Abstract
Primordial follicle formation and the subsequent transition of follicles to the primary and secondary stages encompass the early events during folliculogenesis in mammals. These processes establish the ovarian follicle pool and prime follicles for entry into subsequent growth phases during the reproductive cycle. Perturbations during follicle formation can affect the size of the primordial follicle pool significantly, and alterations in follicle transition can cause follicles to arrest at immature stages or result in premature depletion of the follicle reserve. Determining the molecular events that regulate primordial follicle formation and early follicle growth may lead to the development of new fertility treatments. Over the last decade, many of the growth factors and signaling proteins that mediate the early stages of folliculogenesis have been identified using mouse genetic models, in vivo injection studies, and ex vivo organ culture approaches. These studies reveal important roles for the transforming growth factor β (TGF-β) superfamily of proteins in the ovary. This article reviews these roles for TGF-β family proteins and focuses in particular on work from our laboratories on the functions of activin in early folliculogenesis.
Keywords: TGF-β superfamily, folliculogenesis, growth factors, primordial follicle, activin
Ovarian follicles, the functional units within the female gonad, are comprised of three cell types: oocytes, surrounding granulosa cells, and an external thecal cell layer. Follicles serve to nurture maturation of the oocyte, a process that is critical for successful reproduction. Folliculogenesis starts with the assembly of primordial follicles. In mice, primordial germ cells migrate to the urogenital ridge around embryonic day 11.1 At embryonic day 13.5, synchronous rounds of mitotic division in the female gonad yield clusters of oocytes arranged in syncytia, also referred to as “cysts” or “nests.”2 Germ cell nests arise from incomplete germ cell cytokinesis. The resulting syncytia persist until germ cells undergo a wave of apoptosis near the time of birth.3 During programmed nest breakdown, germ cells are encapsulated by squamous somatic cells (pregranulosa cells) to generate primordial follicles. Primordial follicles assemble during midgestation in humans.4
Enlargement of the oocyte and differentiation of pregranulosa cells from a squamous to a cuboidal morphology are some of the hallmarks of the primordial to primary follicle transition. During the reproductive life-span, some primordial follicles remain dormant; others are recruited to enter the growing follicle pool. This is referred to as initial follicle recruitment.5 Secondary follicles contain two layers of granulosa cells, resulting from granulosa cell proliferation, and an external layer of thecal cells. Thecal cells are the source of androgens, which become aromatized to estrogen in granulosa cells. During the female reproductive cycle, select numbers of follicles mature in response to circulating gonadotropins, steroids, and the local actions of growth factors5. Many of these growth factors are synthesized and secreted by granulosa cells. Follicle development continues until ovulation, when an egg or eggs competent for fertilization are extruded from the ovary, and the remaining somatic cells of the follicle luteinize. The late stages of follicle maturation are beyond the scope of this article but are addressed in many excellent reviews.6–8
Studies within the last decade have uncovered some of the pathways and genes that mediate primordial follicle formation and the transition to primary and secondary follicles. For example, intraovarian steroids (E2), secreted signaling molecules (Wnt4), and transcription factors (Foxl2, Figα, Nobox) impact early folliculogenesis.9,10 Many of these findings stem from knockout studies in mice or ex vivo organ culture experiments. Therefore, this review centers on work performed in the rodent, but comparisons to other species are discussed where appropriate. Skinner and colleagues have described the roles of several growth factors in regulating the primordial to primary follicle transition,11,12 and Ojeda and colleagues (see this issue) detail the functions of neurotrophins during early follicle development. This article provides a broad overview of signaling by transforming growth factor β (TGF-β) superfamily proteins and then focuses on their roles during follicle formation and the early stages of follicle growth.
Transforming Growth Factor Beta Superfamily Signaling
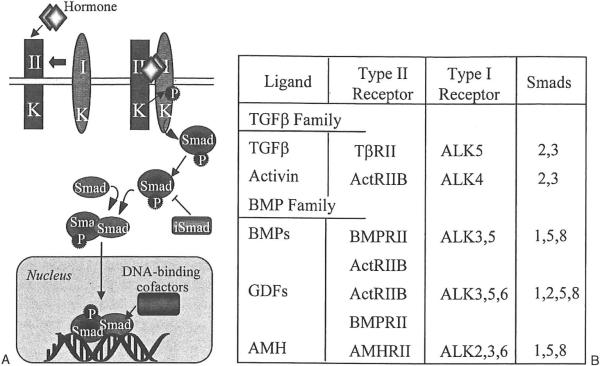
TGF-β superfamily proteins are extracellular, secreted growth factors that function via autocrine or paracrine signaling. TGF-β signaling directs a variety of cell processes during embryonic and postnatal development, including differentiation, apoptosis, proliferation, and cell specification.13 There are > 40 mammalian TGF-β family proteins.14 The two chief subgroups include the growth differentiation factors and bone morphogenetic proteins (GDFs/BMPs) and the activins/TGF-ßs. TGF-β ligands are either homodimeric or heterodimeric molecules that bind to and signal through plasma membrane-spanning serine/threonine kinase receptors.15 Following ligand binding to a type II receptor, type II receptors recruit and transphosphorylate type I receptors.15 Type I receptors are also known as activin-like kinases, or ALKs. BMPs bind type I receptors first or may bind type II/ALK complexes on the cell surface.16 Phosphorylation of ALKs within a serine-rich juxtamembrane domain leads to the activation of receptor Smad proteins, molecules that transduce TGF-β family signals.17 Receptor-activated Smads are phosphorylated and in turn form a complex with the co-Smad Smad4. Smad complexes translocate into the nucleus and promote TGF-β superfamily target gene transcription.15 TGF-β signal transduction is antagonized by inhibitory Smads, or I-Smads. I-Smads are believed to inhibit signaling by either preventing phosphorylation of type I receptors or by sequestering Smad4.15 Although I-Smads inhibit the signaling of TGF-β superfamily proteins through intracellular means, signaling suppression also occurs outside the cell. Extracellular binding proteins, including follistatin, noggin, and chordin, prevent interaction between TGF-β superfamily ligands and their receptors.18 Figure 1 summarizes TGF-β signaling and lists the receptors and Smad proteins that comprise the core signaling pathway for TGF-β family growth factors.
Figure 1.
(A) Transforming growth factor beta (TGF-β) family cell signaling pathway. TGF-β family ligands bind and signal through serine/threonine kinase receptors on the plasma membrane. Signals are transduced through phosphorylated receptor Smad proteins. Receptor Smad/co-Smad dimers translocate into the nucleus to stimulate target gene transcription. (B) Receptors and Smad proteins that comprise the core signaling pathway for TGF-β and bone morphogenetic proteins (BMP) family ligands. See Shimasaki et al16 for a comprehensive list of TGF-β family ligands and their receptors. AMH, anti-müllerian hormone; GDF, growth differentiation factors.
Transforming Growth Factor Beta
TGF-ßs are homodimeric cytokines that influence cell migration, proliferation, and other vital cellular processes.19 Mammals express three of the five TGF-β isoforms: TGF-β1, TGF-β2, and TGF-β3.19 Little is known about how TGF-ßs affect early folliculogenesis. Depending on the species examined, TGF-β can either support or inhibit preantral follicle survival in vitro.20 In vivo models of TGF-β action in the early ovary are lacking because targeted disruption of TGF-β2, TGF-β3, or the transforming growth factor β type II receptor (TßRII) results in either embryonic or perinatal lethality.14 TGF-βi-null mice develop inflammatory lesions, resulting in postnatal lethality.21 In a 2006 report, Ingman et al found that TGF-βl-null mice maintained on an immunocompromised genetic background have a similar percentage of primordial, primary, and antral follicles compared with controls.22 The generation of conditional TGF-β2 and TGF-β3 knockout mice would shed light on how these proteins function during the initial stages of folliculogenesis.
Inhibin and Activin
Inhibins are disulflde-linked heterodimers of α and β subunits.23 The β subunit shares substantial homology with other TGF-β family proteins, whereas the α subunit is more divergent. The two isoforms of inhibin/activin β subunits are βA and βB. Inhibin A is comprised of α and βA subunits, whereas dimerization of α and βB subunits results in inhibin B formation. Activins are homodimers and heterodimers of the β subunits: activin A (βAßA), activin B (βBßB), and activin AB (βAßB). All subunits (α, βA, and βB) are expressed in granulosa cells of growing follicles24. As an endocrine regulator, inhibin functionally antagonizes activin in the pituitary by inhibiting follicle-stimulating hormone (FSH) production.25,26 Inhibin likely antagonizes activin actions in the ovary as well. Inhibin antagonizes activin through competition for binding to activin receptors.27 Activin signaling may also be decreased through competition for subunit assembly, for instance when the expression of the inhibin ot subunit exceeds that of the activin βA or βB subunits.28 Activin signaling is also attenuated by its binding to the secreted glycoprotein antagonist follistatin.29 Multiple paracrine roles for inhibin and activin are described in the ovary.30 For example, inhibin positively regulates luteinizing hormone–induced androgen synthesis in theca cells.31 Activin stimulates FSH receptor expression in granulosa cells and synergizes with FSH to regulate the proliferation and differentiation of granulosa cells from late-stage follicles30. Less is known about how inhibin and activin function during the initial stages of follicle development.
Activin subunits and receptors are expressed in the germ cell and somatic cell compartments within neonatal mouse32 and human fetal ovaries.33 Ovaries at these stages undergo germ cell nest breakdown and subsequent follicle formation. The ovaries of neonatal mice injected with activin A develop 30% more primordial follicles than controls, although many of these follicles are likely abnormal (Fig. 2).32 An increase in pregranulosa cell and germ cell proliferation is seen in ovaries from mice injected with activin A.32 Germ cell proliferation has also been reported in human ovarian cortical sections cultured with activin.33 These data indicate that local concentrations of activin during the time follicles assemble may determine the size of the ovarian follicle pool, and higher pregranulosa cell and/or germ cell numbers may enhance the efficiency of follicle formation. Inhibin B levels drop more than twofold during the interval of follicle formation in neonatal mice.34 This decrease in inhibin may lower the ratio of inhibin to activin in the neonatal mouse ovary and thus provide a mechanism of sustained activin signaling during the period of follicle formation. Activin A is upregulated in adult mice lacking inhibin α, but it has not been reported whether this occurs in neonatal mice.35 If Activin A is upregulated in neonatal inhibin α−/− mice, then it would be informative to examine the ovarian phenotype of these mice to determine whether there is an increased percentage of primordial follicles in neonatal inhibin α−/− ovaries, which would support findings from activin injection experiments.
Figure 2.
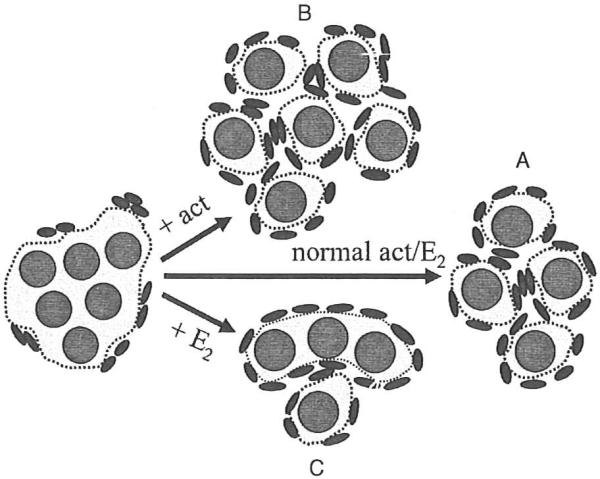
Hormonal regulation of germ cell nest breakdown. (A) Germ cell nests breakdown giving rise to primordial follicles. (B) Increased activin (act) signaling within neonatal mouse ovaries leads to a higher number of primordial follicles compared with controls. (C) Increased estradiol (E2) signaling within neonatal mouse ovaries downregulates activin expression and results in multi-oocytic follicle (MOF) formation.
The ovaries of neonatal mice injected with estradiol (E2), the synthetic estrogen diethylstilbestrol, or the phytoestrogen genistein have decreased activin subunit expression and low levels of phosphorylated Smad2, indicating that activin signaling in this system is attenuated.36 Interestingly, the ovaries of mice treated with these estrogenic compounds develop multi-oocytic follicles (MOFs; Fig. 2).36–40 MOFs, which have two or more germ cells trapped within a follicle boundary, likely arise from the incomplete breakdown of germ cell nests.37,38 Thus these data support the concept that activin is important for germ cell nest breakdown. Germ cell nest breakdown is inhibited in the ovaries of neonatal mice injected with genistein and in mouse ovaries cultured with estrogen.39,41 These data imply that estrogen needs to be suppressed or at low levels to provide an environment permissive for primordial follicle formation in mice.41,42 Estrogen appears to have the opposite effect in nonhuman primates. As with humans, primordial follicle formation begins at midgestation in baboons, a time when estrogen levels are elevated.43 Fetal ovaries have an increased number of germ cell nests and fewer primordial follicles when pregnant baboons are injected with an aromatase inhibitor.44 Inhibin α expression is upregulated in the fetal ovary of these estrogen-suppressed baboons, and activin subunit and receptor expression is unchanged.45 In untreated baboons, activin subunits and receptors are expressed in the fetal ovary; inhibin α expression is minimal.45 These studies suggest that estrogen suppresses inhibin α in the fetal baboon ovary. Although there may be differences in how estrogen regulates inhibin and activin expression in baboon and mouse ovaries, a lower inhibin-to-activin ratio in both species during the initial stages of folliculogenesis may promote follicle formation.
The MOF phenotype described in ovaries of estrogen-treated mice is also found in mouse models where activin synthesis or signaling is decreased: MT-α inhibin, Smad2-DN, and conditional activin βA-null mice.28,46,47 The relatively low number of MOFs found in adult activin-attenuated mouse ovaries implies that other genetic pathways may compensate for activin during the neonatal period or that other pathways are equally important for follicle formation. MOFs were not reported in βB-null ovaries, suggesting that this phenotype is linked directly to βA deficiency or to the perturbation of other growth factors in these animal models. Future rescue experiments, where neonatal βA-null ovaries are cultured with activin, would help clarify a role for activin in mediating the proper encapsulation of germ cells by somatic cells.
Activin signaling likely promotes but may not be critical for proper primordial follicle formation. Adult ovaries lacking both activin βA and βB subunits have similar numbers of primordial, primary, and secondary follicles compared with controls.47 Aside from other signaling proteins promoting follicle formation, the method of generating the conditional βA knockout allele may account for similarities in follicle populations between activin subunit null and control ovaries. Cre-mediated excision of activin βA, specifically in granulosa cells, was achieved using the anti-müllerian hormone receptor 2 (Amhr2) promoter.48 Because Amhr2-Cre is likely most active in granulosa cells once primordial follicles have formed, the timing of activin disruption in conditional βA-null mice may be too late to see a significant effect on follicle formation.49 Activin subunits and receptors are also expressed in the germ cell compartment during the earliest stages of follicle development.32,33,50 A conditional system that achieves activin subunit or receptor disruption in both germ and somatic cells may therefore be useful for determining activin action at these early stages. Ex vivo organ culture studies may serve as a complementary approach to this conditional system, where activin genes are excised from cultured late-embryonic or neonatal rodent ovaries through cell-permeable Cre recombinase.51
It is unclear how activin impacts the growth of primary follicles. In vitro studies have shown that activin stimulates the proliferation of rat granulosa cells and promotes the growth of rat and mouse preantral follicles.52,53 This effect on preantral follicles is age dependent: Activin stimulates the growth of preantral follicles isolated from immature mice but not adult mice.53,54 The preantral follicles isolated in these studies have one to two layers of granulosa cells, so the growth of primary follicles may be influenced by activin. The ovaries of mice overexpressing follistatin arrest at the primary follicle stage,55 making it tempting to speculate that activin is involved in promoting the transition between primary and secondary follicles. Follistatin antagonizes different TGF-β ligands including BMP7,18 so other growth factors may be affected in ovaries overexpressing follistatin. Interestingly, ovaries from Foxl2 mutant mice exhibit early follicular depletion, a potential consequence of low activin A levels.56 This raises the possibility that activin may have the capacity to suppress early follicle growth. Indeed, one study demonstrated that preantral follicle growth is inhibited when preantral follicles are co-cultured with secondary follicles, a source of activin.57 Activin has no effect on the growth of primary follicles in cultured bovine ovarian cortical pieces.58 Further investigation is necessary to determine if activin directly affects primary follicle growth in rodent ovaries.
Anti-Müllerian Hormone
Anti-müllerian hormone (AMH), also referred to as müllerian-inhibiting substance, controls müllerian duct regression during male development.59 In the female, AMH is expressed in granulosa cells of primary and growing follicles within mouse and human ovaries.60–62 Amhr2 receptor mRNA is expressed in granulosa cells of preantral follicles,63 whereas oocytes and granulosa cells of developing follicles express AMH type I receptor mRNAs.64 Ablation of the AMH gene in mice65 augments primordial follicle recruitment, and consequently the ovarian follicle reserve is prematurely depleted.66 These data indicate that AMH inhibits early follicle growth. Similar studies with the Amhr2 knockout mouse have yet to be performed but would be informative in this regard. As discussed in the previous section, Foxl2 mutant ovaries exhibit early follicular depletion.56 This phenomenon may be linked to the downregulation of AMH in the ovaries of Foxl2 mutant mice. Aside from mouse genetic models, findings from two ex vivo culture experiments support a role for AMH in inhibiting follicle growth. Neonatal mouse ovaries cultured in the presence of AMH develop 40% fewer growing follicles compared with controls.60 Similarly, Carlsson et al have shown that the growth of primordial follicles is diminished in human ovarian cortical strips cultured with AMH.67 These studies show that AMH inhibits the primordial to primary follicle transition, a role that may be conserved across species. In one contrasting report, AMH promoted the growth of primordial follicles in human ovarian cortical cultures.68 These cultures were exposed to AMH for a longer period compared with the experiments of Carlsson and coworkers,67,68 so it is unclear if this stimulation had confounding effects on follicle survival.
Increased AMH expression in vivo would be predicted to retard follicle growth in ovaries, but there are no models to support this assertion. Neonatal ovaries of mice that overexpress AMH have fewer germ cells than controls, and these transgenic ovaries degenerate.69 Some AMH transgenic ovaries display retarded follicle growth compared with controls, a potential consequence of ectopic AMH expression in the fetal ovary.70 These phenotypes are reversed when AMH transgenic mice are crossed with Amhr2 knockout mice, indicating that AMH signals specifically through Amhr2.71 An in vivo gain-of-function model for AMH, via inducible transgene expression during the neonatal period, may corroborate current findings from ex vivo experiments in which neonatal ovaries were cultured with AMH. In addition to its role in suppressing initial follicle growth, AMH serves as a marker of female fertility.72 In humans, serum AMH levels decline with age as the ovarian follicle reserve becomes depleted.73 Therefore, serum AMH measurements provide a way to gauge the quality of the ovarian follicle pool.
The mechanisms by which AMH inhibits initial follicle recruitment are unknown. Although Amhr2 mRNA is expressed in the neonatal mouse ovary,60 demonstration of Amhr2 mRNA and protein localization in ovaries at this stage is lacking. Recent evidence suggests that AMH can suppress the stimulation of initial follicle growth by three growth factors: basic fibroblast growth factor, kit ligand, and keratinocyte growth factor.74 From this same study, microarray data showed that AMH decreases the expression of TGF-β family receptors whose ligands are known to promote the primordial to primary follicle transition.74 Thus AMH inhibition of primordial follicle growth may be achieved directly, by interactions with putative Amhr2 receptors on pregranulosa cells of primordial follicles. Inhibition of primordial follicle growth by AMH may also occur indirectly, where AMH signaling decreases the expression of growth factors known to promote the primordial to primary follicle transition, or AMH attenuates the responsiveness of ovarian cells to these growth factors by decreasing the expression of their receptors.74
BONE MORPHOGENETIC PROTEINS AND GROWTH DIFFERENTIATION FACTOR 9
Although initially characterized in the bone, BMPs regulate cell death, growth, and differentiation in a host of tissues.16 Several BMPs play important roles in the postnatal ovary. BMP7 and BMP4 mRNAs are expressed in the thecal cell layer of antral follicles.64 BMPRII transcripts are expressed in oocytes of primary follicles and granulosa cells of developing follicles.64 Immunolocalization studies in neonatal rat ovaries reveal that BMP4 is expressed in stromal cells surrounding primordial follicles.75 Intrabursal injection of BMP7 leads to an increased number of developing follicles.76 To further define BMP7 action in early ovaries, Lee et al cultured neonatal rat ovaries in the presence of BMP7 and found a stimulatory effect on primordial follicle growth.77 Ovaries cultured with BMP4 also have an increased percentage of developing follicles compared with control ovaries.75 These studies suggest that BMP4 and BMP7 are important for the primordial to primary follicle transition. Removal of endogenous BMP4 in ovary cultures with a neutralizing antibody results in progressive oocyte and follicle loss, so BMP4 may be considered a follicle survival factor.75 This property of BMP4 may not correlate with primordial follicle growth. Comparing the numbers of atretic follicles between control and BMP4-treated ovary cultures should answer this question. Type I and type II BMP receptors are expressed in rodent primary follicles.20 Thus BMP4 and BMP7 may act directly on these follicles to promote the transition to secondary follicles.
GDF9 mRNA and protein is expressed exclusively in oocytes of primary and growing follicles within mouse ovaries.78,79 This expression pattern is conserved in both rat and humans.80,81 GDF9 female mice are infertile because follicles arrest at the primary follicle stage, indicating that GDF9 is crucial for primary follicle growth.82 Findings from in vivo and organ culture studies corroborate a role for GDF9 in promoting the advancement of primary follicles to the secondary follicle stage.83,84 Some reports propose that GDF9 may also induce primordial follicle progression.84,85 Given that GDF9−/− ovaries contain primary follicles, this may be considered a minor role for GDF9. The progression of primary follicles to the secondary stage requires granulosa cell proliferation. The granulosa cells of primary follicles in GDF9−/− ovaries have decreased expression of proliferation markers compared with controls.86 Additionally, primary rat granulosa cells treated with GDF9 exhibit an increase in granulosa cell proliferation.87 These studies provide evidence that the transition between primary and secondary follicles is mediated in part through GDF9-induced granulosa cell proliferation. Follicles from GDF9−/− ovaries lack supporting theca cells.82 In addition to its role in stimulating granulosa cell proliferation, GDF9 is believed to regulate thecal cell proliferation or differentiation.88,89
Interestingly, inhibin α is upregulated in granulosa cells of primary follicles in GDF9-null ovaries.86 The interactions between GDF9 and inhibin α were addressed using a mouse genetic model.89 GDF9/inhibin α double-null ovaries contain follicles beyond the primary stage.89 This implies that suppression of inhibin α in the granulosa cells of primary follicles may be required for continued maturation of follicles. βA and βB subunits are upregulated in GDF9−/−/inhibin α−/− ovaries,89 and therefore activin may promote the growth of primary follicles via granulosa cell proliferation. The timing of GDF9 action is species dependent. Primordial follicle formation occurs earlier in hamster ovaries compared with mouse and rat ovaries. GDF9 is expressed in germ cells before follicle formation in the hamster ovary.90 RNA interference (RNAi)-mediated knockdown of this growth factor in cultured fetal hamster ovaries results in fewer primordial follicles, suggesting that GDF9 mediates primordial follicle formation in the hamster.91
Like GDF9, BMP15 expression is restricted to oocytes of primary and growing follicles.92 BMP15 is the closest homologue to GDF9 within the TGF-β superfamily.16 In contrast to the severe ovarian defects and infertility of GDF9-null mice, BMP15-null mice are subfertile with ovaries that are histologically similar to litter-mate controls.93 This phenotype is not seen in sheep, where naturally occurring mutations of BMP15 yield a similar block in the progression of primary follicle growth compared with that found in GDF9-null ovaries.94 Despite the fact that BMP15 is not essential for early follicle maturation in rodents, this growth factor has a supporting role for granulosa cell function.16 BMP15 stimulates the proliferation of primary rat granulosa cells.95 This effect on proliferation is enhanced by concomitant treatment with GDF9, indicating synergism between these TGF-β ligands.87 The synergistic effect on granulosa cell proliferation was not seen in cultured ovine granulosa cells, indicating species-specific differences in the responsiveness to these growth factors.96
CONCLUSIONS AND FUTURE DIRECTIONS
The ovary contains a myriad network of factors that regulate the initial stages of folliculogenesis, and this network includes several TGF-β family proteins (Fig. 3). Adding to this growing list of factors, a distant TGF-β ligand, glial cell-derived neurotrophic factor (GDNF), was recently found to promote the primordial to primary follicle transition.97 The core signaling components (receptors, Smads) are similar for each ligand, yet there are differences in how these growth factors impact follicle formation and the transition between primordial and primary follicles. This diversity likely stems from the temporal control of TGF-β family gene expression and also crosstalk between TGF-β and other cell signaling pathways. For instance, TGF-β signaling can activate the mitogen-activated protein kinase (MAPK) pathway, and crosstalk between these two pathways occurs at the level of Smad–MAPK protein interactions.98 The antagonism of multiple TGF-β ligands by a single modulator, such as follistatin or noggin, provides another method of achieving diversity in cellular responses to TGF-β signaling. For example, inhibin was found to antagonize BMPs at the level of the BMP receptor,99 adding to the complexity of TGF-β family signaling regulation.
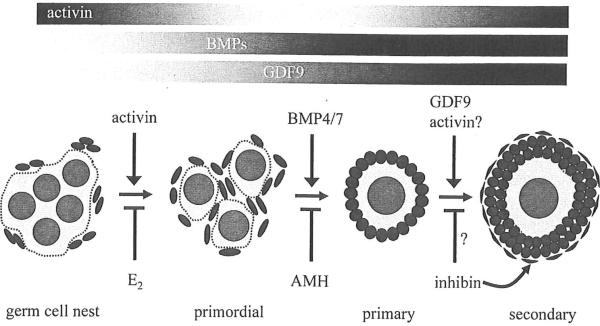
Figure 3.
Early folliculogenesis involves the breakdown of germ cell nests into primordial follicles and the subsequent transition of follicles to the primary and secondary stages. Different transforming growth factor beta (TGF-β) superfamily ligands regulate each of these developmental processes. Activin may play a role in primordial follicle formation. Bone morphogenetic protein 7 (BMP7) and BMP4 promote the transition of primordial follicles to primary follicles, whereas anti-müllerian hormone (AMH) inhibits initial follicle growth. Growth differentiation factor 9 (GDF9) is critical for follicles to advance to the secondary stage. In primary follicles, inhibin may be suppressed by GDF9, which allows for granulosa cell proliferation by activin. Bars indicating the relative activities of these TGF-β family proteins in prepubertal mouse ovaries are also shown (black = high activity). E2, estradiol.
Activin may promote primordial follicle formation in mice, and it can be considered one of the earliest TGF-β family effectors of folliculogenesis. Further investigation is necessary to determine if activin is critical for follicle formation. Activin may also positively regulate the growth of primary follicles, although this might occur in the immature mouse ovary and not in the adult ovary. Thus TGF-β family ligands can have multiple roles during early follicle development, and in some cases these roles may change depending on the maturity of the ovary. There are no known TGF-β family proteins that inhibit primordial follicle formation. AMH inhibits the primordial to primary follicle transition, whereas BMP4 and BMP7 promote this process. Animal knockout models have show that the growth of primary follicles depends, in part, on the suppression of inhibin by GDF9. GDF9 has an essential role in advancing primary follicles to the secondary stage in the mouse ovary. In contrast, GDF9 is important for primordial follicle formation in the hamster ovary. This demonstrates that different species may use a TGF-β family protein in distinct ways, yet the net impact is to ensure follicle maturation.
The initial stages of folliculogenesis can be thought of as a balance of stimulatory and inhibitory inputs, but what tips this balance? For instance, AMH inhibits the transition of primordial follicles to primary follicles, but which factors temper AMH activity to promote follicle transition? Studies aimed at identifying how TGF-β family proteins interact with other signaling proteins and how TGF-β target genes are affected during early folliculogenesis may answer these questions. Our understanding of TGF-β protein function has benefited from gain and loss of function studies in mice. Such studies have shown that perturbation of TGF-β family protein signaling alters the early stages of folliculogenesis. Organ culture studies have provided an alternative approach to studying TGF-β family protein function. The majority of organ culture experiments performed are gain of function experiments, where ovaries are cultured in the presence of a TGF-β family ligand. Although it is possible to block protein function in an organ culture system by using neutralizing antibodies or antagonists, new technologies will likely enhance such loss of function experiments. For example, RNAi has been used extensively as a tool to knock down gene expression in mammalian cells. New gene delivery systems promise to make RNAi more useful in the context of organ cultures. One can envision simultaneously knocking down multiple combinations of TGF-β ligand and receptor mRNAs in ovary cultures to answer questions pertaining to early follicle growth. Ultimately, understanding the events that mediate early follicle development may provide an avenue to better treat female infertility.
ACKNOWLEDGMENTS
Daniel J. Trombly is a fellow of the Reproductive Biology Training Grant (HD00678) and a Chicago Chapter scholar of the Achievement Rewards for College Scientists (ARCS) Foundation. This work was supported by NIH/NICHD P01 HD021921, “Hormonal Signals That Regulate Ovarian Differentiation.”
REFERENCES
- 1.Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240(2):488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- 2.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125(17):3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 3.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234(2):339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagenen G, Simpson ME. Embryology of the ovary and testis: Homo sapiens and Macaca mulatta. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- 5.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 6.Baker SJ, Spears N. The role of intra-ovarian interactions in the regulation of follicle dominance. Hum Reprod Update. 1999;5(2):153–165. doi: 10.1093/humupd/5.2.153. [DOI] [PubMed] [Google Scholar]
- 7.Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12(5):537–555. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- 8.Richards JS. Perspective: the ovarian follicle—a perspective in 2001. Endocrinology. 2001;142(6):2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 9.Epifano O, Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab. 2002;13(4):169–173. doi: 10.1016/s1043-2760(02)00576-3. [DOI] [PubMed] [Google Scholar]
- 10.Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44(12):622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131(6):1007–1015. doi: 10.1530/rep.1.00978. [DOI] [PubMed] [Google Scholar]
- 12.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;ll(5):461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 14.Pangas SA, Matzuk MM. Genetic models for transforming growth factor beta superfamily signaling in ovarian follicle development. Mol Cell Endocrinol. 2004;225(1–2):83–91. doi: 10.1016/j.mce.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 16.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25(1):72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 17.Kaivo-Oja N, Jeffery LA, Ritvos O, Mottershead DG. Smad signalling in the ovary. Reprod Biol Endocrinol. 2006;4:21. doi: 10.1186/1477-7827-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250(2):231–250. [PubMed] [Google Scholar]
- 19.Lutz M, Knaus P. Integration of the TGF-beta pathway into the cellular signalling network. Cell Signal. 2002;14(12):977–988. doi: 10.1016/s0898-6568(02)00058-x. [DOI] [PubMed] [Google Scholar]
- 20.Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11(2):143–160. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingman WV, Robker RL, Woittiez K, Robertson SA. Null mutation in transforming growth factor beta1 disrupts ovarian function and causes oocyte incompetence and early embryo arrest. Endocrinology. 2006;147(2):835–845. doi: 10.1210/en.2005-1189. [DOI] [PubMed] [Google Scholar]
- 23.Muttukrishna S, Tannetta D, Groome N, Sargent I. Activin and follistatin in female reproduction. Mol Cell Endocrinol. 2004;225(1–2):45–56. doi: 10.1016/j.mce.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Woodruff TK, D'Agostino J, Schwartz NB, Mayo KE. Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science. 1988;239(4845):1296–1299. doi: 10.1126/science.3125611. [DOI] [PubMed] [Google Scholar]
- 25.Carroll RS, Corrigan AZ, Gharib SD, Vale W, Chin WW. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol. 1989;3(12):1969–1976. doi: 10.1210/mend-3-12-1969. [DOI] [PubMed] [Google Scholar]
- 26.Woodruff TK, Krummen LA, Lyon RJ, Stocks DL, Mather JP. Recombinant human inhibin A and recombinant human activin A regulate pituitary and ovarian function in the adult female rat. Endocrinology. 1993;132(6):2332–2341. doi: 10.1210/endo.132.6.8504739. [DOI] [PubMed] [Google Scholar]
- 27.Lewis KA, Gray PC, Blount AT, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404(6776):411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 28.McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology. 2001;142(11):5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Takio K, Eto Y, et al. Activin-binding protein from rat ovary is follistatin. Science. 1990;247(4944):836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- 30.Findlay JK. An update on the roles of inhibin, activin, and follistatin as local regulators of folliculogenesis. Biol Reprod. 1993;48(l):15–23. doi: 10.1095/biolreprod48.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod. 1995;10(1):75–81. doi: 10.1093/humrep/10.1.75. [DOI] [PubMed] [Google Scholar]
- 32.Bristol-Gould SK, Kreeger PK, Selkirk CG, et al. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298(1):132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Martins da Silva SJ, Bayne RA, Cambray N, et al. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation before primordial follicle formation. Dev Biol. 2004;266(2):334–345. doi: 10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Weng Q, Wang H, Medan S, et al. Expression of inhibin/activin subunits in the ovaries of fetal and neonatal mice. J Reprod Dev. 2006;52(5):607–616. doi: 10.1262/jrd.18026. [DOI] [PubMed] [Google Scholar]
- 35.Matzuk MM, Finegold MJ, Mather JP, et al. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A. 1994;91(19):8817–8821. doi: 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148(5):1968–1976. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43(3):478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 38.Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34(1):29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- 39.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74(1):161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67(4):1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148(8):3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 42.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144(8):3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 43.Albrecht ED, Pepe GJ. Endocrinology of pregnancy. In: Brans YW, Kuehl TJ, editors. Non-Human Primates in Perinatal Research. John Wiley and Sons; New York, NY: 1988. pp. 13–78. [Google Scholar]
- 44.Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod. 2002;67(4):1148–1156. doi: 10.1095/biolreprod67.4.1148. [DOI] [PubMed] [Google Scholar]
- 45.Billiar RB, Zachos NC, Burch MG, Albrecht ED, Pepe GJ. Up-regulation of alpha-inhibin expression in the fetal ovary of estrogen-suppressed baboons is associated with impaired fetal ovarian folliculogenesis. Biol Reprod. 2003;68(6):1989–1996. doi: 10.1095/biolreprod.102.011908. [DOI] [PubMed] [Google Scholar]
- 46.Bristol-Gould SK, Hutten CG, Sturgis C, et al. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146(12):5228–5236. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- 47.Pangas SA, Jorgez CJ, Tran M, et al. Intraovarian activins are required for female fertility. Mol Endocrinol. 2007;21(10):2458–2471. doi: 10.1210/me.2007-0146. [DOI] [PubMed] [Google Scholar]
- 48.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmprla for müllerian duct regression during male sexual development. Nat Genet. 2002;32(3):408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 49.Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol. 2004;18(4):953–967. doi: 10.1210/me.2003-0301. [DOI] [PubMed] [Google Scholar]
- 50.Drummond AE, Le MT, Ethier JF, Dyson M, Findlay JK. Expression and localization of activin receptors, Smads, and beta glycan to the postnatal rat ovary. Endocrinology. 2002;143(4):1423–1433. doi: 10.1210/endo.143.4.8728. [DOI] [PubMed] [Google Scholar]
- 51.Patsch C, Edenhofer F. Conditional mutagenesis by cell-permeable proteins: potential, limitations and prospects. Handb Exp Pharmacol. 2007;178:203–232. doi: 10.1007/978-3-540-35109-2_9. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Phillips DM, Mather JP. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136(3):849–856. doi: 10.1210/endo.136.3.7867593. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Andoh K, Abe Y, et al. A comparative study on transforming growth factor-beta and activin A for preantral follicles from adult, immature, and diethylstilbestrol-primed immature mice. Endocrinology. 1999;140(6):2480–2485. doi: 10.1210/endo.140.6.6827. [DOI] [PubMed] [Google Scholar]
- 54.Yokota H, Yamada K, Liu X, et al. Paradoxical action of activin A on folliculogenesis in immature and adult mice. Endocrinology. 1997;138(11):4572–4576. doi: 10.1210/endo.138.11.5526. [DOI] [PubMed] [Google Scholar]
- 55.Guo Q, Kumar TR, Woodruff T, et al. Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Mol Endocrinol. 1998;12(1):96–106. doi: 10.1210/mend.12.1.0053. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt D, Ovitt CE, Anlag K, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131(4):933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 57.Mizunuma H, Liu X, Andoh K, et al. Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage. Endocrinology. 1999;140(1):37–42. doi: 10.1210/endo.140.1.6409. [DOI] [PubMed] [Google Scholar]
- 58.Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol. 2000;163(1–2):53–60. doi: 10.1016/s0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 59.Munsterberg A, Lovell-Badge R. Expression of the mouse anti-müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113(2):613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 60.Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 61.Hirobe S, He WW, Lee MM, Donahoe PK. Müllerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology. 1992;131(2):854–862. doi: 10.1210/endo.131.2.1639028. [DOI] [PubMed] [Google Scholar]
- 62.Weenen C, Laven JS, Von Bergh AR, et al. Anti-müullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 63.Baarends WM, Uilenbroek JT, Kramer P, et al. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136(11):4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 64.Shimasaki S, Zachow RJ, Li D, et al. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A. 1999;96(13):7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79(3):415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 66.Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti-müllerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 67.Carlsson IB, Scott JE, Visser JA, et al. Anti-müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt KL, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-müllerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol. 2005;234(1–2):87–93. doi: 10.1016/j.mce.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing müllerian inhibiting substance. Nature. 1990;345(6271):167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- 70.Lyet L, Louis F, Forest MG, et al. Ontogeny of reproductive abnormalities induced by deregulation of anti-müllerian hormone expression in transgenic mice. Biol Reprod. 1995;52(2):444–454. doi: 10.1095/biolreprod52.2.444. [DOI] [PubMed] [Google Scholar]
- 71.Mishina Y, Whitworth DJ, Racine C, Behringer RR. High specificity of müllerian-inhibiting substance signaling in vivo. Endocrinology. 1999;140(5):2084–2088. doi: 10.1210/endo.140.5.6705. [DOI] [PubMed] [Google Scholar]
- 72.Visser JA, Themmen AP. Anti-müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234(1–2):81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 73.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 74.Nilsson E, Rogers N, Skinner MK. Actions of anti-müllerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134(2):209–221. doi: 10.1530/REP-07-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod. 2003;69(4):1265–1272. doi: 10.1095/biolreprod.103.018671. [DOI] [PubMed] [Google Scholar]
- 76.Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65(4):994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- 77.Lee WS, Yoon SJ, Yoon TK, et al. Effects of bone morphogenetic protein-7 (BMP-7) on primordial follicular growth in the mouse ovary. Mol Reprod Dev. 2004;69(2):159–163. doi: 10.1002/mrd.20163. [DOI] [PubMed] [Google Scholar]
- 78.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13(6):1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 79.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9(1):131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 80.Aaltonen J, Laitinen MP, Vuojolainen K, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84(8):2744–2750. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 81.Jaatinen R, Laitinen MP, Vuojolainen K, et al. Localization of growth differentiation factor-9 (GDF-9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF-9 and its novel homolog GDF-9B. Mol Cell Endocrinol. 1999;156(1–2):189–193. doi: 10.1016/s0303-7207(99)00100-8. [DOI] [PubMed] [Google Scholar]
- 82.Dong J, Albertini DF, Nishimori K, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 83.Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod. 2002;67(3):1018–1024. doi: 10.1095/biolreprod.101.002527. [DOI] [PubMed] [Google Scholar]
- 84.Vitt UA, McGee EA, Hayashi M, Hsueh AJ. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology. 2000;141(10):3814–3820. doi: 10.1210/endo.141.10.7732. [DOI] [PubMed] [Google Scholar]
- 85.Hreinsson JG, Scott JE, Rasmussen C, et al. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87(1):316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- 86.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13(6):1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 87.McNatty KP, Juengel JL, Reader KL, et al. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function. Reproduction. 2005;129(4):473–480. doi: 10.1530/rep.1.0511. [DOI] [PubMed] [Google Scholar]
- 88.Spicer LJ, Aad PY, Allen DT, et al. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol Reprod. 2008;78(2):243–253. doi: 10.1095/biolreprod.107.063446. [DOI] [PubMed] [Google Scholar]
- 89.Wu X, Chen L, Brown CA, Yan C, Matzuk MM. Interrelationship of growth differentiation factor 9 and inhibin in early folliculogenesis and ovarian tumorigenesis in mice. Mol Endocrinol. 2004;18(6):1509–1519. doi: 10.1210/me.2003-0399. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod. 2004;70(3):577–585. doi: 10.1095/biolreprod.103.023234. [DOI] [PubMed] [Google Scholar]
- 91.Wang C, Roy SK. Expression of growth differentiation factor 9 in the oocytes is essential for the development of primordial follicles in the hamster ovary. Endocrinology. 2006;147(4):1725–1734. doi: 10.1210/en.2005-1208. [DOI] [PubMed] [Google Scholar]
- 92.Dube JL, Wang P, Elvin J, et al. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12(12):1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 93.Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 94.Galloway SM, McNatty KP, Cambridge LM, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25(3):279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 95.Otsuka F, Yao Z, Lee T, et al. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem. 2000;275(50):39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- 96.McNatty KP, Juengel JL, Reader KL, et al. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction. 2005;129(4):481–487. doi: 10.1530/rep.1.00517. [DOI] [PubMed] [Google Scholar]
- 97.Dole G, Nilsson E, Skinner MK. Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis. Reproduction. 2008;135(5):671–682. doi: 10.1530/REP-07-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24(37):5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 99.Wiater E, Vale W. Inhibin is an antagonist of bone morphogenetic protein signaling. J Biol Chem. 2003;278(10):7934–7941. doi: 10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]