g). Heterologous expression of Vv GT6 cDNAs was performed as follows. After cultivating the transformant cells at 37°C until the OD600 of the culture reached 0.5, the culture was immediately chilled on ice for 10 min, followed by the addition of isopropyl 1-β-d-thiogalactoside to the medium at a final concentration of 0.5 mM and further cultivation at 15°C for 24 h. The cells were then harvested by centrifugation (10 min, 5000g).
All subsequent operations were conducted at 0 to 4°C. The harvested cells were suspended in buffer A (20 mM HEPES-NaOH, pH 7.5, containing 14 mM 2-mercaptoethanol) supplemented with 20 mM imidazole. The cell suspension was sonicated eight times for 15 min, and the resulting cell debris was removed by centrifugation (15,000g, 15 min) followed by filtration with a 0.22-μm filter (Millipore). The supernatant was applied to a 1-mL His SpinTrap (GE Healthcare Bio-Sciences) that had been equilibrated with buffer A containing 20 mM imidazole, followed by centrifugation (70g, 30 s). The column was washed with 5 mL of equilibration buffer, followed by washing with 5 mL of buffer A containing 100 mM imidazole and then with 5 mL of buffer A containing 500 mM imidazole. The enzyme-containing fractions were concentrated using a Microcon YM-30 ultrafiltration device (Millipore), followed by substitution with buffer A. SDS-PAGE was performed according to the method of Laemmli (1970), and the proteins in the gels were visualized using Coomassie Brilliant Blue R 250.
Phylogenetic Analysis
The nucleotide sequences of Vv GTs and the related UGTs were aligned by taking consideration of codon position using ClustalW bundled in MEGA 4.1 (Thompson et al., 1994; Tamura et al., 2007). All positions containing gaps and missing data were eliminated from the further analysis. The unrooted phylogenetic trees of them were reconstructed by neighbor-joining methods from translated amino acid sequences. The alignment used to generate the tree shown in Figure 1B is available as Supplemental Data Set 1 online. The neighbor-joining tree was reconstructed by MEGA 4.1 with the matrix of the evolutionary distances calculated by Poisson correction for the multiple substitutions. The reliability of reconstructed tree was evaluated by bootstrap test with 1000 replicates.
Test for Positive Selection
To assess the degree of natural selection on Vv GTs, the rate ratio of nonsynonymous to synonymous substitution (ω) was estimated by the codon substitution model implemented in CODEML program of PAML 4.3 package (Yang, 2007). The likelihood analysis of branch-specific model (two-ratio model; model = 2, NS sites = 0) was performed to estimate the ratio ω for the ancestral lineages of Vv GT2~6 (foreground branches) and the ratio ω for all other branches (background branches). The likelihood analysis of the one-ratio model (M0 model; model = 0, NS sites = 0), which assumes the same ratio for all branches, was also performed as a null model comparing with the branch-specific model.
Site-Directed Mutagenesis
According to the previously published method of Noguchi et al. (2007), in vitro mutagenesis of the Vv GT5 and Vv GT6 genes was performed using recombinant PCR with the pENTR-Directional-TOPO vector (Invitrogen) containing the wild-type cDNAs as the templates and the specific mutagenic oligonucleotide primers shown below to obtain the following site-directed mutants: Vv GT5-R140W, Vv GT6-P19T, Vv GT5-R140W-T19P, and Vv GT6-Q373H. The amplified fragments were digested with NdeI and BglII, and the resulting DNA fragments were ligated with pET-15b (Novagen), as described above. Individual mutation was verified by DNA sequencing of both strands: VvGT5-R140W-Fw, 5′-GGGTGGCAATTTGGACTGCTG-3′; VvGT5-R140W-Rv, 5′-CAGCAGTCCAAATTGCCACCC-3′; VvGT5 T19P R140W-Fw, 5′-TTCCCCCCCCATACAGC-3′; VvGT5 T19P R140W-Rv, 5′-TAGCTGTATGGGGGGGG-3′; VvGT6-P19T-Fw, 5′-TTCCCCACCCATGCAGCTA-3′; VvGT6-P19T-Rv, 5′-TAGCTGCATGGGTGGGGAA-3′; VvGT6-S142A-Fw, 5′-ATTTGGACTGCTGCGCTCTGCTAC-3′; VvGT6-S142A-Rv, 5′-TGAGCAGAGCGCAGCAGTCCAAAT-3′; VvGT6-Q373H-Fw, 5′-TTCTTTGGAGATCATTGTATCGACA-3′; VvGT6-Q373H-Rv, 5′-TGTCGATACAATGATCTCCAAAGAA-3′.
Enzyme Assays
The standard reaction mixture (100 μL) consisted of 100 μM sugar acceptor, 1 mM UDP-sugar, 50 mM glycine-NaOH (pH 9.1, for Vv GT5) or 50 mM HEPES-NaOH (pH 7.4, for Vv GT6), 14 mM 2-mercaptoethanol, and enzyme (typically 50 ng). Prior to addition of the enzyme, the mixture was preincubated at 30°C for 15 min, and the reaction was started by addition of the enzyme. After incubation at 30°C for 15 min, the reaction was stopped by the addition of 100 μL of 40% (by volume) acetonitrile containing 0.1% (by volume) trifluoroacetic acid. After centrifugation, the supernatant (100 μL) was subjected to reversed-phase HPLC using a Gilson 305 system equipped with an online Gilson model 231 autosample injector.
HPLC conditions for the analysis of flavonols and their glycosides were as follows: column, YMC J'sphere ODS M80 (4.6 × 150 mm; YMC Co.); flow rate, 0.7 mL/min; solvent A, 0.2% (by volume) formic acid in water; solvent B, 0.2% formic acid in a 9:1 (by volume) mixture of acetonitrile and water. After injection (100 μL) into a column that had been equilibrated with 10% solvent B (by volume), the column was initially developed isocratically with 10% solvent B for 3 min, followed by a linear gradient from 10 to 40% solvent B for 10 min and then by a linear gradient from 40 to 90% solvent B for 10 min. The column was then washed isocratically with 90% solvent B for 1 min, followed by a linear gradient from 90 to 10% solvent B for 1 min. There was a 10-min delay before the next injection to ensure re-equilibration of the column. The chromatograms were obtained with detection at 360 nm.
HPLC conditions for the analysis of anthocyanidins and their glycosides were as follows: column, Asahipak ODP-50 4E (4.6 × 250 mm; Shodex); flow rate, 0.7 mL/min; solvent A, 0.5% (by volume) trifluoroacetic acid in water; solvent B, 0.5% trifluoroacetic acid in a 1:1 (by volume) mixture of acetonitrile and water. After injection (100 μL) into a column that had been equilibrated with 30% solvent B (by volume), the column was initially developed isocratically with 30% solvent B for 3 min, followed by a linear gradient from 30 to 65% solvent B for 10 min and then by a linear gradient from 65 to 100% solvent B for 1 min. The column was then washed isocratically with 100% solvent B for 5 min, followed by a linear gradient from 100 to 30% solvent B for 1 min. The column was equilibrated with 30% solvent B for 10 min before the next injection. The chromatograms were obtained with detection at 520 nm.
Peak identification of each component was confirmed post-run by photodiode array spectroscopy analysis from 220 to 600 nm (for anthocyanin) using a Shimadzu SPD M20A system. Regioisomers of flavonol glucuronides were also identified by their λmax values (see Supplemental Table 2 online). The amounts of flavonoids were determined from peak integration using authentic samples for calibration.
The HPLC conditions used for the analyses of the phenolics flavones, isoflavones, flavanones, coumarins, capsaicin, caffeic acid, ferulic acid, salicylic acid, and trans-resveratrol have been described previously (Noguchi et al., 2008, 2009b).
Enzyme Kinetics
Assays of the initial velocity of UGT-catalyzed reactions were performed under steady state conditions using a standard assay system (see above) and various substrate concentrations. The apparent Km and kcat values and their standard errors for the sugar donors and acceptors in the presence of a saturating concentration of the counter substrate were determined by fitting the initial velocity data to the Michaelis-Menten equation using a nonlinear regression method (Leatherbarrow, 1990).
Quantitative RT-PCR
Total RNA was prepared from an array of organs and tissues, including the leaves, stems, and developing berries, of cv Cabernet Sauvignon and cv Pinot Noir using the RNeasy plant mini kit (Qiagen). Total RNA was treated with DNase I (RNase-free; TaKaRa). First-strand cDNA synthesis was performed starting with 0.5 μg of total RNA using a kit (Transcriptor first-strand cDNA synthesis kit; Roche). The Vv GT5, Vv GT6, Vv GT1, and Vv FLS1 cDNAs in the mixture were quantified by quantitative real-time PCR with the specific primers: Vv GT5, 5′-GCTCCATCTCCTCTGCTCAAA-3′ and 5′-GAAAGCACAAGGTCCTCT-3′; Vv GT6, 5′-GGTTCCCTGGTTGGCAATTT-3′ and 5′-GCACCCGCCCCACAACCTT-3′; Vv GT1, 5′-CCCACCGCCGGTTATACC-3′ and 5′-CGACCGAGGTGGGTTTTCT-3; and, Vv FLS1, 5′-AAACCACCTACTTACAGAGG-3′ and 5′-ACCTAACCCCAGTGACAGAC-3′. Real-time PCR was performed using a 7500 Real-Time PCR system (Applied Biosystems) and a QuantiTect SYBR Green RT-PCR Kit (Qiagen). Relative transcription levels were analyzed by a ΔΔCt method (Applied Biosystems) after normalization to transcription of an internal standard (cDNA encoding V. vinifera ubiquitin 2), which was quantified using the primers 5′-TCCAGGACAAGGAAGGGATTC-3′ and 5′-GCCATCCTCAAGCTGCTTTC-3′. The results are presented as the average ± se of three independent determinations.
Flavonoid Analysis
Weighed quantities of frozen organs and tissues of grapevine (1 to 2 g) were dried in a lyophilizer. The flavonoids were extracted from the dried samples with 8 to 16 mL of 0.1% trifluoroacetic acid (by volume) in a 1:1 mixture of acetonitrile and water as follows: 0.1 g fresh weight/mL with sonication (38 kHz) for 20 min. In the case of dried fruits, a sample (10 g) was pulverized in liquid nitrogen using a mortar. The flavonoids were extracted with 20 mL of 0.1% trifluoroacetic acid (by volume) in a 1:1 mixture of acetonitrile and water. The extract was diluted with water (two equivalents), embedded on a water-equilibrated C-18 Sep-Pak column (Vac20cc; Waters), and eluted with 20 mL of 9:1 mixture of acetonitrile and water after washing with 50 mL water. The extracts were centrifuged at 1000g for 10 min, and the supernatant was filtered through a 0.45-μm filter and analyzed for flavonoids by LC-MS using an LCMS-IT-TOF apparatus (Shimadzu) equipped with a YMC-Pack polymer C18 column (2.0 × 150 mm; s6μm, YMC). HPLC was performed at a flow rate of 0.2 mL/min using 0.1% (by volume) formic acid in water as solvent A and 0.1% formic acid in a 9:1 (by volume) mixture of acetonitrile and water as solvent B. After injection of sample into a column that had been equilibrated with 20% solvent B (by volume), the column was developed with a linear gradient from 20 to 60% solvent B for 15 min and then was washed isocratically with 60% solvent B for 10 min. The chromatograms were obtained with detection over a wavelength range of 220 to 500 nm using an SPD-M10A photodiode array detector (Shimadzu). The online mass spectrometry analyses (scan range, 100 to 1000 m/z) were performed using the interfaces in negative mode with 4.5 kV and in positive mode with 3.5 kV applied to the spray tip. Identification was based on a combination of HPLC retention time and UV and mass spectrometry spectral data. The m/z value for quercetin 3-O-glucuronide was 477.077 [M–H]–. The 3-O-glucoside and galactoside of quercetin in crude samples could not be separated from one another under these analytical conditions; therefore, these compounds were identified as quercetin 3-O-glucoside/galactoside (m/z 463.09 [M–H]–). Flavonols were quantified by absorption at 350 nm.
Homology Modeling
The crystal structure of Vv GT1 was used to construct homology models of Vv GT5 and Vv GT6 using the Homology module installed in the Insight II molecular modeling system (Molecular Simulations). The UDP-Glc bound in Vv GT1 was inserted into the constructed Vv GT5 and Vv GT6 models. The glucose portion of UDP-Glc was replaced with glucuronic acid or galactose moieties as needed. Kaempferol was placed in the sugar acceptor binding site. Two water molecules in the crystal structure were inserted into the model structures to form hydrogen bonds with the substrates, UDP-Glc or kaempferol, and with amino acid residues of Vv GT1. Before optimization, initial model structures of the corresponding mutants (e.g., the R140W mutant of Vv GT5 or the Q373H and P19T mutants of Vv GT6) were constructed by replacing the relative amino acid residues of Vv GT5 and Vv GT6, respectively. Structure optimization of each model was performed using the molecular mechanics and molecular dynamics simulation program Discover3 (Molecular Simulations).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or Genbank/EMBL/DDBJ databases under the following accession numbers: Vv GT1 (UGT78A5), AAB81683 (cv Shiraz); Vv GT2 (UGT78A6), CAO67330 (cv Pinot Noir); Vv GT2-like (UGT78A7), CAO67328 (cv Pinot Noir); Vv GT3 (UGT78A10), CAO43305 (cv Pinot Noir); Vv GT4 (UGT78A8), CAO67332 (cv Pinot Noir); Vv GT4-like (UGT78A9), CAO67329 (cv Pinot Noir); Vv GT5 (UGT78A11), CAN74919 (cv Pinot Noir) and AB499074 (cv Cabernet Sauvignon); Vv GT6 (UGT78A12), AB499075 (cv Cabernet Sauvignon); Vv GT5 promoter (1.5 kb), AB541989; Vv GT6 promoter (1.5 kb), AB541990; At3GlcT (UGT78D2), AAM91139; Ph3GlcT, BAA89008; Ph3GalT, AAD55985; Pf F7GAT (UGT88D7), BAG31948; Pf 7GlcT (UGT88A7), AB362992; Gm IF7GlcT (UGT88E3), BAF64416; Ac GalT, BAD06514; Bp UGAT (UGT94B1), BAD77944; Vl RSgt, ABH03018; Vm 3GalT, AB009370; Ih 3GlcT, BAD83701; Zm 3GlcT (Bronze 1), AAV64215; and V. vinifera ubiquitin 2, CAO48597. Vv GT1 structural information is available in the Protein Data Bank (pdb code 2C1Z)
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Bifunctional Activities of Vv GT6.
Supplemental Figure 2. Superposition of Structural Models of Michaelis Complexes of GATs from Different Phylogenetic Clusters (Vv GT5, Pf F7GAT [UGT88D7 of Perilla frutescens], and Bp UGAT [UGT94B1 of Bellis perennis]).
Supplemental Figure 3. A Structural Model of the R140W Mutant of Vv GT5 Docked with UDP-GA and Kaempferol.
Supplemental Figure 4. A Structural Model of the Q373H Mutant of Vv GT6 Docked with UDP-Gal.
Supplemental Figure 5. Maximum Parsimony Analysis of the Vv GT Cluster.
Supplemental Table 1. Sequence Identity among Vv GT cDNAs.
Supplemental Table 2. HPLC Analysis of Quercetin Monoglucuronides and 3-O-Glucoside.
Supplemental Data Set 1. Text File of the Alignment Used to Generate the Phylogenetic Tree Shown in Figure 1B.
Supplemental Results 1. Thermodynamic Consideration of the Effects of R140W Substitution on Kinetic Parameters of Vv GT5.
Supplemental Results 2. Maximum Likelihood Analysis of Vv GT Genes.
Acknowledgments
We thank N. Goto-Yamamoto (National Research Institute of Brewing, Hiroshima, Japan) for providing a plant sample of V. vinifera cv Shiraz and N. Watanabe and S. Fujimoto (Suntory Liquors Ltd., TOMI NO OKA Winery, Yamanashi, Japan) for the gifts of cv Cabernet Sauvignon and cv Pinot Noir. We thank Keiko Yonekura-Sakakibara (RIKEN, Yokohama, Japan) for providing UDP-rhamnose. We also thank A. Ohgaki (Suntory Wellness), Y. Matsuo, N. Tsuruoka, and H. Toyonaga (Suntory Holdings) for their technical support.
References
- Aharoni A., Gaidukov L., Khersonsky O., Mc Q., Gould S., Roodveldt C., Tawfik D.S. (2005). The ‘evolvability’ of promiscuous protein functions. Nat. Genet. 37: 73–76 [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Baur J.A., et al. (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J.D., Arnold F.H. (2009). In the light of directed evolution: pathways of adaptive protein evolution. Proc. Natl. Acad. Sci. USA 106: 9995–10000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton R. (2001). The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 52: 67–87 [Google Scholar]
- Bowles D.J., Isayenkova J., Lim E.-K., Poppenberger B. (2005). Glycosyltransferases: Managers of small molecules. Curr. Opin. Plant Biol. 8: 254–263 [DOI] [PubMed] [Google Scholar]
- Butterweck V., Jürgenliemk G., Nahrstedt A., Winterhoff H. (2000). Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 66: 3–6 [DOI] [PubMed] [Google Scholar]
- Campbell J.A., Davies G.J., Bulone V., Henrissat B. (1997). A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 326: 929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Munoz N., Gomez-Alonso S., Garcia-Romero E., Gomez M.V., Velders A.H., Hermosin-Gutierrez I. (2009). Flavonol 3-O-glycosides series of Vitis vinifera cv. Petit Verdot red wine grapes. J. Agric. Food Chem. 57: 209–219 [DOI] [PubMed] [Google Scholar]
- Copeland R.A. (2000). Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. (New York: Wiley-VCH; ). [Google Scholar]
- Corder R., Douthwaite J.A., Lees D.M., Khan N.Q., Viseu Dos Santos A.C., Wood E.G., Carrier M.J. (2001). Endothelin-1 synthesis reduced by red wine. Nature 414: 863–864 [DOI] [PubMed] [Google Scholar]
- Corder R., Mullen W., Khan N.Q., Marks S.C., Wood E.G., Carrier M.J., Crozier A. (2006). Red wine procyanidins and vascular health. Nature 444: 566. [DOI] [PubMed] [Google Scholar]
- Coutinho P.M., Deleury E., Davies G.J., Henrissat B. (2003). An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328: 307–317 [DOI] [PubMed] [Google Scholar]
- Czemmel S., Stracke R., Weisshaar B., Cordon N., Harris N.N., Walker A.R., Robinson S.P., Bogs J. (2009). The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 151: 1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais D.L., Rausher M.D. (2008). Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature 454: 762–765 [DOI] [PubMed] [Google Scholar]
- Downey M.O., Mazza M., Krstic M.P. (2007). Development of a stable extract for anthocyanins and flavonols from grape skin. Am. J. Enol. Vitic. 58: 358–364 [Google Scholar]
- Ford C.M., Boss P.K., Høj P.B. (1998). Cloning and characterization of Vitis vinifera UDP-glucose:flavonoid 3-O-glucosyltransferase, a homologue of the enzyme encoded by the maize Bronze-1 locus that may primarily serve to glucosylate anthocyanidins in vivo. J. Biol. Chem. 273: 9224–9233 [DOI] [PubMed] [Google Scholar]
- Fujita A., Goto-Yamamoto N., Aramaki I., Hashizume K. (2006). Organ-specific transcription of putative flavonol synthase genes of grapevine and effects of plant hormones and shading on flavonol biosynthesis in grape berry skins. Biosci. Biotechnol. Biochem. 70: 632–638 [DOI] [PubMed] [Google Scholar]
- Hall D., De Luca V. (2007). Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 49: 579–591 [DOI] [PubMed] [Google Scholar]
- Heller W., Forkmann G. (1994). Biosynthesis of flavonoids. The flavonoids, Harbone J.B., (Washington, DC: Chapman & Hall/CRC; ), pp. 499–535 [Google Scholar]
- Hmamouchi M., Es-Safi N., Lahrichi M., Fruchier A., Essassi E.M. (1996). Flavones and flavonols in leaves of some Moroccan Vitis vinifera cultivars. Am. J. Enol. Vitic. 47: 186–192 [Google Scholar]
- Jürgenliemk G., Boje K., Huewel S., Lohmann C., Galla H.-J., Nahrstedt A. (2003). In vitro studies indicate that miquelianin (quercetin 3-O-β-D-glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med. 69: 1013–1017 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Nishikawa T., Shiba Y., Saito S., Murota K., Shibata N., Kobayashi M., Kanayama M., Uchida K., Terao J. (2008). Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries. J. Biol. Chem. 283: 9424–9434 [DOI] [PubMed] [Google Scholar]
- Kubo A., Arai Y., Nagashima S., Yoshikawa T. (2004). Alteration of sugar donor specificities of plant glycosyltransferases by a single point mutation. Arch. Biochem. Biophys. 429: 198–203 [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R.J. (1990). Using linear and non-linear regression to fit biochemical data. Trends Biochem. Sci. 15: 455–458 [DOI] [PubMed] [Google Scholar]
- Mato M., Ozeki Y., Itoh Y., Higeta D., Yoshitama K., Teramoto S., Aida R., Ishikura N., Shibata M. (1998). Isolation and characterization of a cDNA clone of UDP-galactose: flavonoid 3-O-galactosyltransferase (UF3GaT) expressed in Vigna mungo seedlings. Plant Cell Physiol. 39: 1145–1155 [DOI] [PubMed] [Google Scholar]
- Matsuno M., et al. (2009). Evolution of a novel phenolic pathway for pollen development. Science 325: 1688–1692 [DOI] [PubMed] [Google Scholar]
- Matus J.T., Loyola R., Vega A., Pena-Neira A., Bordeu E., Arce-Johnson P., Alcalde J.A. (2009). Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 60: 853–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern P.E., Hartung U., Badler V., Glusker D.L., Exner L.J. (1997). The beginnings of wine making and viniculture in the ancient Near East and Egypt. Expedition 39: 3–21 [Google Scholar]
- Miller K.D., Guyon V., Evans J.N., Shuttleworth W.A., Taylor L.P. (1999). Purification, cloning, and heterologous expression of a catalytically efficient flavonol 3-O-galactosyltransferase expressed in the male gametophyte of Petunia hybrida. J. Biol. Chem. 274: 34011–34019 [DOI] [PubMed] [Google Scholar]
- Noguchi A., Horikawa M., Fukui Y., Fukuchi-Mizutani M., Iuchi-Okada A., Ishiguro M., Kiso Y., Nakayama T., Ono E. (2009a). Local differentiation of sugar donor specificity of flavonoid glycosyltransferase in Lamiales. Plant Cell 21: 1556–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A., et al. (2009b). Identification of an inducible glucosyltransferase from Phytolacca americana L. cells that are capable of glucosylating capsaicin. Plant Biotechnol. 26: 285–292 [Google Scholar]
- Noguchi A., Saito A., Homma Y., Nakao M., Sasaki N., Nishino T., Takahashi S., Nakayama T. (2007). A UDP-glucose:isoflavone 7-O-glucosyltransferase from the roots of soybean (Glycine max) seedling. J. Biol. Chem. 282: 23581–23590 [DOI] [PubMed] [Google Scholar]
- Noguchi A., Sasaki N., Nakao M., Fukami H., Takahashi S., Nishino T., Nakayama T. (2008). cDNA cloning of glycosyltransferases from chinese wolfberry (Lycium barbarum L.) fruits and enzymatic synthesis of a catechin glucoside using a recombinant enzyme (UGT73A10). J. Mol. Catal., B Enzym. 55: 84–92 [Google Scholar]
- Ober D. (2005). Seeing double: Gene duplication and diversification in plant secondary metabolism. Trends Plant Sci. 10: 444–449 [DOI] [PubMed] [Google Scholar]
- Offen W., Martinez-Fleites C., Yang M., Kiat-Lim E., Davis B.G., Tarling C.A., Ford C.M., Bowles D.J., Davies G.J. (2006). Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 25: 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E., Ruike M., Iwashita T., Nomoto K., Fukui Y. (2010). Co-pigmentation and flavonoid glycosyltransferases in blue Veronica persica flowers. Phytochemistry 71: 726–735 [DOI] [PubMed] [Google Scholar]
- Osmani S.A., Bak S., Imberty A., Olsen C.E., Moller B.L. (2008). Catalytic key amino acids and UDP-sugar donor specificity of a plant glucuronosyltransferase, UGT94B1: Molecular modeling substantiated by site-specific mutagenesis and biochemical analyses. Plant Physiol. 148: 1295–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. (1948). Nature of forces between large molecules of biological interest. Nature 161: 707–709 [DOI] [PubMed] [Google Scholar]
- Price S.F., Breen P.J., Valladao M., Watson B.T. (1995). Cluster sun exposure and quercetin in Pinot noir grapes and wine. Am. J. Enol. Vitic. 46: 187–194 [Google Scholar]
- Renaud S., de Lorgeril M. (1992). Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339: 1523–1526 [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki H., Ichimaida F., Yamaguchi M.A., Iwashita T., Fukui Y., Hemmi H., Nishino T., Nakayama T. (2005). UDP-glucuronic acid:anthocyanin glucuronosyltransferase from red daisy (Bellis perennis) flowers. J. Biol. Chem. 280: 899–906 [DOI] [PubMed] [Google Scholar]
- Schwarz M., Picazo-Bacete J.J., Winterhalter P., Hermosín-Gutiérrez I. (2005). Effect of copigments and grape cultivar on the color of red wines fermented after the addition of copigments. J. Agric. Food Chem. 53: 8372–8381 [DOI] [PubMed] [Google Scholar]
- Soundararajan R., Wishart A.D., Rupasinghe H.P., Arcellana-Panlilio M., Nelson C.M., Mayne M., Robertson G.S. (2008). Quercetin 3-glucoside protects neuroblastoma (SH-SY5Y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis. J. Biol. Chem. 283: 2231–2245 [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- The French-Italian Public Consortium for Grapevine Genome Characterization (2007). The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449: 463–467 [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T., Jones P. (2000). Glycosyltransferases in plant natural product synthesis: Characterization of a supergene family. Trends Plant Sci. 5: 380–386 [DOI] [PubMed] [Google Scholar]
- Wang X. (2009). Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 583: 3303–3309 [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K., Tohge T., Niida R., Saito K. (2007). Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. J. Biol. Chem. 282: 14932–14941 [DOI] [PubMed] [Google Scholar]
- Yang Z. (2007). PAML 4: A program package for phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24: 1586–1591 [DOI] [PubMed] [Google Scholar]
- Yoshitama K., Ishikura N., Fuleki T., Nakamura S. (1992). Effect of anthocyanin, flavonol co-pigmentation and pH on the color of the berries of Ampelopsis brevipedunculata. J. Plant Physiol. 139: 513–518 [Google Scholar]