Abstract
TNF receptor superfamily member 25 (TNFRSF25; also known as DR3, and referred to herein as TNFR25) is constitutively and highly expressed by CD4+FoxP3+ Tregs. However, its function on these cells has not been determined. Here we used a TNFR25-specific agonistic monoclonal antibody, 4C12, to study the effects of TNFR25 signaling on Tregs in vivo in mice. Signaling through TNFR25 induced rapid and selective expansion of preexisting Tregs in vivo such that they became 30%–35% of all CD4+ T cells in the peripheral blood within 4 days. TNFR25-induced Treg proliferation was dependent upon TCR engagement with MHC class II, IL-2 receptor, and Akt signaling, but not upon costimulation by CD80 or CD86; it was unaffected by rapamycin. TNFR25-expanded Tregs remained highly suppressive ex vivo, and Tregs expanded by TNFR25 in vivo were protective against allergic lung inflammation, a mouse model for asthma, by reversing the ratio of effector T cells to Tregs in the lung, suppressing IL-13 and Th2 cytokine production, and blocking eosinophil exudation into bronchoalveolar fluid. Our studies define what we believe to be a novel mechanism for Treg control and important functions for TNFR25 in regulating autoaggression that balance its known role in enhancing autoimmunity.
Introduction
The TNF superfamily (TNFSF) consists of at least 19 ligands and 30 receptors (TNFRSFs) that are differentially expressed by both lymphoid and nonlymphoid cells. In CD3+ T cells, TNFSF signals function usually in TCR-dependent ways to support various phases of an immune response, including polarization, expansion, effector function, contraction, memory, and death (1, 2). TNFRSF25 (also known as DR3, and referred to herein as TNFR25) is one of the more recently discovered TNFSF members and is expressed primarily by CD4+ and CD8+ T and NKT cells (3–9). The ligand for TNFR25, TL1A (also known as TNFSF15), is expressed by some endothelial cells and is rapidly induced on dendritic cells and macrophage/monocytes following TLR4 or FcγR signaling (10–12). In vitro studies demonstrate that TNFR25 signaling on CD4+, CD8+, or NKT cells increases IL-2, IL-4, and IFN-γ production subsequent to TCR activation or costimulation by IL-12 and IL-18 (10, 13, 14). TNFR25 signaling also lowers the threshold of CD4+ T cells to TCR-induced proliferation in the absence of CD28 costimulation by an IL-2–dependent mechanism (10, 15).
Activation of TNFR25 by TL1A exacerbates disease pathology in experimental asthma, inflammatory bowel disease (IBD), RA, and EAE (3, 10, 16–18). In each of these studies, antigen-dependent TNFR25 stimulation of Th1-, Th2-, or Th17-polarized and TCR-activated effector T cells (Teffs) enhances the production of the relevant effector cytokines from each Th cell subset. TNFR25 signals are not required for the differentiation of naive CD4+ T cells toward Th1, Th2, or Th17 lineages (10). In several of these reports, mouse models with genetic ablation of TNFR25 or TL1A (16, 18) or transgenic mouse models expressing a dominant-negative TNFR25 or systemic antibody blockade of TL1A were studied (3, 17). No immune abnormalities or disease susceptibilities have been observed in mouse models deficient in TL1A or TNFR25 or in autoaggressive disease models in which normal signaling of TL1A to TNFR25 is inhibited. Furthermore, in each of these reports, expression of TNFR25 or TL1A produces a proinflammatory phenotype that appears more hazardous to the animal than in the absence of TNFR25 or TL1A.
To our knowledge, there have been no reports to date examining the role of TNFR25 on CD4+FoxP3+ Tregs, although it has been reported that Tregs express TNFR25 (16). Given the importance of Tregs in preventing lethal autoimmunity (19), expression of TNFR25 by Tregs, and function of TNFR25 in the pathogenesis of multiple autoaggressive disease models, we decided to study the role of TNFR25 on the function of Tregs. Our investigation revealed that TNFR25 was highly expressed by Tregs, but not CD4+FoxP3– conventional T cells (Tconvs). In vivo stimulation of TNFR25 in the absence of exogenous antigen using an agonistic antibody, clone 4C12, led to rapid and selective proliferation of preexisting Tregs, but not Tconvs, to 30%–35% of all CD4+ T cells within 4 days of 4C12 treatment; this effect was dependent upon TCR engagement with MHC class II (MHC-II) and IL-2 signaling. Treg expansion by TNFR25 protected against lung inflammation upon airway antigen challenge of sensitized mice. These data demonstrate what we believe to be a novel role for TNFR25 as a regulator of Tregs, which may protect from disease pathogenesis in allergic asthma. Furthermore, in vivo expansion of Tregs with TNFR25 agonists may provide a translatable method, as an alternative to IL-2– or ex vivo–based approaches, to facilitate the clinical use of Treg therapy in humans.
Results
TNFR25 is highly expressed by Tregs.
To our knowledge, there are no reports to date demonstrating a function for TNFR25 on Tregs, although a single report has documented TNFR25 expression by Tregs (16). To confirm expression of TNFR25 by Tregs, Tconvs and Tregs were single-cell sorted from FoxP3 reporter mice to greater than 99% purity and subsequently analyzed by flow cytometry for expression of TNFR25 as well as GITR (also known as TNFRSF18), OX40 (also known as TNFRSF4 or CD134), and 4-1BB (also known as TNFRSF9 or CD137). Sorting of live Tregs was made possible by use of FoxP3-RFP reporter mice (FIR mice) expressing a red fluorescent protein (RFP) knockin transgene from a bicistronic construct under the FoxP3 promoter (20). Whereas TNFR25, OX40, GITR, and 4-1BB were all expressed by both Tregs and Tconvs, the greatest relative difference in expression levels was observed by very high expression of TNFR25 in Tregs compared with low expression by Tconvs (Figure 1A). The differential expression of TNFR25 between Tregs and Tconvs suggested that TNFR25 may play an important role in the function of Tregs, which we decided to study.
Figure 1. TNFR25 stimulates rapid proliferation of CD4+FoxP3+ cells in vivo.
(A) Differential expression of TNFR25, GITR, OX40, and 4-1BB on CD4+FoxP3– Tconvs and CD4+FoxP3+ Tregs. TNFRSF expression was determined by flow cytometry on highly purified Tconvs and Tregs from splenocytes harvested from untreated FIR mice. (B) Kinetics and dose-dependent expansion of Tregs in peripheral blood after 4C12 injection. FIR mice were injected i.p. with the indicated amounts of purified 4C12. The mice were bled daily, and FoxP3-RFP expression was analyzed in peripheral blood cells by flow cytometry. (C) Treg expansion was compared after treatment with other TNFR-agonistic antibodies. Mice were injected i.p. with the indicated antibodies (100 μg) on day 0. Mice were bled daily as in B for 6 days, and the percentage of peripheral blood Tregs relative to total CD4+ T cells on day 4 was determined. Data were reproduced in more than 8 independent experiments. Error bars indicate mean ± SEM. Significance was determined by Student’s t test (B) or 1-way ANOVA with Tukey post-test (C). *P < 0.05; **P < 0.01; ***P < 0.001.
TNFR25 stimulation rapidly expands Tregs in vivo.
We previously reported the generation of a TNFR25 agonistic antibody, clone 4C12 (3). By use of FIR mice, we were able to continuously monitor the frequency and phenotype of the Treg population in peripheral blood following 4C12 treatment. Injection i.p. of 4C12 induced rapid and highly reproducible expansion of Tregs in vivo (Figure 1B). This expansion was maximal at 4 and 5 days after 4C12 injection, with Tregs constituting 30%–35% of the total CD4+ T cells in the peripheral blood at the peak of the response. 4C12-expanded Tregs persisted in the peripheral blood and all tissue sites examined for 2 weeks, slowly contracting to levels seen in unstimulated mice. The site of injection did not play a role in this expansion, as demonstrated by equivalent Treg expansion following 4C12 injection either i.p., s.c., or i.v. (data not shown). Treg expansion following 4C12 injection was dose dependent, with maximal responses seen with a dose of only 10 μg, corresponding to approximately 0.4 mg/kg body weight (Figure 1B). Treatment of FIR mice with purified mouse TL1A-Ig fusion protein (100 μg) induced Treg expansion with a magnitude and with kinetics similar to those of 4C12 treatment (data not shown). Detection of RFP expression in FIR mice faithfully reports the presence of FoxP3 transcripts; however, the possibility exists that it may not guarantee expression of FoxP3 protein because FoxP3 and RFP are independently translated from the FoxP3-RFP transcript. Therefore, expansion of CD4+FoxP3+ cells following 4C12 administration was confirmed in wild-type mice by staining with FoxP3 antibodies and in FoxP3-GFP knockin reporter mice, which express a FoxP3-GFP fusion protein (data not shown).
Among Treg-expressed TNFR members, TNFR25 is unique in causing Treg expansion.
The TNFRSF members GITR and OX40, which we observed to be expressed by Tregs (Figure 1A), affect Treg activity and proliferation (21–27). There are also reports that stimulation of Tregs by 4-1BB can modulate both activity and proliferation of these cells (28, 29). Furthermore, stimulation of CD4+FoxP3– cells by TNFRSF member CD27 (also known as TNFRSF7) has been reported to induce FoxP3 expression (30). Given the overlap between either functional suppression or induction of Tregs between TNFR25 and these other TNFSF members, we compared Treg expansion in vivo after stimulation of TNFR25, OX40, 4-1BB, GITR, or CD27. In all cases, we used well-characterized agonistic monoclonal antibodies to the respective receptor to trigger specific signaling. These studies demonstrated that TNFR25 was unique among the TNFRSF members examined in its ability to selectively induce expansion of Tregs (Figure 1C). It was recently reported that OX40-induced Treg expansion required depletion of IL-4, IL-6, and IFN-γ (31). In contrast, TNFR25-induced Treg expansion in vivo required no additional manipulations.
MHC-II and IL-2 signals are required for TNFR25-induced Treg proliferation.
In vitro, Treg proliferation can be induced with various combinations of TCR-stimulating antibodies, APCs, and IL-2 signals (32–35). We attempted to induce Treg proliferation in vitro using many different combinations of anti-CD3 and anti-CD28 antibodies, recombinant IL-2, TGF-β, and retinoic acid with or without TNFR25 agonistic antibody; in all cases, TNFR25 stimulation failed to enhance Treg proliferation in vitro, which indicated that additional signals were required (data not shown and Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI42933DS1). Because TNFR25 has been reported to influence the responsiveness of CD4+ T cells to TCR signals (10, 15), we determined whether TNFR25-induced Treg proliferation depends upon TCR signaling in vivo. MHC-II–deficient (Cd74–/–) or Cd4–/– mice were adoptively transferred with total CD4+ cells containing 106 CD4+FoxP3+ cells purified from FIR mice. Because Cd74–/– mice are deficient in CD4+ T cells, we used Cd4–/– mice as a control population to control for any homeostatic expansion that may occur following adoptive transfer into a CD4+ T cell–depleted environment. Mice were treated with 4C12 or isotype control antibody 2 days after adoptive transfer, and the percentage and absolute number of Tregs were determined at days 4 and 6 after antibody injection (Figure 2, A and B). Although Tregs expanded to a similar degree in wild-type and Cd4–/– mice, MHC-II molecules were required for TNFR25-induced Treg proliferation in vivo. The percentage of adoptively transferred Tregs in Cd74–/– mice was lower than that in Cd4–/– mice, because Cd74–/– mice had a greater number of CD4+ cells at baseline than did Cd4–/– mice (Figure 2A). Comparison of the absolute numbers of adoptively transferred Tregs, however, showed equivalent numbers from the 2 groups (Figure 2B). These studies demonstrate a requirement for MHC-II signals in TNFR25-induced Treg proliferation, which indirectly implies that TCR signaling is required for Tregs to become permissive to TNFR25 signaling, similar to the known requirement for TNFR25 signaling in Tconvs (10, 15). To provide additional evidence that TCR signals are required for TNFR25-induced Treg proliferation, mice were pretreated with cyclosporin-A or FK506, and Treg numbers were analyzed subsequent to treatment with the 4C12 or isotype control antibodies (Figure 2, C and D). Similar to what was observed in the absence of MHC-II signals, TNFR25 triggering in the presence of cyclosporin-A or FK506 failed to induce Treg proliferation. The requirement for cognate self-antigen in the MHC-II is currently unknown and under further investigation, but such a requirement may provide an additional explanation for Treg selectivity of TNFR25 (in addition to the selective expression of TNFR25 on Tregs; Figure 1) in the absence of exogenous antigen.
Figure 2. TNFR25-induced Treg expansion requires TCR and IL-2 signaling.
CD4+ cells were highly purified by FACS sorting from FIR mice and adoptively transferred into Cd74–/– or Cd4–/– mice. Following adoptive transfer, recipient mice were treated with either 4C12 or isotype control antibody, and the percentage (A) and absolute number (B) of FoxP3+ cells was analyzed 4 days after antibody treatment. FIR mice were treated with cyclosporin-A (C) or FK506 (D) or a vehicle control from day –1 through day 4 by i.p. injection as described in Methods. Mice were treated with either 4C12 antibody or IgG control antibody, and the proportion of FoxP3+ cells relative to total CD4+ cells in the peripheral blood was analyzed on day 4. Tg+ Il2rb–/– (E) or Cd80–/–Cd86–/– (F) mice were analyzed for the proportion of CD4+FoxP3+ cells relative to total CD4+ splenocytes 4 days after treatment with either 4C12 or isotype control antibody, compared with C57BL/6 control mice. Data are mean ± SEM of at least 2 independent experiments, with 3 or more mice per group per experiment. **P < 0.01; ***P < 0.001.
It has previously been reported that TNFR25 signaling increases the responsiveness of Tconvs to IL-2 signals subsequent to TCR signals in the absence of CD28 costimulation (10, 15). Given the requirement for both MHC-II and NFAT activation for TNFR25-induced proliferation of Tregs (Figure 2, A–D), we next determined whether IL-2 or CD80/86 signals are additionally required. Treg expansion in Cd80–/–Cd86–/– mice and in mice expressing a thymic targeted transgenic IL-2 receptor (IL-2R) β chain in Il2rb–/– mice (Tg+ Il2rb–/– mice) was determined 4 days after injection of 4C12 (Figure 2, E and F). These data demonstrated that TCR and IL-2R signaling, but not CD80 or CD86 costimulation, was required for TNFR25-induced Treg expansion in vivo, which indirectly suggests that CD28 and CTLA-4 signaling in Tregs is not a requirement for TNFR25-induced proliferation. Furthermore, because combined TNFR25, TCR stimulation, and IL-2 signaling failed to induce Treg proliferation in vitro, we conclude that additional signals are required; these are also under investigation.
TNFR25-stimulated Tregs are hyperresponsive to IL-2–induced proliferation ex vivo.
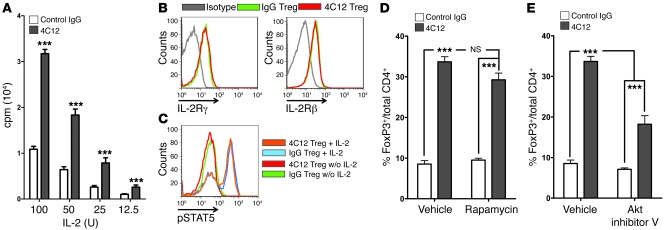
Although the requirements for TNFR25-induced Treg proliferation in vitro remain elusive, we observed that Tregs purified from mice treated with TNFR25 agonistic antibodies were hyperresponsive to IL-2–induced proliferation ex vivo (Figure 3A). These data corroborate the importance of IL-2 signals in TNFR25-induced Treg expansion (Figure 2E) and suggest that TNFR25 triggering induces Treg expansion by influencing the sensitivity of Tregs to IL-2 signals. We envisioned several potential mechanisms that could explain this observation: (a) TNFR25 could increase the expression of IL-2R subunits on Tregs; (b) TNFR25 could enhance STAT5 activation in Tregs; (c) TNFR25 could enhance mammalian target of rapamycin (mTOR) activation in Tregs; and (d) TNFR25 could enhance PI3K/Akt activation in Tregs. To determine the expression of the IL-2R α, β, and γ chains, flow cytometry was performed on Tregs undergoing expansion in vivo subsequent to treatment with the 4C12 antibody and compared with IgG control antibody–treated isolated Tregs (Figure 3B). Although the expression of the IL-2R α chain (i.e., CD25) actually decreased following exposure to 4C12 (Supplemental Figure 1), expression of the β and γ chains (i.e., CD122 and CD132, respectively) remain unchanged on Tregs isolated from mice treated with 4C12 and isotype control antibodies (Figure 3B), effectively eliminating the first potential mechanism. To determine whether phosphorylation of STAT5 was enhanced in 4C12-treated mice, Tregs were isolated from mice treated 4 days previously with 4C12 or isotype control antibody and exposed to IL-2 ex vivo (10 ng/ml for 15 minutes). Subsequent staining of these Tregs with phosphospecific antibodies demonstrated that STAT5 phosphorylation was not enhanced in Tregs isolated from 4C12-treated mice compared with control mice (Figure 3C), effectively eliminating the second potential mechanism. Subsequently, TNFR25-induced Treg proliferation in vivo was unchanged in the presence of the mTOR inhibitor rapamycin (Figure 3D), eliminating the third potential mechanism (36). Finally, to determine whether Akt signaling was required for TNFR25-induced Treg proliferation, mice were treated with TNFR25 agonistic antibodies or control antibody in the presence or absence of the Akt1/2/3 selective inhibitor triciribine (referred to herein as Akt inhibitor V; ref. 37). Selective inhibition of Akt activation was sufficient to inhibit TNFR25-induced Treg proliferation from 33.69% ± 1.253% in vehicle-treated controls to 22.43% ± 1.352% (n = 6) when treated once daily with Akt inhibitor V (P < 0.001; data not shown) and to 18.20% ± 2.117% (n = 3) when treated twice daily with Akt inhibitor V (P = 0.0003; Figure 3E).
Figure 3. TNFR25-expanded Tregs are hyperresponsive to IL-2 and require Akt activation.
(A) CD4+FoxP3+ cells were purified from FIR mice on day 4 after treatment with control IgG or 4C12 antibodies and incubated with the indicated amounts of IL-2 in vitro. CD4+FoxP3+ cell proliferation was measured on day 3 of the culture by incorporation of tritiated thymidine. (B) CD4+FoxP3+ cells were purified as in A, and the surface expression of IL-2Rγ (CD132) or IL-2Rβ (CD122) was determined by flow cytometry. (C) CD4+FoxP3+ cells purified from FIR mice 4 days after treatment with IgG or 4C12 antibody were analyzed for expression of pSTAT5 15 minutes after treatment with 10 ng/ml IL-2 in vitro. (D and E) FIR mice were treated once daily with rapamycin (D) or twice daily with Akt inhibitor V (E) or a vehicle control from day –1 through day 4 by i.p. injection as described in Methods. Mice were treated with either 4C12 or control IgG antibody on day 0, and the proportion of FoxP3+ cells relative to total CD4+ cells in the peripheral blood was analyzed on day 4. Data are mean ± SEM of at least 2 independent experiments, with 2 or more mice per group per experiment. ***P < 0.001.
Comparison of TNFR25- and IL-2 antibody complex–induced Treg expansion in antigen-naive mice.
The only other agent that selectively expands Tregs in vivo was reported by Boyman et al. through use of an IL-2/anti–IL-2 antibody complex (IAC; see Methods and ref. 38). Thus, in vivo Treg expansion was directly compared after treatment with either 4C12 or IAC (Supplemental Figure 1A). The magnitude and kinetics of Treg expansion were similar following treatment with 4C12 and IAC in vivo. However, the contraction of expanded Tregs was prolonged after 4C12 compared with IAC treatment. In contrast to TNFR25-expanded Tregs, which expressed intermediate levels of CD25, IAC-expanded Tregs expressed high levels of CD25 (Supplemental Figure 1B), as previously reported (39). No other differences in expression of CD11a, CD28, CD45RA, CD62L, CD127, intra- or extracellular CTLA-4, OX40, PD-1, IL-17A, or IFN-γ were found in comparing Tregs expanded by 4C12 with Tregs expanded by IAC (data not shown).
In vivo Treg expansion by TNFR25 reduces allergic lung inflammation.
To determine whether 4C12-expanded Tregs prevent inflammation in a disease model, we tested whether this treatment could reduce inflammation if induced in a well-characterized model of allergic lung inflammation, OVA/alum-primed mice followed by airway OVA challenge. Mice were primed with OVA/alum, as previously described (3), on days 0 and 5 and then treated with 4C12 or hamster IgG on day 12. At the time of maximal Treg expansion 4 days later, the airways were challenged with OVA aerosolized in PBS or with a PBS saline control. Maximal expansion of Tregs was confirmed by monitoring Tregs in the peripheral blood during this period (Figure 4A). 4C12-induced Treg expansion following OVA/alum sensitization was slightly delayed in the first 2 days compared with expansion in nonsensitized mice, but Tregs then rapidly expanded to a higher proportion (50%–55%) of total CD4+ T cells by day 4 (Figure 4, B and C). Mice were sacrificed 3 days after aerosolization, and bronchoalveolar lavage fluid (BALF), bronchial lymph nodes, and lung tissue were analyzed.
Figure 4. In vivo Treg expansion by TNFR25 inhibits inflammation in allergic asthma.
Allergic asthma was induced by immunization with OVA/alum followed by aerosol challenge with OVA/PBS, as described in Methods. (A) Peripheral blood was collected and analyzed for the fraction of CD4+FoxP3+ cells relative to total CD4+ T cells from OVA/alum-immunized mice compared with nonimmunized mice following treatment with either 4C12 or isotype control antibody. (B) Total lung cells were harvested and analyzed by flow cytometry. The total number of each indicated cell population are shown. (C) The percentage of Tregs out of total CD4+ T cells. (D) BALF was collected 3 days after aerosolization with OVA/PBS. The total number of eosinophils is shown. (E) Total RNA was extracted from total lung cells and used for real-time RT-PCR. Expression of IL-4, IL-5, IL-13, and FoxP3 in 4C12- or isotype control–treated mice is shown relative to saline-aerosolized control lung cells. (F) Lungs were harvested and sectioned for histological sections. H&E and PAS images were obtained for each treatment group; representative images are shown. Data were repeated in 4 independent experiments with at least 3 mice per group per experiment. (G) PAS-stained sections were quantitated using Image J software as described in Methods; 2 representative images were quantitated from each of 5 or more mice from 2 separate experiments. All data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus respective control, 1-way ANOVA with Tukey post-test.
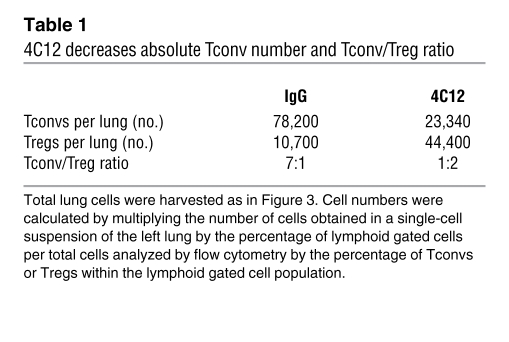
The total number of cells isolated from the lungs was unchanged between control- and 4C12-treated animals (data not shown). Consistent with this observation, the number of CD4+ and CD8+ T cells within the lungs was similar between control- and 4C12-treated mice; however, in 4C12-treated mice, the number of Tregs was significantly increased (Figure 4B). Analysis of the composition of Tregs within the lung tissue revealed that 7 days after 4C12 administration (i.e., 3 days after aerosolization), the frequency of Tregs in the lungs remained at 55% of all CD4+ T cells compared with 22% in hamster IgG–treated mice (Figure 4C). Because it has previously been reported that the balance of Tconvs to Tregs is a better predictor of disease pathogenesis than merely the total Treg number (40, 41), the Tconv/Treg ratio was determined in lung tissue (Table 1). To confirm that the phenotype of lung-infiltrating Tregs was consistent with the phenotype of TNFR25-expanded Tregs in disease-free mice, lung-infiltrating Tregs were analyzed and found to be indistinguishable from lung-infiltrating Tregs isolated from IgG-treated mice in terms of GITR, OX40, PD-1, CD44, CD62L, and CD69 expression (data not shown).
Table 1 .
4C12 decreases absolute Tconv number and Tconv/Treg ratio
Consistent with analysis of lung tissue cells, the total number of cells isolated from BALF was significantly increased following challenge with aerosol containing OVA, but not with saline aerosol control, in all conditions, but was markedly reduced by 4C12 treatment (data not shown). The total number of eosinophils within the BALF roughly mirrored the total number of BALF cells, and pretreatment with 4C12 significantly reduced the severity of airway eosinophilia (Figure 4D).
The proinflammatory cytokines IL-4, IL-5, and IL-13 have been strongly implicated in the pathogenesis of allergic lung inflammation (42–44). To determine whether expression of these cytokines was reduced by pretreatment with 4C12, total RNA was extracted from flash-frozen lungs 3 days after aerosolization and analyzed by RT-PCR. The expression of IL-4, IL-5, and IL-13 among lung-infiltrating CD4+ cells was significantly reduced following treatment with 4C12, but remained elevated following treatment with isotype control antibody, compared with saline-aerosolized controls (Figure 4E). As an additional control, the level of FoxP3 RNA expression was analyzed; this mirrored the same relative proportions of FoxP3-expressing CD4+ cells, as seen by flow cytometry (Figure 4, compare C and E). Lung tissue histology confirmed these findings, demonstrating reduced lymphocyte infiltration and airway mucus production following 4C12 treatment compared with saline-aerosolized controls (Figure 4, F and G).
TNFR25 expands Tregs without activating or expanding Tconvs.
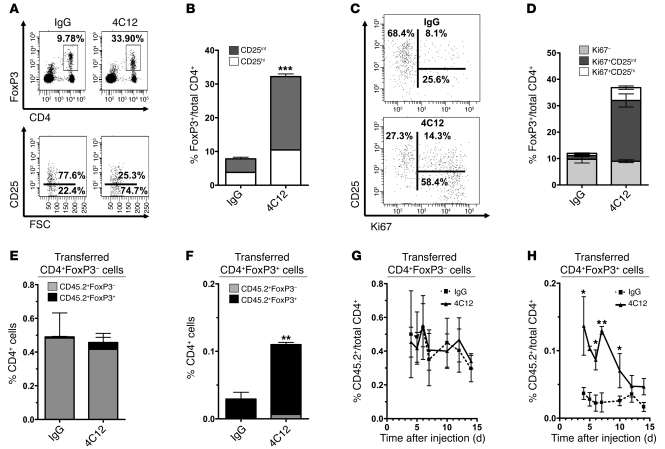
To determine the phenotype of the 4C12-expanded Tregs, we analyzed CD4+FoxP3+ cells isolated from peripheral lymph nodes, mesenteric lymph nodes, and spleens from mice that had been injected with 4C12 or IgG isotype control. 4C12-expanded Tregs were predominantly CD4+FoxP3+CD25int cells and were expanded in all secondary lymphoid organs analyzed (Figure 5, A and B, and Supplemental Figure 2, A and D). 4C12 treatment did not alter the expression of CD11a, CD28, CD45RA, CD62L, CD127, intra- or extracellular CTLA-4, OX40, PD-1, IL-17A, or IFN-γ by Tregs (data not shown). Although all CD4+FoxP3+ cells remained GITR+ following treatment with 4C12, the proportion of CD4+FoxP3+ cells that expressed GITR shifted in favor of the CD25int subset following 4C12 treatment (Supplemental Figure 2B). The αEβ7 integrin (i.e., CD103) has previously been reported to be expressed by a highly suppressive subset of CD4+FoxP3+ that can be either CD25+ or CD25– (45, 46). Analysis of CD103 expression revealed increased expression of CD103 by 4C12-expanded Tregs, but not control Tregs (Supplemental Figure 2C). Importantly, analysis of CD4+FoxP3– and CD8+ cells following treatment with 4C12 revealed that TNFR25 signaling did not increase the absolute number or proportion of either of these cell populations (data not shown). To determine whether treatment with 4C12 stimulated the proliferation of non-Tregs, CD4+ Tconvs and CD8+ T cells were stained with the proliferation marker Ki67. Treatment with 4C12 in the absence of exogenous antigen did not increase Tconv or CD8+ T cell proliferation (data not shown). Moreover, staining of CD8+ cells and FoxP3–CD4+ cells for CD44, CD62L, and CD69 revealed no differences between 4C12- and IgG-treated mice (data not shown). Thus, TNFR25 signaling selectively expands Tregs without inducing expansion or activation of CD4+ or CD8+ effector cells in the absence of exogenous antigen.
Figure 5. TNFR25 stimulation leads to Treg expansion in vivo by inducing proliferation of existing CD4+FoxP3+CD25int cells.
(A) FIR mice were treated with IgG or 4C12 on day 0, and splenocytes were harvested 4 days later and analyzed by flow cytometry. Representative plots are shown. (B) Mean CD25hi and CD25int Treg numbers in splenocytes 4 days after the indicated treatments. (C) Representative dot plots were pregated on CD4+FoxP3+ cells. Percentages indicate the contribution of each phenotype toward the total fraction of CD4+FoxP3+ cells. (D) Mean proportion of Ki67+ and Ki67– cells among CD25hi and CD25int Tregs as in A. Data are mean ± SEM. (E–H) CD4+FoxP3– and CD4+FoxP3+ cells were sorted from CD45.2+ FIR mice to greater than 99% purity, and 2 × 106 cells from each subset were adoptively transferred into CD45.1 congenic B6-SJL mice. Mice were injected i.p. 24 hours later with 20 μg 4C12 or IgG. (E and F) Histograms showing percentage of transferred CD45.2+FoxP3+ and CD45.2+FoxP3– cells out of total CD4+ cells on day 5 after adoptive transfer. (G and H) Kinetics of transferred cell contraction following 4C12 or hamster IgG treatment. Data are mean ± SEM for 3 mice per group for each of 2 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
TNFR25 stimulation induces proliferation of preexisting Tregs in vivo.
The increase in Tregs following 4C12 treatment could result either from de novo FoxP3 expression or from the proliferation of CD4+FoxP3+ cells. To differentiate between these possibilities, we determined the expression of Ki67 on CD4+FoxP3+ cells (Figure 5, C and D). As was suggested by the increase in the ratio of CD4+FoxP3+CD25int to CD4+FoxP3+CD25hi cells, the majority of Ki67+ cells was CD4+FoxP3+CD25int in mice treated with 4C12. A smaller proportion (~27%) of CD4+FoxP3+ cells did not stain for Ki67 (Figure 5, C and D), and the majority of these cells were CD25hi. It remains unclear whether the observed proliferation of CD25int cells following 4C12 treatment resulted from selective stimulation of CD25int cells or whether Tregs were stimulated to proliferate regardless of CD25 expression, which was then reduced during proliferation.
Increased proliferation by CD4+FoxP3+CD25int cells does not conclusively rule out the possibility that TNFR25 signaling could stimulate de novo FoxP3 expression by CD4+FoxP3– cells. To examine this possibility, we performed adoptive transfer experiments by infusing highly purified (>99% purity) CD4+FoxP3– or CD4+FoxP3+ cells from CD45.2+ FIR mice into CD45.1+ congenic B6/SJL mice. These studies allowed us to track adoptively transferred CD45.2+ cells following treatment with 4C12 in CD45.1+ hosts and monitor persistence, induction, or silencing of FoxP3-RFP by adoptively transferred CD45.2+CD4+ cells (Figure 5, E–H). We deliberately chose to perform these experiments in fully immunocompetent mice to avoid any complications that may arise from homeostatic expansion of Tregs following adoptive transfer into genetically or experimentally immunodeficient strains. Transfer of 2 × 106 sorted cells (Supplemental Figure 3A) into immunocompetent CD45.1+ recipients was sufficient to detect a rare, but easily distinguishable, population of CD45.2+CD4+ cells in the peripheral blood for at least 2 weeks after adoptive transfer (Figure 5, G and H). TNFR25 stimulation of recipient mice by 4C12 after adoptive transfer did not stimulate de novo FoxP3 expression by CD4+FoxP3– cells (Figure 5E), which remained at 0.5% frequency and FoxP3– regardless of 4C12 or control antibody treatment. The frequency of FoxP3+ cells after adoptive transfer of 2 × 106 cells was 0.04% of the CD4 cells in peripheral blood in mice treated with control antibody and increased to 0.11% in 4C12-treated mice (Figure 5F), a 3-fold increase in FoxP3+CD45.2+ cell frequency. This result was consistent with the extent of expansion of FoxP3+ Tregs by the TNFR25 agonistic antibody in nontransferred mice (Figure 1B). 4C12 treatment selectively stimulated the proliferation of CD4+FoxP3+ cells, which maintained FoxP3 expression following expansion (Figure 5F). The data show that TNFR25 signaling stimulates primarily increased proliferation of CD4+FoxP3+CD25int cells, resulting in a systemic increase in Tregs. These studies also demonstrated that while the adoptively transferred CD4+FoxP3– cells did not expand at any time following 4C12 treatment (Figure 5G), the expansion of adoptively transferred CD4+FoxP3+ cells followed kinetics similar to those of expansion and contraction of endogenous Tregs in FIR mice (compare Figure 5H and Figure 1B). Importantly, the adoptively transferred CD4+FoxP3+ cells maintained FoxP3 expression both during and after expansion (Figure 5, F and H, and data not shown), which suggests that the observed contraction of the expanded Treg pool results from cell death rather than from loss of FoxP3 expression. If the expanded pool of adoptively transferred CD45.2+CD4+FoxP3+ cells were losing FoxP3 expression at any point throughout the course of the experiment, the fraction of CD4+FoxP3– cells within the CD45.2+CD4+ cells would have increased; however, this did not occur. Less than 5% of adoptively transferred CD4+FoxP3– cells exhibited FoxP3 expression (Figure 5E), and less than 5% of transferred CD4+FoxP3+ cells lost FoxP3 expression (Figure 5F), over the course of the experiment. Such minor instabilities in FoxP3 expression have been reported previously (47), and likely explain these observations.
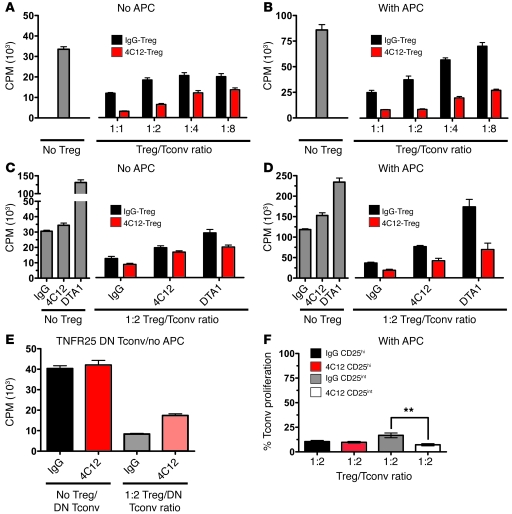
TNFR25-expanded Tregs are highly suppressive ex vivo.
To determine whether 4C12-expanded Tregs retain suppressive activity, we purified Tregs from FIR mice 4 days after treatment with either 4C12 or IgG isotype control antibody (Supplemental Figure 3A). These Treg subsets were then used in a traditional proliferation assay, as previously described (48). Purified Tregs from 4C12-treated mice suppressed proliferation of CD4+CD25– cells to a greater degree than did those from isotype control antibody–treated mice (Figure 6, A–D). Suppression of Tconv proliferation by 4C12-expanded Tregs was observed in both the presence and the absence of APCs in the in vitro suppression assay (Figure 6, compare A and B). To determine whether addition of 4C12 during the suppression assay modulates the suppressive activity of Tregs, identical assays were performed in the presence of 4C12, DTA1 (a GITR-agonistic antibody known to release Tconvs from Treg-mediated suppression; ref. 26), or isotype control antibody (Figure 6, C and D). The presence of agonistic TNFR25 or GITR antibodies partially restored Tconv proliferation, with both antibodies producing a similar effect in the absence of APCs regardless of whether the Tregs were obtained from 4C12- or IgG isotype control–treated mice (Figure 6C). Interestingly, in the presence of APCs, DTA1 induced the proliferation of Tconvs in the presence of Tregs from IgG isotype control–treated mice to a greater extent than with Tregs from 4C12-treated mice (Figure 6D). The presence of APCs did not significantly alter the partial restoration of Tconv proliferation in the presence of 4C12. Controls also demonstrated that the stimulatory effect of 4C12 on Tconvs alone was minimal, and significantly less than the stimulatory effect of GITR on Tconvs (Figure 6, C and D). To further demonstrate that inhibition of Treg-suppressive activity by 4C12 was specific to the effect of TNFR25 expressed by Tregs and not Tconvs, suppression assays were performed using transgenic Tconvs expressing a dominant-negative TNFR25 (ref. 3 and Figure 6E). These data demonstrated that inhibition of Treg-suppressive activity by TNFR25 signaling occurred under conditions in which only Tregs express a functional TNFR25, which indicates that this effect is the result of signaling by TNFR25 on Tregs and not Tconvs. Notably, Tregs expanded in vivo with 4C12 and then subjected to the in vitro suppression assays (Figure 6, A and B) were highly suppressive under conditions in which the 4C12 antibody was no longer present. Only when the 4C12 antibody was maintained in the course of the suppression assay was partial inhibition of Treg-suppressive activity observed. Because 4C12 induced the proliferation of CD25int Tregs, and in some studies the level of CD25 expression is predictive of the suppressive activity of Tregs (49), we compared the suppressive activity of CD25hi and CD25int Tregs sorted from mice following treatment with 4C12 or isotype control antibody (Figure 6F and Supplemental Figure 3B). The suppressive activity did not depend on the level of CD25 expression, since CD25hi and CD25int Tregs were both highly suppressive in the proliferation assay (Figure 6F), as has recently been reported (49). Interestingly, the 4C12-expanded CD25int Tregs had slightly greater suppressive activity than did the CD25int Tregs from IgG-treated mice (Figure 6F). This finding indicates that the increased suppressive activity of 4C12-expanded compared with IgG-treated Tregs (Figure 6, A–D) is attributable, at least in part, to the activity of CD25int cells.
Figure 6. Suppressive activity of in vivo–expanded Tregs.
Tregs were sorted from 4C12- and IgG isotype control–injected mice on day 4 and subjected to a standard in vitro suppression assay using CD4+FoxP3–CD25– cells as Tconvs and soluble α-CD3 (2 μg/ml) for 72 hours (96-well, round bottom plate). The assay was performed in the absence (A and C) or presence (B and D) of 1:1 APCs. (A and B) Treg/Tconv ratios were as indicated. (C and D) IgG, 4C12, or DTA1 (10 μg/ml) antibodies were added to the suppression assay, and the Treg/Tconv ratio was kept constant at 1:2. (E) Tconvs from TNFR25 dominant-negative (DN) mice (3) and Tregs from wild-type mice were used. IgG or 4C12 antibodies (10 μg/ml) were added to the suppression assay; the Treg/DN Tconv ratio was 1:2. (F) CD4+CD25hi and CD4+CD25int Tregs from IgG- or 4C12-injected mice were used. 3H-thymidine was added for the last 6 hours before the assay was analyzed on a scintillation counter. Percent Tconv proliferation was calculated as a percentage of the total counts obtained in wells containing Tconvs in the absence of Tregs. Data are mean ± SEM with at least 4 samples for each condition in each of 2 independent experiments with more than 6 mice per group per experiment. **P < 0.01.
Discussion
Members of the TNFR family have been recognized as important costimulators of immune effector cell responses and as inducers of apoptosis. Here we identified in TNFR25 a nonredundant function as regulator of Tregs, which we believe to be novel. TNFR25 mediated robust expansion of Tregs in vivo in immune competent mice, while at the same time partially restraining their suppressive activity. To our knowledge, no other physiological signals — including those of other TNFR family members — have been reported to exert similar activity on Tregs. Further, the observation that TNFR25 signals induced Treg expansion with magnitude and kinetics similar to the only other reported reagent to selectively expand Tregs (IAC; ref. 38) indicates that TNFR25 agonists may provide a translatable alternative to IL-2–based therapies for therapeutic use in humans.
The current understanding of TNFR25 and TL1A strongly implicates this receptor/ligand pair in the generation of pathogenic inflammation in various disease models. To date, there is not a single report to our knowledge that identifies a role for TNFR25 or TL1A in maintaining health or preventing disease, which indicates that such a role had evaded discovery. The availability of the TNFR25 agonistic antibody 4C12 enabled the study of TNFR25 on various T cell subsets in a setting in which the temporal availability of TNFR25 signals, inflammatory signals, and exogenous antigen could be independently controlled. The identification of a protective role for TNFR25-expanded Tregs in allergic lung inflammation does not contradict previous studies implicating TL1A in the exacerbation of allergic lung inflammation (3), because in the current studies, TNFR25 signaling preceded antigen exposure, whereas in previous studies, TNFR25 signals followed antigen challenge. Rather, the differential high expression of TNFR25 by Tregs in contrast to the low expression by Tconvs suggests that the sequence of exposure of T cells to antigen, costimulatory signals, or TL1A may govern whether a particular inflammatory response is suppressed by Tregs or induced by Tconvs. In the current studies, treatment with TNFR25 agonists prior to airway antigen challenge induced the preferential accumulation of Tregs, but not Tconvs, within the airways and was associated with reduced production of IL-4, IL-5, and IL-13 as well as reduced eosinophilia and mucus production in the bronchoalveolar space.
The requirement for MHC-II and IL-2 signals, but not CD80/86 costimulation, for TNFR25-induced Treg proliferation echoes prior findings for TNFR25 signaling on Tconvs (10, 15). Although MHC-II and IL-2 signals were required for TNFR25-induced Treg proliferation, provision of TCR and IL-2 signals were not sufficient to induce proliferation of Tregs in vitro, which suggests that additional signals are required; these are currently under investigation. Although the requirement for MHC-II strongly implicates the Treg-expressed TCR in TNFR25-induced Treg proliferation, these data are indirect. As additional evidence for a role of the TCR in this process, we observed that TNFR25 triggering could not induce Treg proliferation in the presence of the NFAT inhibitors cyclosporin-A or FK506, providing evidence that signaling events downstream of the TCR influence Treg proliferation. These data indicate that both Tregs and Tconvs may become permissive to TNFR25 signaling subsequent to TCR ligation and that the Treg-selectivity of TNFR25 agonistic antibodies may be at least in part due to the availability of self-antigen under noninflammatory conditions. Whether persistent TCR stimulation with self-antigen also contributes to the increased expression of TNFR25 in Tregs as compared to Tconvs, or whether this difference is maintained by unrelated signaling pathways, is also not known.
Given the observation that at least 2 additional receptor pathways (IL-2R and TCR) were required for TNFR25-triggered Treg proliferation, the confluence of signaling pathways downstream of these receptors leading to Treg proliferation is likely to be complex. A clue as to how these pathways may interact was provided by our observation that ex vivo, TNFR25-triggered Tregs were hyper-responsive to IL-2 signals. We subsequently determined that the PI3K/Akt pathway provided a link downstream of the IL-2R that was important for TNFR25-induced Treg proliferation. These data echo previous reports indicating that PTEN-mediated inhibition of the PI3K/Akt pathway restricts the proliferation of Tregs downstream of IL-2 signaling (50). Identification of MHC-II, IL-2R, NFAT, and Akt provide a tangible starting point for elucidating the signaling events downstream of TNFR25 triggering that culminates in Treg proliferation, but many additional studies are required to elucidate the molecular mechanisms of cross-talk among these various pathways.
TNFR25-induced Treg expansion occurred with kinetics and magnitude similar to those of Treg expansion induced by IAC, but resulted in an increase in the proportion of CD25int rather than CD25hi cells. The importance of this observation is unknown; however, the increase in CD25 expression by Tregs following exposure to IAC suggests a positive feedback loop driven by the increased availability of IL-2, as has been reported previously (51). In the case of TNFR25-induced Treg expansion, the concentration of IL-2 was not manipulated, so the resulting decrease in CD25 expression by proliferating Tregs may result from increased competition for endogenous IL-2 from an expanding Treg population. Interrogation of other Treg-expressed surface markers revealed few differences between TNFR25- and IAC-expanded Tregs, although some, including GITR, fluctuated between the CD25int and CD25hi populations. The only marker analyzed that was consistently increased following 4C12 treatment was CD103, which contributes to the retention of Tregs within tissues (52, 53).
Our present results complement recent data reporting roles for TNFR25 stimulation in the induction of inflammatory responses with the inhibition of Treg-suppressive activity; together, these data are suggestive of unified theory for the role of TL1A/TNFR25 interactions in both induction and resolution of tissue inflammation. The precise mechanism by which TNFR25 stimulation both induces the proliferation of Tregs and inhibits their suppressive activity remain unclear, in part because the signaling pathways activated by TNFR25 signals are not well understood, but are being investigated further. In addition, it is unknown whether TNFR25-induced expansion of Tregs in vivo is dependent upon the recognition of self-antigen, although such a requirement is suggested by the requirement for MHC-II signals; similar to IAC, the conditions necessary for TNFR25-induced Treg expansion in vitro remain unclear and are under further investigation. We hypothesize that the identification of a requirement in vivo for MHC-II to permit TNFR25-induced Treg proliferation indicates that TCR engagement is a general requirement for TNFR25-induced T cell costimulation, and that the Treg selectivity of TNFR25 in the absence of exogenous antigen is maintained both by the preferential expression of TNFR25 by Tregs and by the availability of self-antigen presented by MHC-II. The increased responsiveness of Tregs to TNFR25 stimulation from immunized versus nonimmunized mice is also intriguing and may indicate distinct functions for TNFR25 in primary versus secondary immune responses. Regardless of the mechanism, in light of the importance of TNFR25 signaling to the pathogenesis of a growing number of inflammatory diseases (e.g., asthma, IBD, EAE, and RA), it is important to understand the spatiotemporal role that TNFR25 signaling exerts on various CD4+ T cell subsets. It is highly likely that, similar to OX40 (31), the temporal context of TNFR25 signaling may differentially guide inflammatory or regulatory immunity. The unique ability of TNFR25 signals to rapidly expand and transiently inhibit CD4+FoxP3+ natural Tregs may have important consequences for the treatment of autoimmune disease, chronic infection, transplantation, and cancer.
Methods
Mice.
Wild-type C57BL/6 mice were purchased from Charles River Laboratories. FIR mice on a B6 background (provided by R. Flavell, Yale University, New Haven, Connecticut, USA; ref. 20), FoxP3-GFP mice (provided by A. Rudensky, Memorial Sloan-Kettering Cancer Center, New York, New York, USA; refs. 54, 55), and CD45.1 SJL, Cd74–/–, Tg+ Il2rb–/– (56), Cd80–/–Cd86–/–, and Cd4–/– mice were bred in our animal facility. Tl1a–/– mice were purchased from Lexicon Genetics Inc. and backcrossed into a C57BL/6 background by Speed Congenics. Mice were used at 6–12 weeks of age and were maintained in pathogen-free conditions at the University of Miami animal facilities. All animal use procedures were approved by the University of Miami Animal Care and Use Committee.
Antibodies and reagents.
Commercial antibodies for use in flow cytometry were purchased from BD Biosciences — Pharmingen or from eBioscience. The Armenian Hamster IgG isotype control was bought from eBioscience. DTA1 (anti-GITR) was obtained from BioXCell, and LG.3A10 (anti–IL-27) was from BioLegend, and 158321 (anti–4-1BB) was obtained from R&D Systems. Recombinant mouse IL-2 and anti-IL-2 monoclonal antibody, clone JES6-1A12, were purchased from eBioscience. IAC was generated by incubating 10,000 U recombinant mouse IL-2 with 5 μg JES6-1A12 for 15 minutes at 25°C. Armenian hamster hybridomas producing antibodies to mouse TNFR25 (4C12, agonistic) were generated as described previously (3). 4C12 (anti-TNFR25) and OX-86 (anti-OX40) were produced in hollow fiber bioreactors (Fibercell Systems) and purified from serum-free supernatants on a protein G column (GE Healthcare). Rapamycin (Rapamune; Wyeth) was used at 75 μg/kg/d as previously described (36). Cyclosporin-A (25 mg/kg/d), FK506 (3 mg/kg/d), and Akt inhibitor V (triciribine, 1.5 mg/kg daily or twice per day as indicated) were purchased from Calbiochem/EMD and administered by i.p. injection as previously described (37, 57).
Flow cytometry and cell sorting.
Single-cell suspensions were prepared from spleen and lymph nodes. 106 cells were preblocked with anti-mouse CD16/CD32 and stained with different antibody combinations. Intracellular staining was performed according to standard procedures. Flow cytometric analysis was performed on a BD FACS LSR II instrument and DIVA or FlowJo software. Cell sorting was done using a FACSAria cell sorter (BD) after enrichment of splenocytes for CD4+ T cells using the EasySep Mouse CD4+ T cell Pre-Enrichment Kit from Stem Cell Technologies.
Real-time RT-PCR.
Total RNA was extracted from flash-frozen colonic or lung tissue sections and reverse transcribed using the RNeasy Mini Kit and the QuantiTect Reverse Transcription Kit from QIAGEN, respectively. Real-time PCR was performed in duplicate on an ABI 7300 Light Cycler using TaqMan probes from Applied Biosystems. Samples were normalized to β-actin.
Adoptive transfer.
For studies in Figure 2, A and B, total CD4+ cells were FACS sorted from FIR mice, and the percentage of FoxP3+RFP+ cells was determined after sorting. Total CD4+ cells containing 106 FoxP3+ cells were adoptively transferred i.v. into Cd74–/– or Cd4–/– mice on day –2. On day 0, mice were treated with 4C12 antibody or isotype control. For studies in Figure 4, 2 × 106 FACS-sorted CD4+FoxP3– or CD4+FoxP3+ cells from CD45.2+ FIR mice were adoptively transferred via i.v. injection into CD45.1 congenic SJL mice; 1 day later, 10 μg 4C12 was administered by i.p. injection. The expansion of transferred cells was followed by FACS daily (starting after 3 days) in peripheral blood cells.
In vitro suppression assays.
CD4+CD25– cells (1 × 105) were plated in 96-well round-bottomed plates and activated with 2 μg soluble anti-CD3 (2C11) antibody in the presence or absence of APCs (1:1 ratio) and CD4+FIR+ Tregs at different ratios. Control IgG, 4C12, or DTA1 antibodies were added as indicated at a concentration of 10 μg/ml. Cultures were incubated for 72 hours and pulsed with 3H-thymidine (1 μCi/well; Perkin Elmer) for the last 6 hours. Incorporated isotope was measured by liquid scintillation counting (Micro Beta TriLux counter; Perkin Elmer).
Allergic asthma induction.
Mice were sensitized by i.p. injection of 66 μg OVA (crystallized chicken egg albumin, grade V; Sigma-Aldrich) adsorbed to 6.6 mg alum (aluminum potassium sulfate; Sigma-Aldrich) in 200 μl PBS on day 0, with a i.p. boost on day 5. On day 12, mice were injected i.p. with either 20 μg anti-TNFRSF25 agonistic antibody 4C12 or 20 μg goat anti-hamster IgG isotype control (Jackson ImmunoResearch Laboratories Inc.) in 200 μl PBS. On day 16, mice were aerosol challenged with 0.5% OVA (Sigma-Aldrich) in PBS for 1 hour using a BANG nebulizer (CH Technologies) into a Jaeger-NYU Nose-Only Directed-Flow Inhalation Exposure System (CH Technologies). On day 19, mice were sacrificed, lungs were perfused with PBS, and bronchoalveolar lavages were obtained. Lung lobes were processed for RNA, for flow cytometry analysis, or for lung histology as described previously (3). Draining bronchial lymph nodes were also procured for subsequent RNA analysis as well as flow cytometry analysis. Quantification of periodic acid-Schiff–stained (PAS-stained) lung sections was performed using MacBiophotonics Image J software by color deconvolution (using the H PAS vector) followed by thresholding of images (color “2,” set to 100) and counted using the nucleus counter (limits set between 100 and 1,000).
Statistics.
All graphing and statistical analysis were performed using ABI Prism. Paired analysis was performed using 2-tailed Student’s t test. Multiple variable analysis was performed using 1-way ANOVA and Tukey post-test. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We would like to thank Jim Phillips, Shannon Opiela, and Jay Enten of the SCCC flow cytometry core facility as well as George McNamara of the Analytical Imaging Core Facility for expert technical assistance in these studies. We would also like to thank Emily Leibovitch and Eva Fisher for discussion and expert review of this manuscript. Financial support was provided by grants NCI-5PO1CA109094-03 and NIAID-5RO1AI061807-05 to E.R. Podack and C-120776-01 and NIAID-46689-5A1 to R.B. Levy.
Footnotes
Conflict of interest: E.R. Podack, T.H. Schreiber, D. Wolf, and V.V. Deyev have patent applications relevant to material described in this manuscript.
Citation for this article: J Clin Invest. 2010;120(10):3629–3640. doi:10.1172/JCI42933.
References
- 1.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205(5):1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodmer JL, et al. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95). Immunity. 1997;6(1):79–88. doi: 10.1016/S1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 5.Screaton GR, et al. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci U S A. 1997;94(9):4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan KB, et al. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene. 1997;204(1–2):35–46. doi: 10.1016/S0378-1119(97)00509-X. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan AM, et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274(5289):990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 8.Kitson J, et al. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;384(6607):372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 9.Marsters SA, et al. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-kappa B. Curr Biol. 1996;6(12):1669–1676. doi: 10.1016/S0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 10.Meylan F, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29(1):79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178(7):4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 12.Cassatella MA, et al. Soluble TNF-like cytokine (TL1A) production by immune complexes stimulated monocytes in rheumatoid arthritis. J Immunol. 2007;178(11):7325–7333. doi: 10.4049/jimmunol.178.11.7325. [DOI] [PubMed] [Google Scholar]
- 13.Papadakis KA, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172(11):7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 14.Papadakis KA, et al. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005;174(8):4985–4990. doi: 10.4049/jimmunol.174.8.4985. [DOI] [PubMed] [Google Scholar]
- 15.Migone TS, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16(3):479–492. doi: 10.1016/S1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 16.Pappu BP, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205(5):1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takedatsu H, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135(2):552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bull MJ, et al. The Death Receptor 3-TNF-like protein 1A pathway drives adverse bone pathology in inflammatory arthritis. J Exp Med. 2008;205(11):2457–2464. doi: 10.1084/jem.20072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102(14):5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105(7):2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 22.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205(4):825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202(7):885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7(8):907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3(2):135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 27.van Olffen RW, et al. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. J Immunol. 2009;182(12):7490–7500. doi: 10.4049/jimmunol.0802751. [DOI] [PubMed] [Google Scholar]
- 28.Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H. CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early but not late phases of tumor development in a B cell lymphoma model. J Immunol. 2007;178(11):6840–6848. doi: 10.4049/jimmunol.178.11.6840. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, et al. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol. 2007;66(4):435–440. doi: 10.1111/j.1365-3083.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25 T cells. Blood. 2007;110(7):2537–2544. doi: 10.1182/blood-2007-03-082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruby CE, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183(8):4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earle KE, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115(1):3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godfrey WR, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104(2):453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 34.Golovina TN, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181(4):2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hippen KL, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7):2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karst AM, Dai DL, Cheng JQ, Li G. Role of p53 up-regulated modulator of apoptosis and phosphorylated Akt in melanoma cell growth, apoptosis, and patient survival. Cancer Res. 2006;66(18):9221–9226. doi: 10.1158/0008-5472.CAN-05-3633. [DOI] [PubMed] [Google Scholar]
- 38.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 39.Shatry A, Levy RB. In situ activation and expansion of host tregs: a new approach to enhance donor chimerism and stable engraftment in major histocompatibility complex-matched allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(7):785–794. doi: 10.1016/j.bbmt.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteiro JP, Farache J, Mercadante AC, Mignaco JA, Bonamino M, Bonomo A. Pathogenic effector T cell enrichment overcomes regulatory T cell control and generates autoimmune gastritis. J Immunol. 2008;181(9):5895–5903. doi: 10.4049/jimmunol.181.9.5895. [DOI] [PubMed] [Google Scholar]
- 42.Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 43.Huang SK, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155(5):2688–2694. [PubMed] [Google Scholar]
- 44.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 45.Leithauser F, Meinhardt-Krajina T, Fink K, Wotschke B, Moller P, Reimann J. Foxp3-expressing CD103+ regulatory T cells accumulate in dendritic cell aggregates of the colonic mucosa in murine transfer colitis. Am J Pathol. 2006;168(6):1898–1909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao D, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112(5):2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106(6):1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Walsh PT, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116(9):2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malek TR, Ashwell JD. Interleukin 2 upregulates expression of its receptor on a T cell clone. J Exp Med. 1985;161(6):1575–1580. doi: 10.1084/jem.161.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174(9):5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 53.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204(6):1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 56.Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164(6):2905–2914. doi: 10.4049/jimmunol.164.6.2905. [DOI] [PubMed] [Google Scholar]
- 57.Medyouf H, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13(6):736–741. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.