Abstract
We have previously demonstrated that cyclothiazide (CTZ) is a potent convulsant drug inducing robust epileptiform activity in hippocampal neurons both in vitro and in vivo. Here we further establish an animal model for CTZ-induced behavioral seizures in freely moving rats. Microinjection of CTZ into left ventricle dose-dependently induced robust seizure behaviors within three hours after administration. At doses of 0.75 μmol, CTZ induced Racine score IV-V seizure behaviors in 71% (n=14) of the rats tested. In addition, CTZ also induced epileptiform EEG activity accompanying behavioral seizures. The convulsant action of CTZ on both behavior and EEG was blocked by pre-treatment with clinical anticonvulsant drug diazepam (n=5). In conclusion, our results demonstrate that CTZ is capable of inducing behavioural seizures in intact animals. Since CTZ acts on both GABAergic and glutamatergic systems, this new animal epilepsy model will be useful for anticonvulsant drug testing and general epilepsy research.
Keywords: Cyclothiazide, seizure behavior, epileptiform EEG, freely moving rats
Introduction
Epilepsy is a chronic neurological disorder that affects approximately 1% of the population globally (Browne & Holmes, 2001). Common features of epilepsy include the manifestation of recurrent seizures due to abnormally synchronized hyperexcitatory activity (Prince and Connors, 1986). Several animal epilepsy models have been developed, including chemical models using pilocarpine and kainic acid (KA) (Nadler et al.,1978; Ben-Ari & Lagowska,1978; Truski et al., 1983, 1984). However, human epilepsy is a complex syndrome with a variety of causes, and it is important to develop diversified animal models to fully understand the cellular mechanisms of epilepsy.
Cyclothiazide (CTZ) not only blocks AMPA receptor desensitization (Patneau et al.1993; Trussell et al. 1993; Yamada & Tang, 1993; Zorumski et al. 1993; Barnes-Davies & Forsythe, 1995; Mennerick& Zorumski, 1995), but also inhibits GABAA receptors (Deng & Chen, 2003). By enhancing excitatory glutamatergic neurotransmission (Diamond & Jahr, 1995; Bellingham & Walmsley, 1999; Ishikawa & Takahashi, 2001) and suppressing inhibitory GABAergic neurotransmission (Deng & Chen, 2003), CTZ has been shown to be a potent convulsant drug (Qi et al., 2006). Furthermore, the BDNF-TrkB receptor signaling pathway appears to mediate the CTZ-induced epileptiform activity (Wang et al., 2009). However, whether CTZ can act as a convulsant to establish a useful behavioral seizure model is still not explored.
In this study, we report that microinjection of CTZ into the lateral ventricle induced typical seizure behaviors in freely moving rats. Accompanying the seizure behaviors, we found that electroencephalograph (EEG) recording also showed robust epileptiform activities within three hours of drug injection. Thus, CTZ-induced animal epilepsy model is potentially useful for epilepsy research, especially in unraveling the mechanisms relating to glutamatergic and GABAergic neurotransmission during epileptogenesis.
Results
CTZ dose-dependently induced seizure behaviors in rats
All the behavioral experiments were performed on freely moving rats at least 5 days after initial cannula implantation surgery. After surgery recovery, CTZ (0.25 μmol in 5 μL DMSO, once per day for consecutive 1-3 days) or DMSO alone (5 μL, once per day for 3 consecutive days) was slowly injected into the left ventricle via pre-implanted cannula in a 5 - 10 min time window. Consistent with our hypothesis, CTZ induced typical seizure behaviors in the majority of rats tested and in a dose-dependent manner (Table 1, Supplemental movie 1). Ranging from 10 to 30 minutes after CTZ injection, animals displayed abnormal behaviors such as rotation, salivating, eye blinking, and ear moving, followed by progressing to generalized seizures such as head nodding, unilateral forelimb clonus, rearing with bilateral forelimb clonus, rearing and falling, corresponding to Racine score II to V (Fig 1). All the animals in control group treated with DMSO didn't show such apparent seizure behaviors, except 2 out of 8 animals exhibiting facial automatisms (Racine I). All animals didn't exhibit exophthalmus after CTZ or DMSO i.c.v. injection.
Table 1.
CTZ induced dose-dependent seizure behavior in rats
| Treatment | DMSO | CTZ |
DIZ+CTZ | ||
|---|---|---|---|---|---|
| 0.25μmol | 0.5μmol | 0.75μmol | |||
| n | 8 | 5 | 4 | 14 | 5 |
| Seizure number | |||||
| Stage II | - (0/8) | - (0/5) | 15 (1/4) | 3.8±1.4 (10/14)* | 3 (1/5) |
| Stage III | - (0/8) | 5 (1/5) | 2.7±1.7 (3/4) | 6.9±2.4 (12/14)* | 1 (1/5)# |
| Stage IV V | - (0/8) | - (0/5) | 1 (1/4) | 3.0±0.8 (10/14)** | - (0/5)# |
| | |||||
| Seizure duration (s) | |||||
| Stage II | - (0/8) | - (0/5) | 505 (1/4) | 95.9±42.5 (10/14) | |
| Stage III | - (0/8) | 39 (1/5) | 29.7±17.2 (3/4) | 63.2±28.6 (12/14)* | |
| Stage IV V | - (0/8) | - (0/5) | 12.0 (1/4) | 83.5±36.8 (10/14)* | |
| | |||||
| Seizure score | 0.3±0.2 | 0.6±0.6 | 2.5±0.9 | 3.9±0.4** | 0.6±0.6## |
Group data on CTZ dose dependent induced seizure behavior number, siezure duration and seizure score, in compare with DMSO control and anticonvulsant drug diazepam pretreatment.
P<0.05
P<0.005 in compare with DMSO control
P<0.05
P<0.005 in compare with CTZ 0.75 μmol group. (the blankets in the table indicate the number of rats responded to the drug verses number of rats tested)
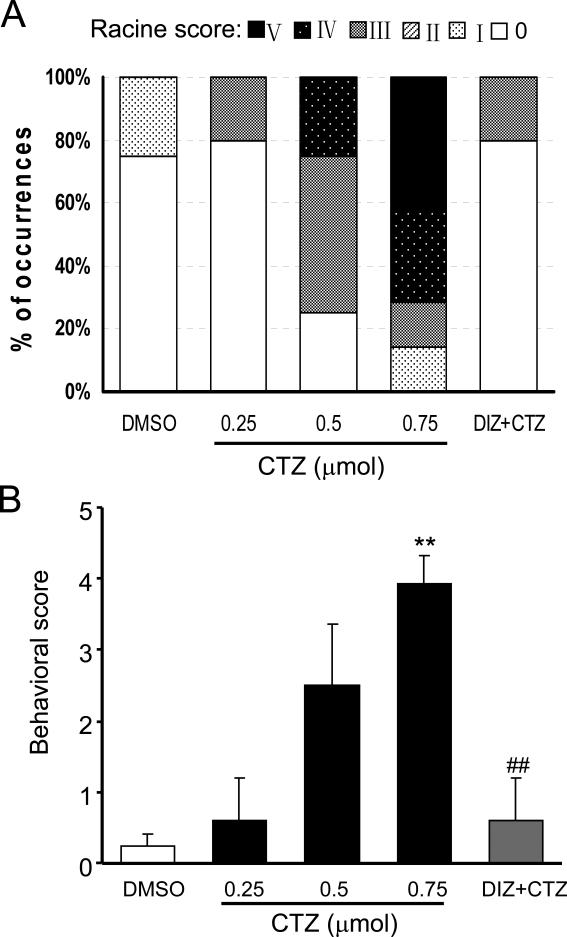
Fig. 1. Dose-dependent effect of CTZ in inducing seizure behaviors in freely moving rats.
Bar histograms showing (A) the percentage of rats had Racine score I - V behavior and (B) the behavioral seizure score of the animals in DMSO control (n=8), CTZ at 0.25 μmol (n=5), 0.5 μmol (n=4) and 0.75 μmol (n=14), as well as CTZ (0.75μmol) after diazepam (10 mg kg-1, n=5) groups. ** P<0.005 compared to the control group; ## P<0.005 compared to the CTZ at 0.75 μmol group.
CTZ-induced seizure behaviors were dose-dependent. Higher doses of CTZ induced higher Racine behavior grade (Fig. 1 and Table 1). None of the seizure behaviors at Racine grade II or above were observed in DMSO control group (n=8), and only 1 rat in 0.25 μmol group (n=5) had Racine grade III behavior (20%). In contrast, in 0.5 μmol (n=4) drug testing group, CTZ induced Racine grade III, IV, and V behaviors in 50%, 25%, and 0%, respectively; and in 0.75 μmol (n=14) group, CTZ induced Racine grade III, IV, and V behaviors in 14%, 29%, and 43%, respectively (Fig 1). The seizure score, calculated according to Racine behavioral grade (see the method section and Zhao et al., 2008), showed a dose-dependent increase after CTZ administration. CTZ at medium and high doses induced seizure score of 2.5±0.9 (n=4, 0.5 μmol) and 3.9±0.4 (n=14, 0.75 μmol), respectively. The score of 0.75 μmol group was significantly higher (P<0.001) than that of DMSO control group (seizure score of 0.3±0.2, n=8) (Table 1, Fig 1).
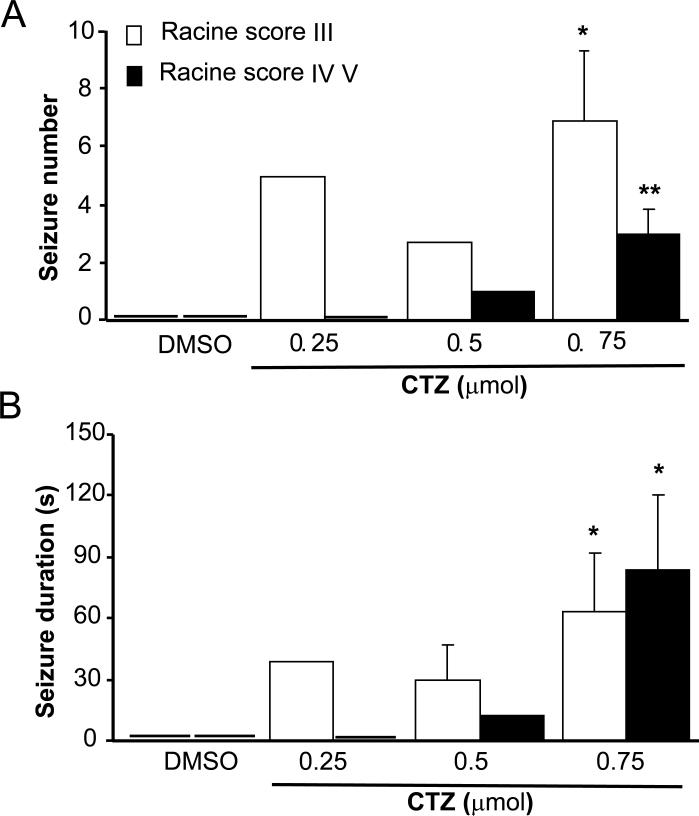
In addition to inducing seizures in higher percentage of tested rats, high doses of CTZ also induced longer duration and more frequent seizure behaviors during our 3-hour testing period. Quantitative analysis revealed that CTZ dose-dependently increased both the seizure number and seizure duration (Table 1 and Fig 2). On average, CTZ at 0.75 μmol dose (n=14) induced Racine grade III behavior 6.9±2.4 times and Racine grade IV-V behavior 3.0±0.8 times in 3 hrs, significantly higher than that of DMSO group (0 times for either grade III or grade IV-V, n=8; P<0.02 and P<0.005, respectively). Furthermore, seizure duration for Racine grade III and IV-V behavior induced by CTZ at 0.75 μmol dose (n=14) were 63.2±28.6 and 83.5±36.8 seconds, respectively, also significantly longer than that of DMSO control group (n=8, P<0.05).
Fig 2. Behavioral characterization of seizures elicited by CTZ in freely moving rats.
Bar histograms showing the group data of CTZ-induced seizure in freely moving rats on (A) seizure numbers, and (B) seizure duration. * P<0.05, **P<0.005 compared to DMSO control group.
CTZ induced robust epileptiform EEG activity in freely moving rats
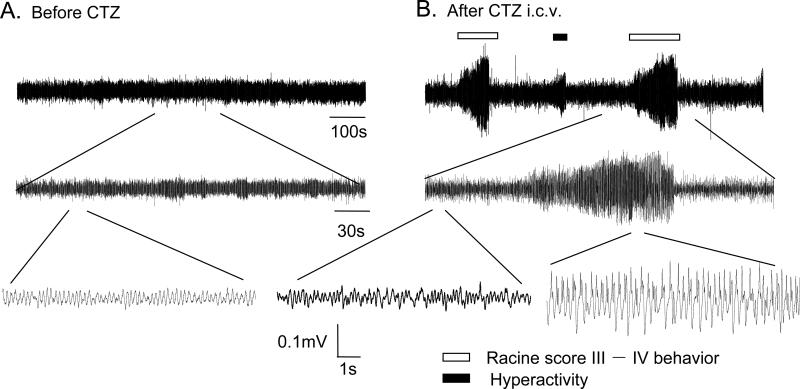
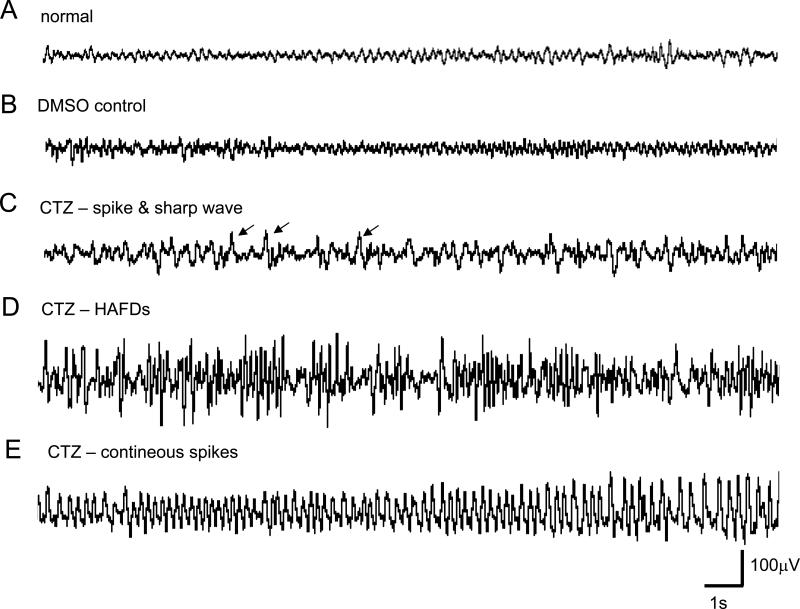
In addition to the observation of seizure behavior induced by CTZ, we also studied the EEG response following high dose of CTZ injection in freely moving rats. CTZ (0.25 μmol, i.c.v., once per day for 3 consecutive days) induced epileptiform EEG activity associated with typical seizure behaviors (Fig 3). During behavioral seizures, EEG displayed high amplitude bursting activities which were never observed before CTZ injection (Fig 3A). In fact, CTZ induced several distinct discharge forms of EEG (Fig 4). DMSO alone did not change the EEG discharges. However, CTZ injection led to epileptiform activities including isolated spikes or sharp waves (interictal discharges), HAFDs, and continuous spikes with high amplitude (ictal discharges) (Fig 4).
Fig. 3. Representative traces showing epileptiform EEG activities associated with behavioral seizures in a freely moving rat.
Traces showing the original EEG recordings (A) before and (B) after CTZ injection in one anaesthetized rat. Part of the top traces taken from indicated area were expanded to illustrate the detail (middle and bottom traces). Seizure behavior-associated epileptiform EEG activity was marked with bar on top of the traces.
Fig. 4. Representative example of epileptiform EEG patterns elicited by CTZ.
(A) Control EEG recording; (B) EEG recording after DMSO administration; (C-E) EEG recording after CTZ administration: (C) spike or sharp wave (indicated by arrows), (D) HAFDs, (E) continuous spikes with high amplitude (> 15 s).
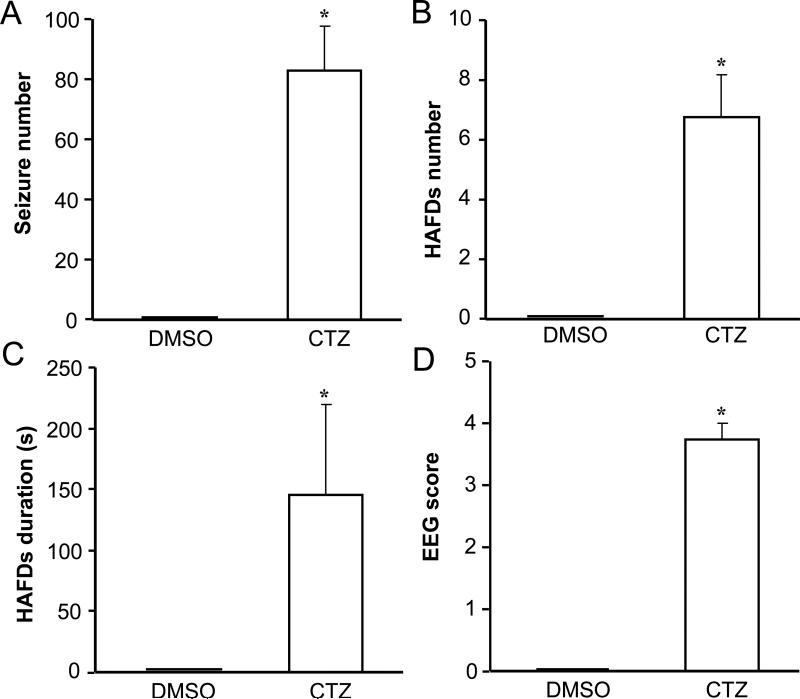
CTZ at 0.75 μmol (n=4) induced epileptiform EEG activities with an average seizure numbers of 83.0±14.6, HAFD numbers of 6.8±1.4, and HAFD duration of 145.3±74.3 s, all significantly more than those from the DMSO group (0 for each component; n=4, P<0.015, P<0.02, and P<0.015, respectively) (Fig 5). The convulsive EEG score of 3.8±0.3 in CTZ group was also significantly higher than that of the DMSO control group (0, P<0.005), which was consistent with the seizure behaviors of animals. In addition, the episodes of HAFDs and continuous spikes with high amplitude were highly coincident with the behavioral seizures with Racine grade III or more (Fig 3).
Fig. 5. Typical electroencephalographic characteristics of seizures elicited by CTZ.
Bar histograms showing the group data of DMSO control and CTZ-induced (0.75 μmol, n=8) epileptiform EEG components of (A) spontaneous seizure number, (B) HAFDs number, (C) HAFDs duration, (D) EEG score. *P<0.05, **P<0.005 compared to the DMSO control group.
Effects of diazepam on CTZ-induced seizure activity
In a separate group of rats (n=5), CTZ (0.25 μmol for 3 consecutive days, total dose of 0.75 μmol) was injected after a pre-injection of diazepam each day to study whether CTZ-induced seizure could be inhibited by a clinical anticonvulsant drug. Diazepam (10 mg kg-1, i.p.) administered 10 min before CTZ injection prevented the convulsant action of CTZ, with only 1 rat showing a short duration of Racine III behavior in 5 rats tested (20% rats to Racine III), significantly less than that of CTZ alone (86% rats showed Racine III or above seizure behaviors, n=14, P<0.05) (Fig 1). The seizure score in diazepam-pretreated animals was significantly reduced from 3.9±0.4 (n=14, CTZ) to 0.6±0.6 (n=5, diazepam+CTZ) (P<0.005). Therefore, result indicates that GABA system is involved in CTZ induced seizure activities, as previously demonstrated by in vitro electrophysiological experiments (Deng and Chen 2003; Qi et al., 2006).
Discussion
In this study, we report a new animal epilepsy model induced by intraventricular administration of CTZ. Our results demonstrate that, in freely moving rats, CTZ dose-dependently induces typical seizure behaviors, which are associated with typical epileptiform EEG.
CTZ enhances excitatory glutamatergic neurotransmission (Diamond & Jahr, 1995; Bellingham &Walmsley, 1999; Ishikawa & Takahashi, 2001) and suppresses inhibitory GABAergic neurotransmission (Deng & Chen, 2003), resulting into a one-way tilt toward hyperexcitation. Our recent electrophysiological studies demonstrated that CTZ can induce epileptiform activity in rat hippocampal neurons both in vitro and in vivo (Qi et al. 2006, Wang et al., 2009), leading us to hypothesize that CTZ is capable of inducing behavioral seizures in animals. Indeed, we found that CTZ is a potent convulsant to elicit seizure behaviors such as facial automatisms, WDS, forelimb clonus, rearing and falling. Such behavioral seizures induced by CTZ may be due to a significant tilt of the balance between excitation and inhibition after CTZ's dual effects on both excitatory and inhibitory neurotransmission (Deng & Chen, 2003).
In current experiments, animals received CTZ at 3 different regimes: 0.25 μmol injection for either once, twice, or three times. In our initial testing experiments, rats received a single bolus injection (slowly) of either 0.5 or 1 μmol CTZ exhibited running, jumping, and generalized tonic-clonic seizure with very short latency after CTZ injection, and led to animal death within 1 hour (n=4). When we reduced the CTZ dose to a single injection of 0.25 μmol, it only induced low Racine score seizure behaviors. However, after repeated CTZ administration at the same dose (0.25 μmol) next day or the day after, high Racine score behaviors were observed. We found that after 3 consecutive administration of CTZ at 0.25 μmol (total of 0.75 μmol), 86% of the rats showed Racine score III and above behaviors and 71% of rats had Racine score IV and V behaviors. Interestingly, with this experimental regime, CTZ did not induce any animal death during our 3-day experimental period, even though the majority of the rats had Racine score IV or V behavior. This phenomenon may suggest that our CTZ-induced seizure animal model has advantage over other classical chemical-induced animal models, such as KA, PTZ, and pilocarpine models, which all showed high mortality (Ben-Ari & Lagowska,1978; Kebriaeezadeh, 2008; Nadler et al., 1978; Truski, 1983, 1984; Zhao et al., 2008). This is consistent with our previous in vitro study showing that CTZ almost induced no cell death in cultured hippocampal neurons but KA induced a significant cell death (Qi et al., 2006). The low mortality in comparison to the other models makes it more suitable for seizure research and anticonvulsant drug test.
The CTZ-elicited seizure model had dose-dependent characteristics. Low-dose CTZ (0.25 μmol) did not elicit apparent behavioral seizure while high dose (0.75 μmol) induced animal limbic motor seizures and generalized seizures, together with epileptiform electroencephalographic activity. In a number of animals, we also administered with 1 μmol CTZ (0.25 μmol in 4 consecutive days) and found that 6 out of 8 animals were fully kindled (score IV or V), similar to the 0.75 μmol dose. Thus, the injection of 0.25 μmol CTZ for 3-times is a suitable administration regime for establishing behavioral seizure models.
In previous studies, CTZ administered either by microinjection into the area tempestas (Fornai et al., 2005) or by microdialysis into dorsal hippocampi (Fedele, 1997) failed to induce seizures, except some “wet dog shakes” in the later study. The simple explanation for the discrepancy is the dose of CTZ used and the route injected. In our current study, a single injection of 0.25 μmol into the left ventricle is equivalent to a concentration of 100 μM in cerebral fluid (~2 ml), much higher than the dose of 1.2 nmol CTZ used for microinjection. The high concentration of CTZ in the cerebral fluid will certainly affect many more brain regions than that of low dose and local microinjection. Thus, it is not surprise that our current experimental regime revealed the true nature of CTZ as a potent convulsant for inducing seizure behaviors predicated from our previous electrophysiological studies (Qi et al., 2006, Wang et al., 2009). Many brain areas could be affected by the i.c.v. injection of the CTZ, and our dye injection experiments indeed revealed that the drug was diffused to a wide range of brain structures surrounding the whole ventricles. Thus it is difficult to pinpoint - the original site(s) where seizure is induced by CTZ in the current study. However, our previous in vivo electrophysiological results have demonstrated that CTZ microinjected into the left lateral ventricle, similar to current experimental regime, induced progressive epileptiform activities in hippocampal CA1 neurons (Qi et al., 2006; Wang et al., 2009) and the first epileptic sign (double population spike peaks) could be observed as early as 6 min after CTZ injection (Qi et al., 2006). Thus we hypothesize that the initiation site of the CTZ-induced seizure is within hippocampus. However, we cannot rule out the possibility of other brain regions contributing to the seizure behaviors observed in this study, which will be further studied using multiple electrophysiological techniques in freely moving animals.
As a newly developed animal seizure model, CTZ model is different from and has advantage over other chemical-induced seizure animal models, besides mortality mentioned above. CTZ acts as a neuromodulator and concurrently affects both glutamatergic and GABAergic neurotransmission systems to induce seizures (Patneau et al.1993; Trussell et al. 1993; Yamada & Tang, 1993; Zorumski et al. 1993; Barnes-Davies & Forsythe, 1995; Mennerick & Zorumski, 1995; Deng & Chen, 2003). In contrast, kainic acid and ibotenic acid induce seizures as glutamate receptor agonists, and PTZ (pentylenetetrazole) elicits seizures as an antagonist of GABAA receptors. Thus, CTZ model seems to be more versatile in regulating neural network activities and useful for identifying new therapeutic approaches for epilepsy.
In conclusion, CTZ is a potent convulsant capable of inducing animal epilepsy with typical seizure behaviors and epileptiform EEG activities. This new animal seizure model, due to its modulation of both glutamatergic and GABAergic transmission, may shed new light in the research of epilepsy pathogenesis and help screening new antiepileptic drugs.
Experimental Procedure
Animals
The behavioral and electroencephalographic experiments were performed on male Sprague Dawley rats weighing 250-280g (supplied by Research Institute of Surgery, Chinese Third Military Medical University, Congqing, China). All animals were maintained in an air-conditioned room with controlled temperature at 23±1°C under a light/dark cycle with lights on from 7:00 am to 7:00 pm and given food and water ad libitum. They were housed separately in plastic cages. All experiments were carried out under the approval of the Institutional Committees of Laboratory Animals, Chongqing Technology and Business University and Fudan University, and in accordance with Chinese governmental regulation.
Surgery
CTZ cannot pass through blood-brain barrier. Thus, our studies were carried out by intra-ventricle injection of CTZ. The animals were anaesthetized with sodium pentobarbital (60 mg kg-1, i.p.) and mounted in a stereotaxic apparatus with body temperature maintained at 37°C with a Harvard homeothermic blanket. Scalp was removed and skull was exposed. A little bur hole was made, and the tip of the guide cannula (22GA) was placed into left lateral ventricle (AP -0.3 mm, ML 1.3 mm, DP 4.0 mm) (Qi et al., 2006, Wang et al., 2009) . Two other bur holes were drilled and little screws fitted, with one screw serving as recording electrode in left skull above the hippocampus (AP -3.8 mm and ML 2.0 mm) and the other as reference electrode above the forehead. Two screws were then connected to a connector-plug with wires for later connecting to recording leads. The guide cannula, screws and connector-plug were then affixed to the skull with dental acrylic. After surgery, animals were allowed to recover for at least 5 days before the experiments.
Measurement of seizure behavior and EEG
Canula-implanted animals were randomly divided into following experimental groups:
DMSO group: 5 μl DMSO (i.c.v.), one injection per day for three consecutive days
CTZ group 1 (low dose): 0.25 μmol (i.c.v.), one injection
CTZ group 2 (middle dose): 0.25 μmol (i.c.v.) for one injection per day, two consecutive days
CTZ group 3 (high dose): 0.25 μmol (i.c.v.) for one injection per day, three consecutive days.
Diazepam + CTZ group: 10 mg Kg-1 (i.p.) diazepam + 0.25 μmol (i.c.v.) CTZ for one injection per day, three consecutive days.
All behavioral tests were carried out between 2:00 pm and 7:00 pm. The animals were first placed in a plastic cage and acclimatized for at least half an hour before experiments. Before and after drug injection, behaviors were continuously monitored for a period of 1 and 3 hours with video recording, respectively.
Behavioral seizures of all rats were scored using 5-graded Racine Score system as previously defined (Racine, 1972). Briefly, Racine score I, facial clonus; score II, head nodding; score III, unilateral forelimb clonus; score IV, rearing with bilateral forelimb clonus; score V, rearing and falling (loss of postural control). The assessment of the severity was based on the latency, seizure duration, seizure number, and seizure score in the 3 hours recording period immediately after CTZ or DMSO administration. A seizure episode was defined as the time window from the beginning of seizure onset to the recovery from the seizure. The seizure score in an animal was calculated based on the maximal behavioral seizure grade observed (Bai et al., 2006, Zhao et al., 2008). Behavioral seizure of grade IV and V are considered to represent kindled motor seizures as previously reported by Lothman and Williamson (1994).
For EEG recordings, electrophysiological signals were amplified (x1000) and filtered (0-50Hz) using NeuroLog system (Digitimer Ltd, Heartford Shire, UK), digitized with CED Micro 1401 (Cambridge Electronic Design, Cambridge, UK) and recorded in a personal computer using Spike 2 software (version 6.0, Cambridge Electronic Design, Cambridge, UK). An electrographic spontaneous seizure was defined as a high-frequency (>5 Hz) high-amplitude (twice larger than baseline) discharge (HAFDs) that lasted for at least 5 sec with frequency of at least 8 Hz and amplitude of at least two times higher than the baseline EEG (Pitkänen et al., 2005, Zhao et al., 2008). The convulsive EEG was divided into four stages: (1) spike or sharp wave, (2) poly spike or wave discharge, (3) continuous spike or spike-and-wave complex (< 15 s), and (4) continuous spike with high amplitude (> 15 s) (Takechi et al., 2008). The electrographic seizure was assessed by counting the number and duration of HAFDs and spontaneous seizures, and EEG scores. The presence of high-voltage EEG seizure activity was used as a seizure marker (Marcelo et al., 2005).
Histology
Similar to previously published work from our lab (Wang et al., 2009), Pontamine Sky Blue (2% in 1M sodium acetate,1 μl) was injected through the pre-implanted cannula, at the end of some experiments, to verify the cannula tip position before the animal was sacrificed. The brain was then removed and coronal section was made at the level where the cannula was inserted. In all cases examined (n=9), the cannula tips were all in the left lateral ventricle and the Pontamine Sky Blue traces were spread all over the ventricles with most staining around the left side lateral ventricle wall (supplemental Figure S1).
Statistical analysis
All data were presented as mean±S.E.M (standard error of the mean). Comparison between multiple groups was executed with NPar Tests (Kruskal-Wallis H test). Comparison between the CTZ group and the other group was executed with Man-Whitney U test. Results were considered significant at P<0.05.
Drugs
Cyclothiazide was purchased from Tocris (Bristol, UK) and dissolved in DMSO in two concentrations of 1 M and 0.2 M for i.c.v. injection; Diazepam was supplied by the Huashan hospital (Fudan University, Shanghai) and dissolved in normal saline in 10 mg ml-1 concentration.
Supplementary Material
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (30770672 to Y.W.; 30828014 to Y.W. and G.C.), Chongqing Science and Technology Commission (CHTC 2007BA5025), the Ministry of Science and Technology of China (2009CB522001), Science and Technology Commission of Shanghai Municipality (09JC1401900) to Y.W. and Chongqing Educational Ministry (KJ0707021, KJ090719) to S-Z.K and M.F. It is also supported by NIH grant (NS054858) to G.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: Disease Related Neuroscience
References
- Bai ZT, Zhao R, Zhang XY, Chen J, Liu T, Ji YH. The epileptic seizures induced by BmK I, a modulator of sodium channels. Exp Neurol. 2006;197:167–176. doi: 10.1016/j.expneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–70. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Lagowska J. Epileptogenic Action of Intra-amygdaloid Injection of Kainic Acid. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences Serie D. 1978;287:813–816. [PubMed] [Google Scholar]
- Browne TR, Holmes GL. Epilepsy. Reply. New Engl J Med. 2001;345:468–469. [Google Scholar]
- Deng LB, Gong C. Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses. PNAS. 2003;100:13025–13029. doi: 10.1073/pnas.2133370100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Fedele E, Conti A, Raiteri M. The Glutamate Receptor/NO/Cyclic GMP Pathway in the Hippocampus of Freely Moving Rats: Modulation by Cyclothiazide, Interaction with GABA and the Behavioural Consequences. Neurophamcol. 1997;36:1393–1403. doi: 10.1016/s0028-3908(97)00112-3. [DOI] [PubMed] [Google Scholar]
- Fornai F, Busceti CL, Kondratyev A, et al. AMPA receptor desensitization as a determinant of vulnerability to focally evoked status epilepticus. Euro J Neurosci. 2005;21:455–463. doi: 10.1111/j.1460-9568.2005.03873.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol. 2001;533:423–31. doi: 10.1111/j.1469-7793.2001.0423a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebriaeezadeh A, Emami S, Ebrahimi M, et al. Anticonvulsant and antiepileptogenic effects of azolylchromanones on lithium-pilocarpine induced seizures and pentylenetetrazole-kindling model of epilepsy. Biomed Pharmacother. 2008;62:208–211. doi: 10.1016/j.biopha.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Williamson JM. Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res. 1994;649:71–84. doi: 10.1016/0006-8993(94)91050-2. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Presynaptic influence on the time course of fast excitatory synaptic currents in cultured hippocampal cells. J Neurosci. 1995;15:3178–92. doi: 10.1523/JNEUROSCI.15-04-03178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Intra-Ventricular Kainic Acid Preferentially Destroys Hippocampal Pyramidal Cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, et al. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–82. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Gröhn O. Experimental febrile seizures require an undetermined factor for induction of hippocampal sclerosis in immature rat brain. Epilepsy Curr. 2005;5:98–100. doi: 10.1111/j.1535-7511.2005.05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JS, Yao J, Fang C, Chen G. Downregulation of tonic GABA currents following epileptogenic stimulation of rat hippocampal cultures. J Physiol. 2006;577:579–590. doi: 10.1113/jphysiol.2006.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues MC, Rossetti F, Foresti ML, et al. Correlation between shaking behaviors and seizure severity in five animal models of convulsive seizures. Epilepsy & Behav. 2005;6:328–336. doi: 10.1016/j.yebeh.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Takechi K, Fujiwara A, Watanabe Y, et al. Participation of GABA-ergic system in epileptogenic activity induced by teicoplanin in mice. Epilepsy Res. 2009;84:127–134. doi: 10.1016/j.eplepsyres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–96. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, et al. Limbic seizures produced by pilocarpine rats: behavioral, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–35. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, et al. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res Rev. 1984;32:37–53. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qi JS, Kong SZ, Jiang M, Chen G. BDNF-TrkB signaling pathway mediates the induction of epileptiform activity induced by a convulsant drug cyclothiazide. Neuropharmacol. 2009;57:49–59. doi: 10.1016/j.neuropharm.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Zhang XY, Yang J, Weng CC, Jiang LL, Zhang JW, Ji YH. Anticonvulsant effect of BmK IT2, a sodium channel-specific neurotoxin in rat models of epilepsy. Brit J Pharmacol. 2008;154:1116–1124. doi: 10.1038/bjp.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Yamada KA, Price MT, et al. A benzodiazepine recognition site associated with the non-NMDA glutamate receptor. Neuron. 1993;10:61–67. doi: 10.1016/0896-6273(93)90242-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.