Abstract
Objective
Schizophrenia is associated with a blunted flush response to niacin. Since niacin-induced skin flushing is mediated by vasodilators derived from arachidonic acid (AA), we tested whether the blunted flush response to niacin is a marker of AA deficiency.
Methods
Eight concentrations of methylnicotinate were applied to the forearms of 20 adults with schizophrenia and 20 controls. Laser Doppler measurement of blood flow responses was used to derive values for niacin sensitivity (defined as the concentration eliciting half-maximal response, i.e., EC50 value) and efficacy (defined as the maximal evoked blood flow response). RBC membrane fatty acids were analyzed by gas chromatography.
Results
Niacin sensitivity and efficacy were reduced in schizophrenia. In the control group, there was significant correlation between AA levels and niacin sensitivity as well as a trend toward correlation between AA levels and niacin efficacy. In contrast, neither sensitivity nor efficacy of niacin correlated with AA levels in schizophrenia. An expected correlation between the levels of AA and its elongation product (adrenic acid) was absent in schizophrenia. Adrenic acid levels correlated with niacin efficacy in schizophrenia.
Conclusions
The schizophrenia-associated niacin response abnormality involves both diminished sensitivity and reduced efficacy. The lack of expected correlation between levels of AA and adrenic acid suggests homeostatic imbalance within the n-6 polyunsaturated fatty acid (PUFA) pathway in schizophrenia. Though AA levels were unrelated to measures of niacin response in schizophrenia, the correlation between adrenic acid and niacin efficacy in schizophrenia suggests relevance of the n-6 PUFA pathway to the blunted niacin response.
Keywords: Niacin, Schizophrenia, RBC Membrane, Arachidonic Acid, Adrenic Acid
1. Introduction
A blunted skin flush response to niacin is a widely replicated physiological abnormality that appears to occur selectively among a portion of patients with schizophrenia. The niacin response abnormality is significantly more prevalent in schizophrenia than in healthy comparison groups (reviewed in Messamore, 2003). Its prevalence in schizophrenia is significantly higher than in major depression (Bosveld-van Haandel et al., 2006) or bipolar disorder (Liu et al., 2007). The niacin response abnormality appears to be a heritable trait within schizophrenia-affected families (Chang et al., 2009; Lin et al., 2007; Waldo, 1999). Thus, it may represent a novel schizophrenia endophenotype. In contrast to the incompletely-understood etiology of schizophrenia, the mechanism of niacin-induced skin flushing in man is relatively well-characterized. This detailed knowledge, coupled with the accessibility of skin for scientific study, presents a technically feasible, straightforward, and economically attractive opportunity to broaden our understanding of the biochemical changes that accompany schizophrenia.
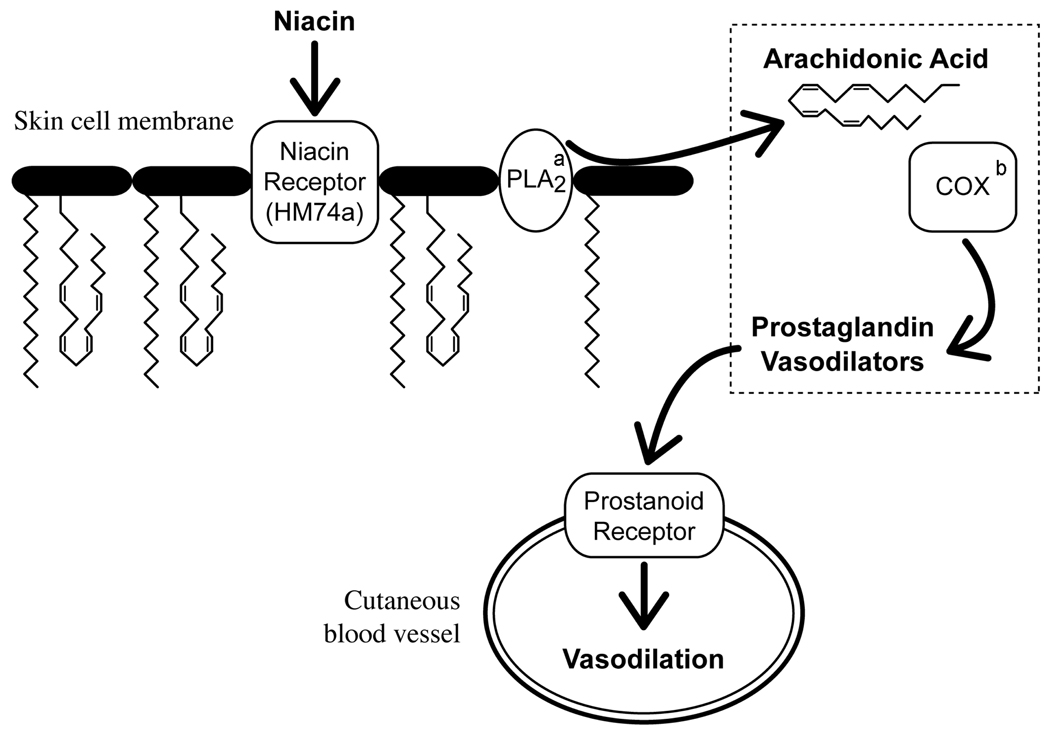
Niacin-induced skin flushing is mediated by prostaglandins derived from arachidonic acid (AA; Figure 1). Niacin binds to a G-protein coupled receptor (HM74a) on skin macrophages (Benyo et al., 2006) and stimulates phospholipase A2 to release AA from cell membrane phospholipids (Tang et al., 2006). Free AA is converted to vasodilatory prostaglandins (Eklund et al., 1979; Morrow et al., 1992) via the sequential action of cyclooxygenase and prostaglandin isomerase enzymes. Activation of prostanoid receptors dilates cutaneous blood vessels (Lai et al., 2007), and a visible skin flush arises from the ensuing increased blood flow. In man, the flush response to niacin can be completely abolished by cyclooxygenase inhibitors such as aspirin or indomethacin (Svedmyr et al., 1977; Wilkin et al., 1982). In mice, the flush response to niacin is eliminated by knocking out genes for either the niacin receptor or cyclooxygenase-1 (Benyo et al., 2005). These findings suggest that niacin-induced skin flushing is mediated by signaling molecules derived from AA.
Fig. 1. The Mechanism of Niacin-Induced Skin Flushing.
a PLA2=phospholipase A2
b COX=cyclooxygenase
Considering the central role of AA-derived mediators in the skin flush response to niacin, it follows that abnormally low niacin sensitivity may reflect disordered AA signaling in schizophrenia. There have been several reports that suggest abnormalities in the turnover or content of AA and other omega-6 polyunsaturated fatty acids (n-6 PUFAs) in schizophrenia (reviewed in Skosnik and Yao, 2003). Glen et al. (1996) found lower levels of AA among schizophrenia patients who did not flush in response to oral niacin, suggesting that niacin subsensitivity is a physiological marker of AA deficiency. We undertook this study to determine whether the niacin response abnormality in schizophrenia is related to red blood cell (RBC) membrane AA levels. We secondarily examined whether the content of related n-6 PUFAs was associated with the illness and/or niacin response.
2. Methods
2.1 Participants
Twenty subjects with schizophrenia and 20 normal controls participated in the study. The diagnosis of schizophrenia was established with the Structured Clinical Interview (SCID) for DSM-IV Axis I Disorders (First et al., 1997). The control group consisted of volunteers who denied family histories of psychotic illnesses and whose responses to the SCID indicated no present or past history of psychiatric illness. Exclusion criteria applicable to either group consisted of: recent use of nonsteroidal anti-inflammatory drugs; neurologic disease or injury; mental retardation; or medical illnesses (such as diabetes, vasculitis, or chronic hypertension) that would affect normal vascular tone or response to niacin stimulus. Participants were asked about their tobacco, alcohol, and drug use during the SCID interview. Self-reported cigarette consumption (in packs per day) was recorded. Responses to questions about alcohol or drug use were recorded in the form of whether or not DSM criteria for a substance use disorder had been met; estimates of consumption (e.g., in drinks per week) were not specifically recorded. Participants whose responses indicated the presence of a substance use disorder (aside from tobacco dependence) active within the past 6 months were excluded. We did not inquire about dietary habits outside of general screening questions to detect neurovegetative signs of mental illness. Written informed consent was obtained from all participants. This study was approved by the institutional review boards for the Portland VA Medical Center and the VA Pittsburgh Healthcare System.
2.2 Niacin Sensitivity Testing
Cutaneous blood flow was measured with a laser Doppler flowmeter (PeriFlux System 5000, Perimed AB, Järfälla, Sweden), equipped with an integrating flow probe that measures the average blood flow of seven spatially discrete tissue volumes (Messamore et al., 2003). To ensure reliable comparison between subjects, the instrument was regularly calibrated to the Brownian motion of latex particles in a standardized solution (Motility Standard PF1001, Perimed, Sweden). The laser Doppler signals from eight recording sites on the anterior surfaces of the forearms were measured. The average laser Doppler signal over one minute of continuous recording was used to define the baseline blood flow at each forearm site. Following basal blood flow measurements, circular patches of laboratory filter paper (9-mm diameter) were placed at each recording site. Twenty-five microliters of aqueous methylnicotinate (molar concentrations of: 10−5, 10−4, 10−3.5, 10−3, 10−2.5, 10−2, 10−1.5 or 10−1) were added to each of the papers. The saturated papers were removed from the recording sites after 5 minutes of methylnicotinate exposure. Fifteen minutes after the saturated papers were removed, blood flow at each site was measured continuously for one minute. This laser Doppler signal was averaged over the minute of recording to obtain a post-niacin blood flow response. Baseline blood flow at each site was subtracted from the site’s post-niacin blood flow response to define the niacin-evoked response. Based on the blood flow response to eight methylnicotinate concentrations on forearm skin, the concentration required to elicit half-maximal blood flow response (EC50) for each participant was derived (Messamore et al., 2003).
2.3 Fatty Acids Analysis
Immediately prior to niacin sensitivity testing, blood samples were collected from the antecubital vein. Approximately 4.5 ml of whole blood was drawn into a vacutainer tube containing buffered sodium citrate anticoagulant. The tube was then centrifuged at 100 × g for 15 minutes. Hemoglobin-free RBC ghost membranes were prepared according to the method of Dodge et al. (1963). Briefly, after separation of overlying plasma, the pellet was resuspended in 15 ml of cold isotonic saline/HEPES (0.5%) buffer and then centrifuged at 750 × g for 7 minutes. This washing step was repeated two additional times. The final washed pellet was frozen immediately at −70°C. The stored RBC samples were then batched and shipped under dry ice to Dr. Yao’s laboratory within 4 months of blood draw.
Lipids were extracted from RBC ghost membranes according to the procedure of Rose and Oklander (1965). Fatty acid methyl esters were prepared according to the method of Ichihara et al. (1996). Diheptadecanoyl lecithin (Matraya, Inc., Pleasant Gap, PA) was added as an internal standard for quantification.
The fatty acid methyl esters were analyzed on a Hewlett-Packard capillary gas chromatograph, Model 5890A, equipped with a hydrogen flame detector (Yao et al., 1994). A 30-meter fused silica SP-2330 column with an inner diameter of 0.32 mm and a 0.20 mm film thickness (Suppelco, Inc., Bellefonte, PA) was used. Samples were run under a spitless injection mode with helium as the carrier gas. Oven temperature was programmed in three stages: stage 1 from 50 to 150°C at a rate of 25°C/min; stage 2 from 150 to 190°C at a rate of 4°C/min; and stage 3 from 190 to 250°C at a rate of 6°C/min, with a final time of 3 min at 250°C. Peak s on the chromatogram were identified by comparing their retention times with those of standard mixtures. To derive their fatty acid content, peaks were integrated with an Agilent ChemStation (Rev A.09.03, Santa Clara, CA) using an internal standard mode.
2.4 Statistical Analysis
EC50 values were generated from nonlinear regression curves (Messamore et al., 2003), with variable (best fit) slopes, using the Prism program (GraphPad Inc., La Jolla, CA). EC50 values were log-transformed to facilitate graphical presentation and to allow for more meaningful statistical comparison. The Komolgorov-Smirnoff test was used to test for normal distribution of data points. Unpaired t-tests were used to compare between-group means. Pearson correlation coefficients were used to test for correlation. Since the primary hypothesis was directional (that lower AA levels would correlate with blunted measures of niacin response), significance testing was one-tailed for correlations between AA levels and values of log EC50 or maximum niacin-evoked blood flow response. Tests of correlation between log EC50 and other fatty acid levels were exploratory; in these cases, two-tailed p values are reported, but are not corrected for multiple comparisons. Stated mean values appear with standard deviations. Analysis of covariance was used to assess the effect of cigarette use on variables of interest such as log EC50 value and fatty acid levels. All but 5 subjects (from either group) reported cigarette consumption of either zero or 1 pack per day; therefore, smoking status was modeled as a dichotomous covariable in analyses of covariance.
3. Results
3.1 Sample Characteristics
A total of 40 people participated in the study: 20 with schizophrenia and 20 controls. There were 17 males in the schizophrenia group. The mean age of the schizophrenia group was 43.4 (± 9.5) years. The control group included 14 males. The mean age of the control group was 44.1 (± 6.7) years. The gender distribution between groups was similar (Fisher’s exact test, p = 0.45). The groups were also similar in age (t = 0.29, df = 38, p = 0.77). Thirteen of 20 (65%) schizophrenic patients were smokers while only 3/20 (15%) of controls smoked.
3.2 Niacin Sensitivity and Efficacy is Reduced in Schizophrenia
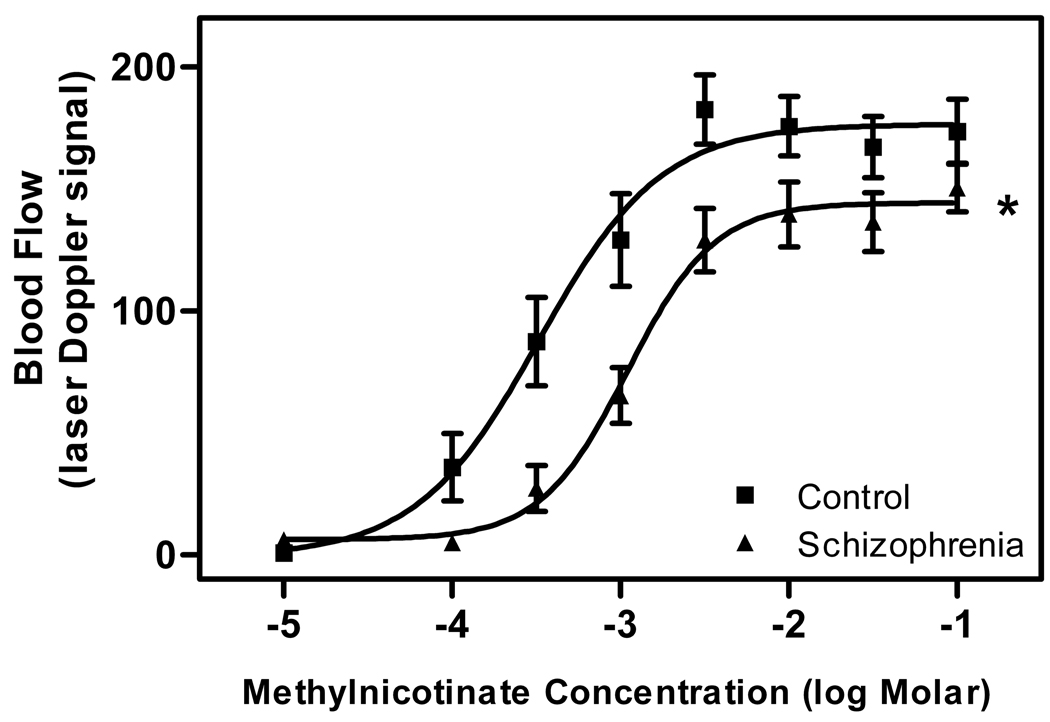
Each group’s mean blood flow responses to increasing concentrations of topical methylnicotinate are shown in Figure 2. The log EC50 value of the schizophrenia group is greater than that of the control group (−2.957 vs. −3.482) and there is no overlap of the 95% confidence intervals (−3.759 to −3.205 for the schizophrenia group; −3.108 to −2.806 for the control group). The maxima of the curves were 176.5 for the control group and 144.3 for the schizophrenia group, and there is no overlap of the 95% confidence intervals (132.2 to 156.3 for the control group; 157.5 to 195.5 for the schizophrenia group). Thus, the dose-response curve of the schizophrenia group differs significantly from that of the control group with respect to log EC50 value and maximal blood flow response.
Fig. 2. Dose-response relationship between topical methylnicotinate concentration and cutaneous blood flow response.
Data are expressed as mean ± SD from 20 control subjects and 20 patients with schizophrenia.
*There is a significant rightward shift and lower asymptote of the curve from the schizophrenia group.
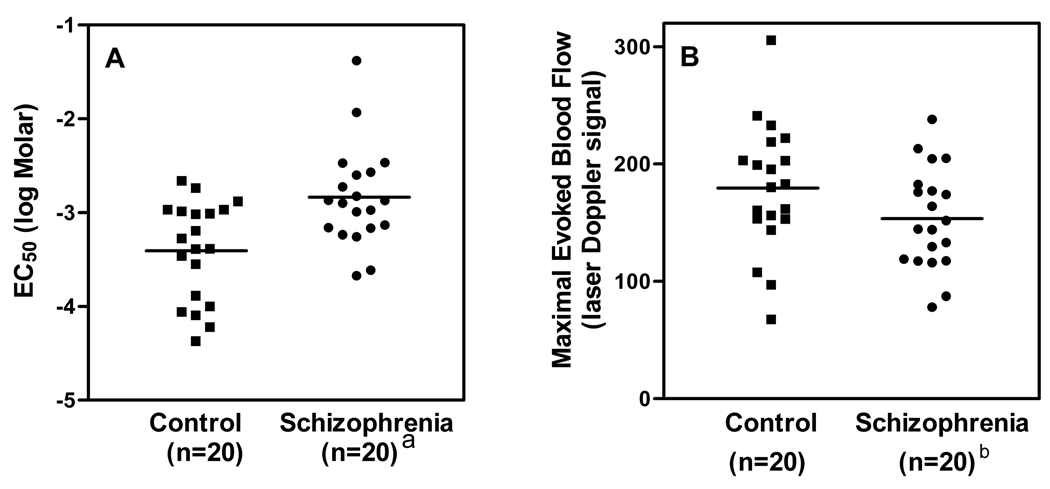
In addition to these group analyses, dose-response data from individual subjects were used to generate log EC50 and maximal response values for each participant. This approach facilitates visual examination of the dispersion of the log EC50 and maximal blood flow response values within each group. It also allows for tests of correlation with fatty acid levels. Log EC50 values from each member of both groups are presented in Figure 3A. The mean log EC50 value in the control group (−3.405 ± 0.528) is significantly lower than in the schizophrenia group (−2.840 ± 0.530; t = 3.373, df = 38, p = 0.002). Smoking status alone accounted for 21% of the variance in log EC50 [F(1,38) = 10.2, p = 0.003]. Group status added 9% to the variance accounted for by smoking alone [F(1,37) = 4.250, p = 0.046] and smoking status was not significant with group in the model [F(1,37) = 3.29, p = 0.078]. The difference between means is significant, even after accounting for smoking status.
Fig. 3. Values of niacin sensitivity and efficacy for each participant in schizophrenia and control groups.
Horizontal lines indicate the mean value of each group. After accounting for smoking status in analysis of covariance, there are significant between-group differences in sensitivity as well as efficacy.
a Significant difference versus control group [F(1,37) = 4.250, p = .046].
b Significant difference versus control group [F(1,37) = 5.89, p = .02].
Individually-derived maximal blood flow response values are presented in Figure 3B. The mean of these values was 179 (± 54) for the control group and 154 (± 43) for the schizophrenia group. Comparison of these means with a t-test suggests a trend toward lower maximal response in the schizophrenia group (t = 1.658, df = 38, p = 0.11). The difference is significant in analysis of covariance [F(1,37) = 5.89, p = 0.02], after accounting for smoking status. Smoking status alone accounted for only 0.12% of the variance [F(1,38) = 0.48, p = 0.5].
3.3 Niacin Response Correlates with AA Levels in Controls, but not in Schizophrenia
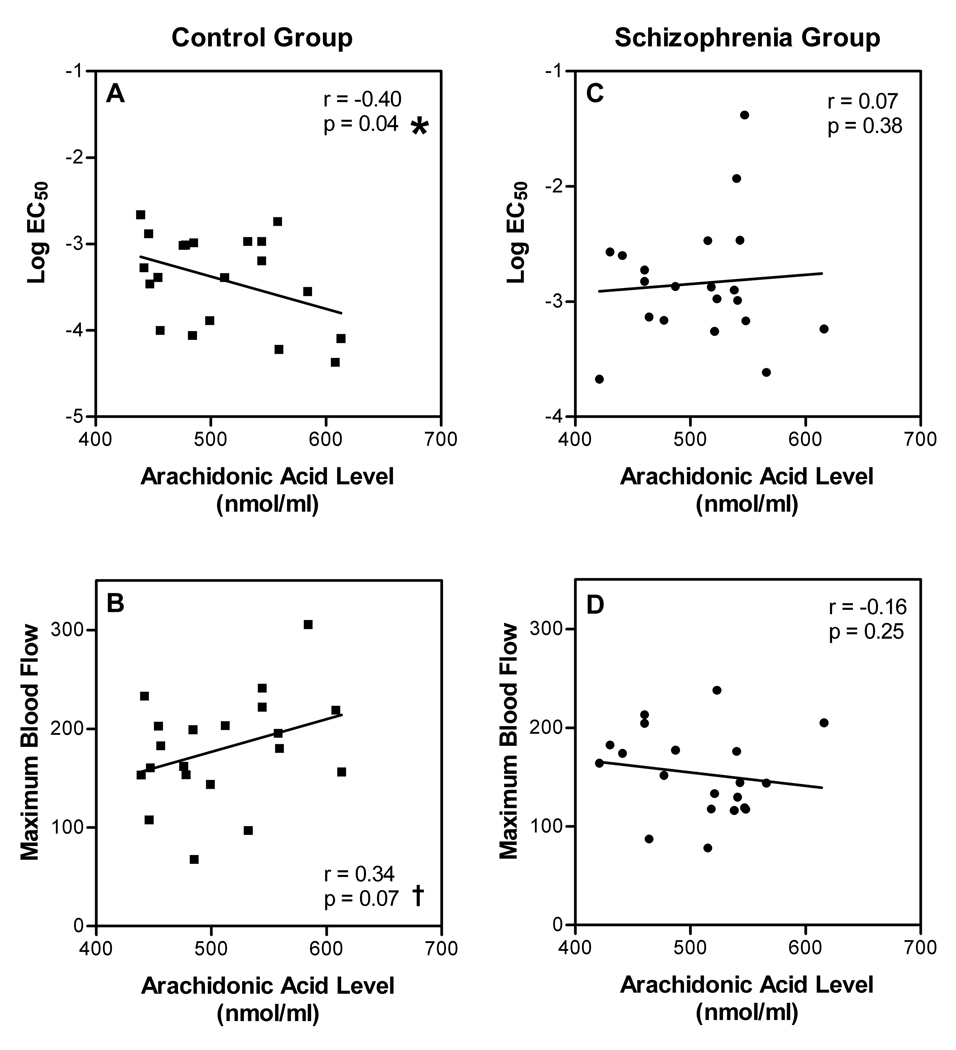
Figure 4 depicts the relationships between AA levels and measures of niacin response in control and schizophrenia groups. In the control group, there was significant negative correlation between AA levels and log EC50 values (Figure 4A; Pearson r = −0.40; p = 0.04). In this group there was also a trend toward correlation between AA levels and maximal evoked blood flow response to methylnicotinate (Figure 4B; Pearson r = 0.34; p = 0.07). In contrast, there was no correlation between AA levels and log EC50 values in the schizophrenia group (Figure 4C; Pearson r = 0.07; p = 0.38). There was also no correlation between AA levels and maximal blood flow responses in the schizophrenia group (Figure 4D; Pearson r = −0.16; p = 0.25).
Fig. 4. Relationship between AA levels and the sensitivity and efficacy of niacin in control and schizophrenia groups.
Individually-derived values of log EC50 and maximal niacin-evoked blood flow response are plotted against RBC membrane AA levels for each participant in the control (n=20) and schizophrenia (n=20) groups. Since the tested hypothesis was directional, significance tests are one-tailed.
*Significant correlation with log EC50 values.
† Trend-level correlation with maximal blood flow response.
To further explore whether abnormal niacin response predicts differences in AA levels, comparisons were made between subgroups high and low on niacin response. Within the schizophrenia group, there was no significant difference between the mean AA levels of the patients whose log EC50 values were in the lowest 25% of the sample and those whose log EC50 values were in the highest 25%. Similarly, AA levels were statistically similar between schizophrenia subgroups formed on the basis of lowest and highest 25% with respect to maximal blood flow response.
3.4 Lack of Expected Correlation Between Levels of AA and Adrenic Acid in Schizophrenia
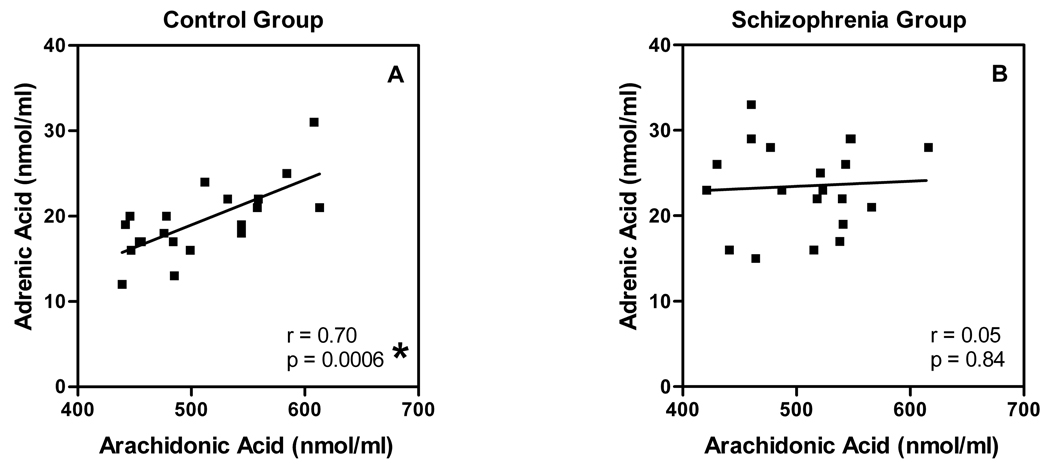
To explore n-6 fatty acid desaturation and elongation processes in our sample, we examined relationships between the measured levels of n-6 precursors and products (Table 1). N-6 PUFA levels were normally distributed in both groups. Levels of oleic acid (18:2 n-6), dihomo-γ-linolenic acid (20:3 n-6), and AA (20:4 n-6) were similar between groups. Levels of adrenic acid (22:4 n-6) appeared significantly elevated when the raw means were compared (t = 2.75, df = 38, p = 0.009). However, after controlling for smoking, which accounted for 18% of the variance [F(1,38) = 8.5, p = 0.006], the difference was not significant [F(1,37) = 2.1, p = 0.15]. Adrenic acid levels were highly correlated with levels of AA in the control group (Pearson r = 0.70, p = 0.0006). However, this correlation was essentially absent in the schizophrenia group (Figure 5; Pearson r = 0.05, p = 0.84).
TABLE 1.
RBC n-6 Polyunsaturated Fatty Acid Levels in Control and Schizophrenia Groups
| n-6 Fatty acids | Control | Schizophrenia | significance |
|---|---|---|---|
| Linoleic Acid (18:2) | 393 ± 74a | 378 ± 66 | ns |
| Dihomo-γ-linolenic Acid (20:3) | 53.6 ± 15.8 | 55.8 ± 14.1 | ns |
| Arachidonic Acid (20:4) | 508 ± 56 | 508 ± 51 | ns |
| Adrenic Acid (22:4) | 19.5 ± 4.2 | 23.4 ± 5.1 | ns |
nmol/mL packed red blood cells.
Fig. 5. The expected correlation between levels of AA and its elongation product adrenic acid is absent in schizophrenia.
RBC membrane levels of AA are plotted against levels of adrenic acid for each member of the control (A) and schizophrenia (B) groups.
*Significant correlation.
3.5 Adrenic Acid Levels Correlate with Niacin Response
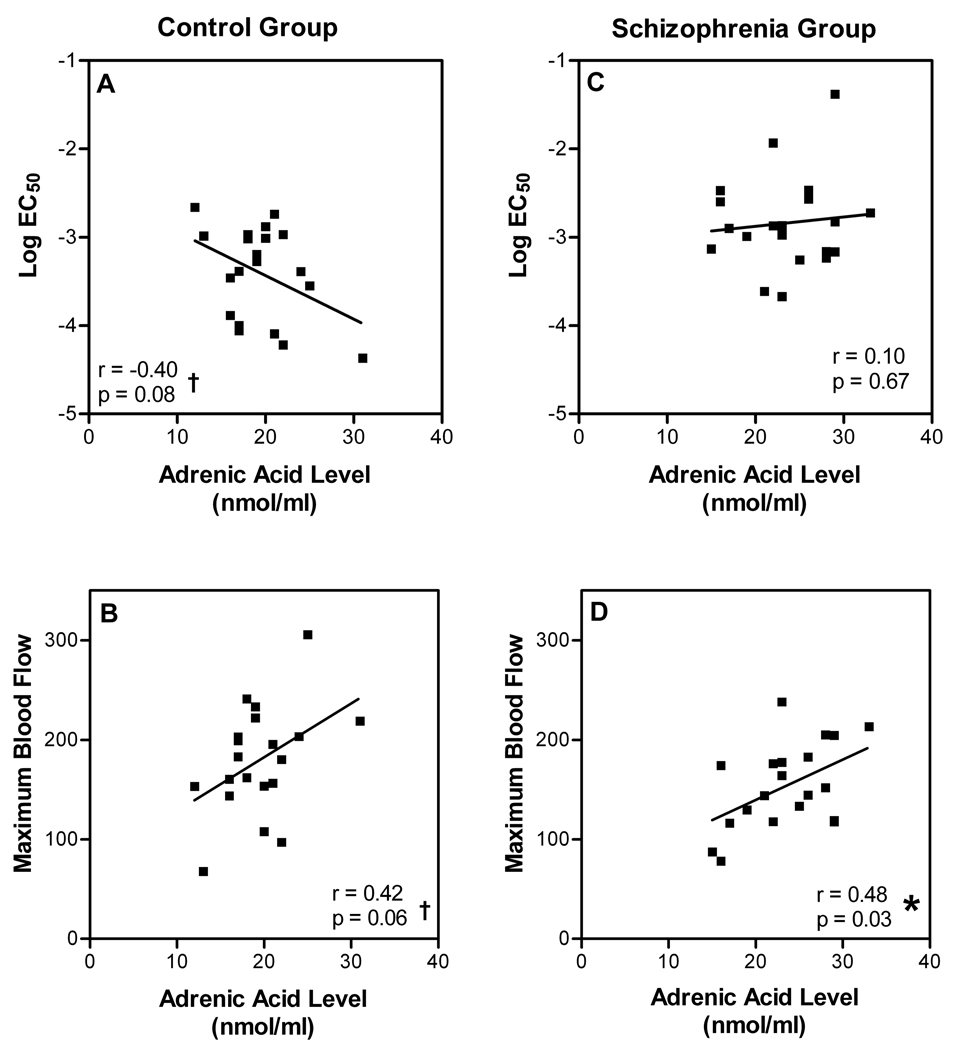
We also explored whether levels of the other n-6 PUFAs correlated with niacin response. Neither 18:2 n-6 nor 20:3 n-6 levels correlated with values of log EC50 or maximal blood flow response in either group. In contrast, we observed trends toward correlation between levels of adrenic acid and log EC50 values (Figure 6A; Pearson r = −0.40, p = 0.08) as well as maximal blood flow responses (Figure 6B; Pearson r = 0.42, p = 0.06) in the control group. In the schizophrenia group, there was no correlation between adrenic acid levels and log EC50 values (Figure 6C; Pearson r = 0.10, p = 0.67). However, there was a significant correlation with maximal blood flow response values (Figure 6D; Pearson r = 0.48, p = 0.03) in the schizophrenia group.
Fig. 6. Relationship between levels of adrenic acid and sensitivity/efficacy of niacin in control and schizophrenia groups.
Individually-derived values of log EC50 and maximal niacin-evoked blood flow response are plotted against RBC membrane levels of adrenic acid for each participant in the control (n=20) and schizophrenia (n=20) groups. Since tests for correlation were exploratory, two-tailed significance tests are not corrected for multiple comparisons.
*Significant correlation.
† Trend-level correlation.
3.6 Niacin Response and AA Levels are Unrelated to Dose of Antipsychotic Medication
All patients in the schizophrenia group were taking antipsychotic medication at the time of their participation. In order to assess the potential influence of medication on measures of niacin response, their antipsychotic medication doses were normalized to chlorpromazine-equivalent doses using the estimates of Rey et al. (1989) for first-generation medications and Woods (2003) for second-generation medications. There were no significant correlations between chlorpromazine-equivalent medication dose and values of log EC50 or maximal blood flow response. There were also no correlations between chlorpromazine-equivalent dose and the levels of AA or any of the other n-6 PUFAs examined.
4. Discussion
Our data extend numerous prior observations that the flush response to niacin is abnormal in a portion of patients with schizophrenia. Our data suggest that this is a complex abnormality, involving reductions in the pharmacological sensitivity to niacin as well as its efficacy. Our data also reveal complexity in the relationship between the niacin response and AA signaling.
Since our method of assessing the flush response involves measuring blood flow changes arising from multiple topical doses of methylnicotinate, we are able to detect possible changes in the pharmacological sensitivity to niacin, as well as possible changes in its efficacy as a vasodilator. Reduced sensitivity (increased EC50 values) to the skin flush effect of niacin in schizophrenia was noted in a prior report (Messamore et al., 2003) and replicated in the present study. Niacin efficacy (the maximal blood flow response to niacin) was lower in the schizophrenia group in our prior study, but the difference was at trend-level significance (p = 0.06). In the present study, we also observe trend-level significance (p = 0.11) to lower efficacy in the schizophrenia group when raw means are compared with a t-test. The efficacy reduction is significant when smoking status is considered in analysis of covariance (p = 0.02). Considering the totality of these observations, we conclude that niacin sensitivity is more robustly affected in schizophrenia, but that niacin’s efficacy as a vasodilator is also reduced.
Considering the role of AA-derived vasodilators in mediating the niacin flush response (Figure 1), and in light of reports of AA deficiency in schizophrenia (Skosnik & Yao, 2003), we postulated that a blunted niacin response would correlate with low AA levels among patients with schizophrenia. However, this is not what we observed. Although AA levels did correlate with niacin sensitivity in the control group, there were no correlations between AA levels and niacin sensitivity or efficacy in the schizophrenia group (Figure 4). Prior investigations of the relationship between AA levels and the niacin flush response in schizophrenia have produced conflicting results. Glen et al. (1996) found that low AA levels predicted absence of a visible skin flush response one hour after a single oral dose of niacin. On the other hand, Maclean et al. (2003) found no correlation between levels of AA and the visually-rated intensity of flush response during a 20 minute period after exposure to four concentrations of topical methylnicotinate. Differences in the routes of administration and measurement of flush response may account for the discrepancies between these studies. We opted to use topical methylnicotinate for this study because blood levels of niacin following oral ingestion are highly variable (Stern et al., 1991) and because the topical method allows for simultaneous exposure to multiple doses. We also employed quantitative, instrumental measurement of the flush response. Our ability to demonstrate correlation between niacin sensitivity and AA levels in the control group suggests that this method is able to detect correlation when present and thus increases confidence in our conclusions that AA levels may contribute to niacin sensitivity under normal conditions, but that AA levels alone do not account for the niacin response abnormality in schizophrenia.
The blunted niacin response in our schizophrenia group occurred in the presence of normal AA levels. Our observation of normal AA levels in schizophrenia is consistent with reports that AA levels can be normal in schizophrenia (Peet et al., 2004). Additionally, AA levels may increase or normalize with long-term medication treatment (Arvindakshan et al., 2003). The patients in our study were chronically ill and all were treated with medication; these factors may explain why we found no evidence of AA deficiency in our sample.
Though levels of AA and other n-6 PUFAs were similar between groups, we observed an intriguing schizophrenia-associated abnormality in the relationship between the levels of AA and its elongation product adrenic acid. Adrenic acid levels normally correlate with those of the parent compound AA (Wijendran et al., 2002). Accordingly, we observed a highly significant positive correlation between levels of AA and adrenic acid in the control group. In contrast, this expected correlation was obliterated in the schizophrenia group (Figure 5), and this between-group difference was significant even after accounting for smoking status. Recently, Yao et al. (2009; 2010) have shown the existence of tightly correlated relationships between certain pairs of precursor and product within biochemical pathways; although many of these correlated relationships persist across disease or medication status, others are lost among patients with schizophrenia. Our findings suggest that the rate or regulation of AA elongation is severely disordered in patients with schizophrenia.
Compared to other fatty acids, there are relatively few reports of adrenic acid changes in schizophrenia. There are reports of decreased levels of adrenic acid in medicated patients (Vaddadi et al., 1986; Yao et al., 1994) and in unmedicated patients (Peet et al., 2004). On the other hand, Richardson et al. (2003) observed significantly elevated adrenic acid levels in adults with schizotypal personality traits. Elevated adrenic acid levels have been associated with the development of diabetes among schizophrenia patients (Krachler et al., 2008).
There were trends for correlation between levels of adrenic acid and measures of niacin response within the control group. This is expected since adrenic acid levels are tightly correlated with AA levels in the control group, and since AA levels were correlated with niacin response in the control group. We unexpectedly observed significant correlation between levels of adrenic acid and niacin’s efficacy as a vasodilator in the schizophrenia group. Though this may be a spurious correlation arising from multiple correlation testing in exploratory data analysis, it could also implicate the relevance and complexity of abnormal AA metabolism as part of the explanation for the niacin response abnormality in schizophrenia.
Prior comparisons of the niacin-induced flushing between medicated and unmedicated schizophrenia patients, or unmedicated non-psychotic relatives, suggest that the niacin response abnormality in schizophrenia is not an artifact of antipsychotic medications (Chang et al., 2009; Lin et al., 2007; Maclean et al., 2003; Shah et al., 2000). Consequently, we did not design the present study to specifically investigate the potential effects of antipsychotic drugs on niacin response. Nonetheless, we found no significant correlations between chlorpromazine-equivalent medication dose and the values of log EC50 or maximal blood flow response. These observations are consistent with earlier findings that niacin response is unrelated to antipsychotic dose (Hudson et al., 1997; Messamore et al., 2003). On the other hand, a study involving repeated measures of niacin sensitivity in medicated patients suggest that second-generation antipsychotic drugs may actually enhance niacin sensitivity (Tavares et al., 2003). Ninety percent of the patients in our present study were taking second-generation antipsychotic drugs. If such drugs do tend to normalize niacin sensitivity, then the potential bias in our sample would be to underestimate the prevalence or magnitude of the niacin response abnormality in schizophrenia. If these medications contributed to normalization of the niacin response in our sample, the data suggest that the mechanism would not require correlation between AA levels and values of EC50 or maximal response.
Several prior reports suggest that cigarette smoking does not affect the flush response to niacin (Chang et al., 2009; Liu et al., 2007; Shah et al., 2000; Smesny et al., 2001). Thus we did not attempt to stringently match our control group to the cigarette use of those in the schizophrenia group. Because the potential impact of cigarette use is frequently considered in the niacin response literature, we asked participants to estimate their average daily cigarette consumption. Analysis of covariance revealed no effect of smoking on niacin efficacy. Although we observed an effect of smoking status on niacin sensitivity, we also found an additional significant effect of group status on niacin sensitivity. Thus, even after considering the effect of smoking, the diagnosis of schizophrenia was associated with reduced niacin sensitivity. Fatty acid levels can be influenced by the composition of the diet and by alcohol intake. The extent to which these environmental factors may influence niacin sensitivity is not known. A potential limitation of our study is that we did not specifically inquire about dietary habits, nor did we attempt to quantify participants’ alcohol consumption.
Based on the mechanism depicted in Figure 1, there are several possible explanations for the finding of niacin subsensitivity in schizophrenia. These include: abnormal signaling at the niacin receptor; abnormal presentation of free AA to cyclooxygenase; abnormal cyclooxygenase activity; abnormal conversion of initial cyclooxygenase products to vasodilatory end products; or abnormal signaling at prostanoid receptors in vascular smooth muscle. Support for abnormal niacin receptor signaling is provided by a report of decreased niacin receptor expression in post-mortem brain from schizophrenia patients (Miller and Dulay, 2008). Presentation of free AA to cyclooxygenase is accomplished by the action of phospholipase A2, which may be abnormally active in niacin-subsensitive schizophrenia (Hudson et al., 1999; Tavares et al., 2003); however, if this was the case in our sample, it did not result in the expected depletion of cell membrane AA stores. Failure to release measurable quantities of vasodilatory prostaglandins is associated with the development of tolerance to the skin flush effect after repeated oral niacin doses (Stern et al., 1991). Any process leading to diminished output of prostaglandins could potentially account for niacin subsensitivity. Abnormal cyclooxygenase activity in schizophrenia was indirectly demonstrated by Das and Khan (1998). Cyclooxygenase action on AA initially produces prostaglandin H2, which is subsequently converted by tissue-specific isomerases to vasodilatory end-products. Isomerase abnormalities have been detected in discrete brain regions from patients with a variety of mental illnesses (Maida et al., 2006). We suggest that one, or some combination, of these other mechanisms will prove to be more relevant than simple AA deficiency as the cause of niacin subsensitivity in schizophrenia. Many of these other mechanisms are compatible with our observation of abnormal niacin response despite normal AA levels in schizophrenia. It is intriguing to note that none of these pathways is specifically targeted by existing antipsychotic medications. To the extent that the niacin response abnormality may represent a schizophrenia endophenotype, discovery of its biochemical mechanism may inform the development of novel strategies to categorize or treat this illness.
The present study represents an attempt to delineate the biochemical mechanism of the abnormal niacin response in schizophrenia. Our data do not support the hypothesis that the abnormality arises strictly from AA deficiency. We nonetheless have detected evidence of impaired homeostatic balance of AA metabolic pathways in schizophrenia. We suggest that some other site in the AA signaling pathway is abnormal among schizophrenia patients with blunted niacin response.
Acknowledgements
We thank Laura Dennis who assisted with the preparation the manuscript. The authors are grateful to Carol Korbanic and Jack Haflett for their technical assistance.
Role of the Funding Source
Funding for this study was provided by: NIMH grant R03 MH070434 (to EM); NIMH grant R01 MH58141 (JKY); and a VA Senior Research Career Scientist Award (JKY). Neither the NIMH nor VA funding sources had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Erik Messamore and Jeffrey K. Yao co-designed the study and co-wrote the protocol. William F. Hoffman contributed to the study design and undertook the statistical analysis. All authors have contributed to and approved the final manuscript.
Conflict of Interest
None of the authors have any actual or potential conflicts of interest, including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence, or be perceived to influence, their work.
References
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol. Psychiatry. 2003;53(1):56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K, Offermanns S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J. Clin. Invest. 2005;115(12):3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol. Pharmacol. 2006;70(6):1844–1849. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- Bosveld-van Haandel L, Knegtering R, Kluiter H, van den Bosch RJ. Niacin skin flushing in schizophrenic and depressed patients and healthy controls. Psychiatry Res. 2006;143(2–3):303–306. doi: 10.1016/j.psychres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Chang SS, Liu CM, Lin SH, Hwu HG, Hwang TJ, Liu SK, Hsieh MH, Guo SC, Chen WJ. Impaired flush response to niacin skin patch among schizophrenia patients and their nonpsychotic relatives: the effect of genetic loading. Schizophr. Bull. 2009;35(1):213–221. doi: 10.1093/schbul/sbm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Khan NS. Increased arachidonic acid induced platelet chemiluminescence indicates cyclooxygenase overactivity in schizophrenic subjects. Prostaglandins Leukot. Essent. Fatty Acids. 1998;58(3):165–168. doi: 10.1016/s0952-3278(98)90109-0. [DOI] [PubMed] [Google Scholar]
- Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Eklund B, Kaijser L, Nowak J, Wennmalm A. Prostaglandins contribute to the vasodilation induced by nicotinic acid. Prostaglandins. 1979;17(6):821–830. doi: 10.1016/0090-6980(79)90055-8. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- Glen AI, Cooper SJ, Rybakowski J, Vaddadi K, Brayshaw N, Horrobin DF. Membrane fatty acids, niacin flushing and clinical parameters. Prostaglandins Leukot. Essent. Fatty Acids. 1996;55(1–2):9–15. doi: 10.1016/s0952-3278(96)90139-8. [DOI] [PubMed] [Google Scholar]
- Hudson CJ, Lin A, Cogan S, Cashman F, Warsh JJ. The niacin challenge test: clinical manifestation of altered transmembrane signal transduction in schizophrenia? Biol. Psychiatry. 1997;41(5):507–513. doi: 10.1016/s0006-3223(96)00112-6. [DOI] [PubMed] [Google Scholar]
- Hudson C, Gotowiec A, Seeman M, Warsh J, Ross BM. Clinical subtyping reveals significant differences in calcium-dependent phospholipase A2 activity in schizophrenia. Biol. Psychiatry. 1999;46(3):401–405. doi: 10.1016/s0006-3223(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Shibahara A, Yamamoto K, Nakayama T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids. 1996;31(5):535–539. doi: 10.1007/BF02522648. [DOI] [PubMed] [Google Scholar]
- Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2008;18(7):503–510. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Lai E, De Lepeleire I, Crumley TM, Liu F, Wenning LA, Michiels N, Vets E, O'Neill G, Wagner JA, Gottesdiener K. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin. Pharmacol. Ther. 2007;81(6):849–857. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- Lin SH, Liu CM, Chang SS, Hwu HG, Liu SK, Hwang TJ, Hsieh MH, Guo SC, Chen WJ. Familial aggregation in skin flush response to niacin patch among schizophrenic patients and their nonpsychotic relatives. Schizophr. Bull. 2007;33(1):174–182. doi: 10.1093/schbul/sbl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Chang SS, Liao SC, Hwang TJ, Shieh MH, Liu SK, Chen WJ, Hwu HG. Absent response to niacin skin patch is specific to schizophrenia and independent of smoking. Psychiatry Res. 2007;152(2–3):181–187. doi: 10.1016/j.psychres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Maclean R, Ward PE, Glen I, Roberts SJ, Ross BM. On the relationship between methylnicotinate-induced skin flush and fatty acids levels in acute psychosis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(6):927–933. doi: 10.1016/S0278-5846(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Maida ME, Hurley SD, Daeschner JA, Moore AH, O'Banion MK. Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease. Brain Res. 2006;1103(1):164–172. doi: 10.1016/j.brainres.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Messamore E. Relationship between the niacin skin flush response and essential fatty acids in schizophrenia. Prostaglandins Leukot. Essent. Fatty Acids. 2003;69(6):413–419. doi: 10.1016/j.plefa.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Messamore E, Hoffman WF, Janowsky A. The niacin skin flush abnormality in schizophrenia: a quantitative dose-response study. Schizophr. Res. 2003;62(3):251–258. doi: 10.1016/s0920-9964(02)00311-0. [DOI] [PubMed] [Google Scholar]
- Miller CL, Dulay JR. The high-affinity niacin receptor HM74A is decreased in the anterior cingulate cortex of individuals with schizophrenia. Brain Res. Bull. 2008;77(1):33–41. doi: 10.1016/j.brainresbull.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Oates JA, Roberts LJ., 2nd Identification of skin as a major site of prostaglandin D2 release following oral administration of niacin in humans. J. Invest. Dermatol. 1992;98(5):812–815. doi: 10.1111/1523-1747.ep12499963. [DOI] [PubMed] [Google Scholar]
- Peet M, Shah S, Selvam K, Ramchand CN. Polyunsaturated fatty acid levels in red cell membranes of unmedicated schizophrenic patients. World J. Biol. Psychiatry. 2004;5(2):92–99. doi: 10.1080/15622970410029917. [DOI] [PubMed] [Google Scholar]
- Rey MJ, Schulz P, Costa C, Dick P, Tissot R. Guidelines for the dosage of neuroleptics, 1: Chlorpromazine equivalents of orally administered neuroleptics. Int. Clin. Psychopharmacol. 1989;4(2):95–104. doi: 10.1097/00004850-198904000-00001. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Cyhlarova E, Ross MA. Omega-3 and omega-6 fatty acid concentrations in red blood cell membranes relate to schizotypal traits in healthy adults. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):461–466. doi: 10.1016/j.plefa.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J. Lipid Res. 1965;6:428–431. [PubMed] [Google Scholar]
- Shah SH, Vankar GK, Peet M, Ramchand CN. Unmedicated schizophrenic patients have a reduced skin flush in response to topical niacin. Schizophr. Res. 2000;43(2–3):163–164. [PubMed] [Google Scholar]
- Skosnik PD, Yao JK. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins Leukot. Essent. Fatty Acids. 2003;69(6):367–384. doi: 10.1016/j.plefa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Smesny S, Riemann S, Riehemann S, Bellemann ME, Sauer H. Quantitative measurement of induced skin reddening using optical reflection spectroscopy - methodology and clinical application. Biomed Tech (Berl) 2001;46(10):280–286. doi: 10.1515/bmte.2001.46.10.280. [DOI] [PubMed] [Google Scholar]
- Stern RH, Spence JD, Freeman DJ, Parbtani A. Tolerance to nicotinic acid flushing. Clin. Pharmacol. Ther. 1991;50(1):66–70. doi: 10.1038/clpt.1991.104. [DOI] [PubMed] [Google Scholar]
- Svedmyr N, Heggelund A, Aberg G. Influence of indomethacin on flush induced by nicotinic acid in man. Acta Pharmacol. Toxicol. 1977;41(4):397–400. doi: 10.1111/j.1600-0773.1977.tb02678.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhou L, Gunnet JW, Wines PG, Cryan EV, Demarest KT. Enhancement of arachidonic acid signaling pathway by nicotinic acid receptor HM74A. Biochem. Biophys. Res. Commun. 2006;345(1):29–37. doi: 10.1016/j.bbrc.2006.04.051. [DOI] [PubMed] [Google Scholar]
- Tavares H, Yacubian J, Talib LL, Barbosa NR, Gattaz WF. Increased phospholipase A2 activity in schizophrenia with absent response to niacin. Schizophr. Res. 2003;61(1):1–6. doi: 10.1016/s0920-9964(02)00281-5. [DOI] [PubMed] [Google Scholar]
- Vaddadi KS, Gilleard CJ, Mindham RH, Butler R. A controlled trial of prostaglandin E1 precursor in chronic neuroleptic resistant schizophrenic patients. Psychopharmacology (Berl) 1986;88(3):362–367. doi: 10.1007/BF00180839. [DOI] [PubMed] [Google Scholar]
- Waldo MC. Co-distribution of sensory gating and impaired niacin flush response in the parents of schizophrenics. Schizophr. Res. 1999;40(1):49–53. doi: 10.1016/s0920-9964(99)00031-6. [DOI] [PubMed] [Google Scholar]
- Wijendran V, Lawrence P, Diau G-Y, Boehm G, Nathanielsz PW, Brenna JT. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J. Lipid Res. 2002;43(5):762–767. [PubMed] [Google Scholar]
- Wilkin JK, Wilkin O, Kapp R, Donachie R, Chernosky ME, Buckner J. Aspirin blocks nicotinic acid-induced flushing. Clin. Pharmacol. Ther. 1982;31(4):478–482. doi: 10.1038/clpt.1982.63. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yao JK, van Kammen DP, Welker JA. Red blood cell membrane dynamics in schizophrenia. II. Fatty acid composition. Schizophr Res. 1994;13(3):217–226. doi: 10.1016/0920-9964(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Jr, Reddy RD, Keshavan MS, Montrose DM, Matson WR, Rozen S, Krishnan RR, McEvoy J, Kaddurah-Daouk R. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol. Psychiatry. 2009 doi: 10.1038/mp.2009.33. Online publication data: (28 April 2009) doi:10.1038/mp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Jr, Reddy RD, Keshavan MS, Montrose DM, Matson WR, McEvoy J, Kaddurah-Daouk R. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS One. 2010;5(3):e9508. doi: 10.1371/journal.pone.0009508. doi:10.1371/journal.pone.0009508. [DOI] [PMC free article] [PubMed] [Google Scholar]