Abstract
Focal adhesion kinase (FAK) has been implicated in tumorigenesis in various cancers; however, it remains unclear how FAK participates in tumor malignancy in vivo. This study seeks to understand the role of FAK activation in gastric cancer progression. Using immunohistochemical staining and Western blotting, we found that pY397 FAK, an autophosphorylation site on FAK activation, was abundant in the cancerous tissues of 21 of 59 patients with gastric carcinomas. We attempted to correlate clinicopathological parameters, including histological types, TNM staging, and cancer recurrence, with the expression of FAK and pY397 FAK in cancerous tissues. Intriguingly, patients with higher levels of pY397 FAK displayed higher incidences of gastric cancer recurrence after surgery and poor 5-year recurrence-free survival. Furthermore, multivariate analyses showed that pY397 FAK was an independent predictor of gastric cancer recurrence. As a result, expression of pY397 FAK is a significant prognostic factor for the recurrence of gastric cancer. Additionally, in vitro studies showed that overexpression of Y397F, a dominant-negative mutant of FAK, in AGS human gastric carcinoma cells impaired cell migration, invasion, and proliferation compared with cells overexpressing wild-type FAK. Thus, activation of FAK through autophosphorylation at Tyr397 leads to the progression of gastric carcinomas by promoting cell migration, invasion, and proliferation. Collectively, our results have provided valuable insights for the development of novel diagnoses and therapeutic targets for gastric cancer treatments.
Gastric cancer is the fifth leading cause of death in Taiwan, accounting for 2400 deaths per year. The progression of gastric cancer depends on hematogenous metastasis, direct invasion, and, mainly, lymphatic spreading. The prognosis of patients with gastric cancer correlates with the level and numbers of lymphatic metastases.1 Curative resection is the mainstay of treatment for gastric cancer; however, up to 40% of patients that undergo radical gastrectomy for gastric cancer develop recurring metastases, 87% of which are distant metastases.2,3 Therefore, it is important to understand the molecular basis by which gastric cancers progress and to develop individualized therapies that target specific molecular events.
Focal adhesion kinase (FAK) is a 125-kDa cytoplasmic protein tyrosine kinase that plays a key role in integrin-mediated signal transduction pathways.4 FAK was originally shown to exhibit increased tyrosine phosphorylation and kinase activity in response to either integrin activation or v-Src–induced transformation.5–7 On engagement of integrins with extracellular matrices, FAK is translocated to focal contact sties and autophosphorylated at its Tyr397 residue, which enables it to couple multiple downstream pathways through its interaction with Src family kinases, Phosphatidylinositol-3 kinase, growth factor receptor bound protein 7 (Grb7), and/or other signaling molecules.8 In vitro studies have shown that mutating FAK Tyr397 to Phe leads to deficiencies in the regulation of both cell migration and cell cycle progression.8
FAK mediates a number of signaling pathways associated with cell adhesion, mitogenesis, motogenesis, and oncogenic transformation,9 suggesting that FAK might contribute to tumor development. It is consistent with the important role of FAK in fundamental aspects of cell behavior and cellular responses, including the regulation of cell adhesion, cell motility, and cell survival. Moreover, a great deal of circumstantial evidence suggests that FAK overexpression contributes to the development of human malignancies. One theme that is consistent throughout many reports is the correlation between elevated FAK protein expression and tumorigenic and/or invasive potential in tumor cells.9 For instance, FAK expression is elevated in cell lines derived from human melanomas, and FAK protein levels correlate with the rate of cell migration on fibronectin.10 FAK is also elevated in primary human sarcomas,11 cervical carcinoma cell lines,12 prostatic carcinoma tumors and cell lines,13 and colon and breast tumors and cell lines.14 More recently, FAK protein expression has also been found to be elevated in ovarian carcinomas15 and associated with liver metastases derived from colon cancers.16 Therefore, we hypothesize that overexpression of FAK in a subset of tumor cells leads to increasing populations of cells with a high propensity for invasion and metastasis. FAK would have a dual role in this regard: overexpression of FAK promotes cell migration and increases cell survival under anchorage-independent conditions.17
In this study, we investigated the correlation between the expression of FAK and its autophosphorylation at Tyr397 in curatively resected gastric cancer specimens in relation to the clinicopathological characteristics of the patients. We found that pY397 FAK is intimately correlated with the recurrence of gastric cancer, which is consistent with the important role of FAK activation in gastric cancer cell migration, invasion, and proliferation.
Materials and Methods
Clinical Specimens
Tissue samples were obtained from 59 patients with primary gastric carcinomas who were not given preoperative chemotherapy and who had undergone resection with curative intent by a single surgeon in our study (Dr. I-Rue, Lai) in the Department of Surgery, National Taiwan University Hospital from July 2000 to March 2005. Patients with distant metastases at initial presentation or patients who received palliative resections were excluded from this study. There were 38 males and 21 females between the ages of 31–89 with a mean of 63.5 years old. The average follow-up time was 48.2 ± 25.5 months after surgery. After surgical resection, tumors and contiguous noncancerous parts from each patient were fixed in 10% buffered formalin and embedded in paraffin. Paired cancerous tissues and noncancerous mucosa were also removed and stored at −80 °C until examined. Clinicopathological factors, including age, gender, surgical procedures, tumor size, histological types by Lauren's classification, lymph node metastases, TNM staging (according to UICC/AJCC),18 and follow-up status were collected and stored in a personal computer database. This study was approved by the institutional review board of National Taiwan University Hospital.
Immunohistochemistry
For immunohistochemical analyses, paraffin-embedded human gastric cancerous tissues were sectioned and stained after antigen retrieval using a 1:200 dilution of the anti-FAK primary antibody (C20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or a 1:250 dilution of the anti-pY397 FAK antibody (Invitrogen, Carlsbad, CA), followed by biotinylated and peroxidase-conjugated secondary antibodies. The sections were then processed using the 3,3′-Diaminobenzidine (DAB) immunostaining assay kit (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) following the manufacturer's instructions. The samples were counterstained with hematoxylin before mounting on coverslips. The samples were then examined under a light microscope (model M1, Zeiss, German) with a ×40 objective lens at room temperature and the images were captured using a Charge-Coupled Device (CCD) camera (model DP71, Olympus, Japan).
Evaluation of Immunohistochemistry
A semiquantitative evaluation system (a Quick score method, or Q-score) was used to determine antigen expression in tissue samples.19 Blood vessels within each sample were used as internal positive controls for FAK expression. Grading of expression was based on both intensity and heterogeneity of the staining patterns. The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The heterogeneity of staining was scored as 0 (0%), 1 (1 to 25%), 2 (26 to 50%), 3 (51 to 75%), or 4 (75 to 100%), depending on the proportion of tumor cells that were positively stained for FAK or pY397 FAK. The Q-score of a given tissue sample, ranging from 0 to 7, is the sum of the intensity and heterogeneity scores, both of which were evaluated independently by two pathologists who were blind to the clinicopathological information. A Q-score ≧ 2 was considered consistent with antigen overexpression, whereas a Q-score <2 was considered to be a normal level of expression. For some rare cases with <5% weakly stained tissues were considered as negative. Protein expression was correlated with clinicopathological data.
Statistical Analyses
The association between FAK or pY397 FAK expression and clinicopathological parameters was evaluated using a χ2 test. Recurrence-free survival was defined as the time period from resection to the date of clinically detected recurrence. Survival curves were depicted using the Kaplan–Meier method. Any differences in survival curves were evaluated by the log-rank test. A P value of <0.05 was considered to be statistically significant.
Cell Culture
The AGS human gastric carcinoma cell line was a kind gift from Dr. Hsinyu Lee (National Taiwan University, Taipei, Taiwan). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Hyclone Laboratories, Inc., Logan, Utah) containing 10% fetal bovine serum (Hyclone Laboratories Inc.) under 5% CO2 in a humidified 37 °C incubator. Cells were infected with adenoviruses harboring wild-type or mutant FAK as indicated. Experiments were conducted 24–48 hours after virus infection.
Recombinant Adenoviruses
Adenoviruses encoding Green Fluorescent Protein (GFP) alone, Myc-6XHis tagged wild-type FAK, Y397F mutant, or FAK-related nonkinase (FRNK) were described previously.20 For adenovirus preparation, adenoviruses were harvested approximately 7 days after infection by several cycles of freezing and thawing in PBS and kept at −80 °C after centrifugation for future use.
Western Blotting
Various adenovirus-infected cells or tissue samples were homogenized, washed twice with ice-cold PBS, and lysed with 1% Nonidet P-40 lysis buffer (20 mmol/L Tris, pH 8.0, 137 mmol/L NaCl, 1% Nonidet P-40, 10% glycerol, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin, and 20 mg/ml leupeptin) on ice for 10 minutes. Subsequently, lysates were collected and clarified by centrifugation for 10 minutes at 4°C, and total protein concentration was determined using the Bio-Rad Protein Assay according to the manufacturer's instructions. Western blotting was performed using anti-FAK (C20), anti-pY397 FAK, anti-Myc, and anti-tubulin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with appropriate horseradish peroxidase-conjugated IgG secondary antibodies. Proteins were detected using the Western Lightning−ECL system (PerkinElmer Inc., Waltham, MA).
BrdU Incorporation Assay
Cells were serum starved for 24 hours in DMEM with 0.5% fetal bovine serum to arrest them in G0 phase. Cells were then washed twice with DMEM and incubated for 24 hours in DMEM supplemented with 10% serum and 100 μmol/L Bromodeoxyuridine (BrdU) (Sigma-Aldrich, St. Louis, MO) to release them from G0. After the cells were washed and incubated, they were fixed, permeabilized, treated with DNase I (New England Biolabs, Ipswich, MA), and processed for immunofluorescent staining with anti-BrdU (1:200, Sigma-Aldrich, St. Louis, MO) and anti-GFP (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) antibodies, as described previously.21 Cells were counted in multiple fields and scored for BrdU-positive staining in each independent experiment.
Boyden Chamber Cell Migration Assays
To perform cell migration assays, a Neuro Probe 48-well chemotaxis Boyden chamber (Neuro Probe Inc., Gaithersburg, MD) was used, as described previously,21 with the following modifications: 2.5 × 104 cells were trypsinized and resuspended in DMEM and then added to each upper well. Cells were allowed to migrate toward the bottom wells containing either fibronectin (10 or 20 μg/ml; Invitrogen Life Technologies, Carlsbad, CA) or epidermal growth factor (EGF; 25 ng/ml; Millipore, Billerica, MA) as the chemo-attractant, or DMEM only as a control, for 10 hours in a 37°C humidified 5% CO2 incubator. At the end of each experiment, cells on the upper side of the polycarbonate membrane were removed and cells on the bottom-side were fixed with methanol for 8 minutes and stained with crystal violet (Sigma-Aldrich, St. Louis, MO). The cells that had migrated were counted from five randomly selected fields from each well under a light microscope equipped with a DP70 CCD camera (Model IX71, Olympus, Japan) at ×20 magnification. Images were captured using Image-Pro Plus software, version 3.0 (Media Cybernetics, Silver Spring, MD).
Matrigel Invasion Assays
BD BioCoat Matrigel invasion chambers (BD Bioscience, Bedford, MA) were used for invasion assays. First, the matrigel chambers were rehydrated in DMEM for 2 hours in a 37°C humidified incubator. After removing the DMEM, 25 ng/ml of EGF, in a total volume of 0.75 ml of DMEM, was added as a chemoattractant to the lower well of the invasion chambers and 2 × 105 AGS cells in 0.5 ml of DMEM were placed into the upper chamber. Cells were incubated for 20 hours to allow them to invade the matrigel. Subsequently, cells were fixed and stained with crystal violet (Sigma-Aldrich, St. Louis, MO). The number of cells that had invaded was counted from five randomly selected fields of each well under a light microscope at ×20 magnification (Model IX71, Olympus, Japan) using Image-Pro Plus software, version 3.0 (Media Cybernetics, Silver Spring, MD).
In-Gel Zymography Assays
Gelatin zymography was performed as previously described.22 Subconfluent cells were grown in serum-free DMEM at 37 °C for 18 hours. The conditioned media were collected, clarified by centrifugation, resolved in nonreducing gels containing 0.05% (wt/vol) gelatin, and processed for zones of degradation activity by zymography.
Results
Increased FAK Expression and Elevated pY397 FAK in Gastric Cancer Tissues
To evaluate whether FAK is involved in tumorigenesis in gastric cancer, we performed immunohistochemical analyses to detect FAK and pY397 FAK on paraffin-embedded specimens from 59 patients diagnosed with gastric carcinoma. The corresponding Q-score analysis shows that 41 cancerous tissues (69.5%) overexpressed FAK (Q-score ≧ 2) and 21 of (35.6%) displayed high levels of pY397 FAK staining in comparison with noncancerous tissue sections (Figure 1A-D). Consistent with this, Western blot analyses from the representative samples show that elevated levels of pY397 FAK were detected in gastric carcinomas, with only few cases exhibiting equal or lower levels of pY397 FAK in cancerous tissues. Total FAK expression was elevated in most of the cancerous tissues (Figure 1E). These data strongly indicate that FAK and its signaling are involved in tumorigenesis in gastric cancer based on the above two approaches.
Figure 1.
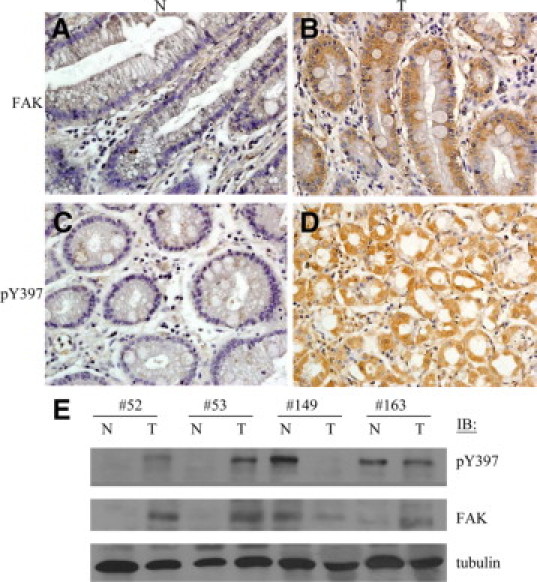
Increased expression and elevated Tyr397 phosphorylation of FAK in gastric cancer tissues. Noncancerous gastric tissues were negatively stained for FAK (A) and phospho-Tyr397 (pY397) FAK (C). In contrast, gastric cancer tissues exhibited high expression levels of FAK (B) and pY397 FAK (D). E: The cancerous (T) and noncancerous (N) gastric tissues were homogenized and subjected to Western blot analyses for detection of FAK and pY397 FAK proteins. Tubulin was used as a loading control.
Expression of pY397 FAK Is Correlated with Poor Survival in Gastric Carcinoma
To explore the clinical significance of pY397 FAK in gastric cancer, the clinicopathological profiles between patients with and without pY397 FAK overexpression were analyzed, as listed in Table 1. These analyses revealed a significant correlation between pY397 FAK and a higher incidence of tumor recurrence (57.1% versus 23.7%, P = 0.011). Moreover, the 5-year recurrence-free survival of the pY397 FAK-positive group was significantly lower than that of the pY397 FAK-negative group in gastric carcinoma tissues (73.2% versus 28.7%, P = 0.017; Figure 2B). A comparison between the clinicopathological profiles of the patients with and without FAK overexpression showed that patients exhibiting FAK overexpression were associated with a higher ratio of intestinal type (Lauren's classification) in this disease (85.2%, P = 0.007; Table 2). However, the incidence of recurrence was not significantly different between the patients with and without FAK overexpression (P = 0.441). In addition, the 5-year recurrence-free survival between the patients with and without FAK overexpression could not be statistically discriminated (54.3% versus 62.5%, P = 0.701; Figure 2A).
Table 1.
Correlation of pY397 Expression and Clinicopathological Factors
| Factor | pY397-positive (n = 21) | pY397-negative (n = 38) | P Value |
|---|---|---|---|
| Sex (M:F) | 15:6 | 23:15 | 0.571 |
| Age, years (mean ± SD) | 62.6 ± 13.0 | 64.5 ± 14.2 | 0.613 |
| Procedure (gastrectomy) | 0.357 | ||
| Subtotal:total | 14:7 | 30:8 | |
| Tumor size, cm | 4.3 ± 2.8 | 3.9 ± 2.4 | 0.560 |
| Lymph node dissected | 29.4 ± 12.5 | 29.7 ± 10.2 | 0.939 |
| Lauren classification | 0.896 | ||
| Intestinal type | 10 | 17 | |
| Diffuse type | 10 | 18 | |
| Mixed type | 1 | 3 | |
| Tumor depth* | 0.637 | ||
| 1:2:3:4 | 3:3:7:8 | 9:7:13:9 | |
| Node metastasis | 0.557 | ||
| No:Yes | 5:16 | 13:25 | |
| UICC staging | 0.415 | ||
| I:II:II:IV | 5:1:4:11 | 12:5:9:12 | |
| Recurrence | 0.011 | ||
| No | 9 | 29 | |
| Yes | 12 | 9 |
pY397-positive, overexpressed pY397; pY397-negative, low or nonexpressed pY397.
M, male; F, female.
Tumor depth: 1, mucosa or submucosa; 2, muscularis propria; 3, subserosa; 4, serosa or extra-serosal involvement.
Figure 2.
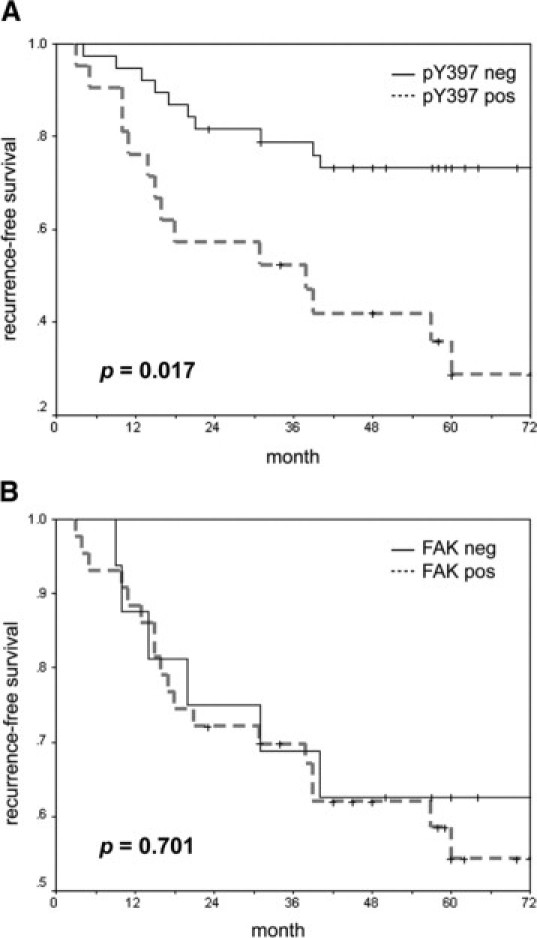
Expression of pY397 FAK in gastric carcinoma correlates with poor survival. A: Recurrence-free survival time was significantly different between the pY397 FAK-positive and pY397 FAK-negative gastric carcinoma groups (P = 0.017). B: Recurrence-free survival was not significantly different between the FAK-positive and FAK-negative gastric carcinoma groups (P = 0.701). Samples were designated positive for overexpression of FAK or pY397 FAK if the Q-scores were greater than or equal to 2. Samples were designated negative for low or no FAK or pY397 FAK if the Q-scores were less than 2, as described in the Materials and Methods section.
Table 2.
Correlation of FAK Expression and Clinicopathological Factors
| Factor | FAK-positive (n = 43) | FAK-negative (n = 16) | P Value |
|---|---|---|---|
| Sex (M:F) | 28:15 | 9:7 | 0.897 |
| Age, years (mean ± SD) | 64.0 ± 13.3 | 63.3 ± 14.8 | 0.549 |
| Procedure (gastrectomy) | 0.259 | ||
| Subtotal:total | 32:11 | 13:3 | |
| Tumor size, cm | 4.2 ± 2.3 | 3.9 ± 3.1 | 0.244 |
| Lauren classification | 0.007 | ||
| Intestinal type | 23 | 4 | |
| Diffuse type | 20 | 9 | |
| Mixed type | 0 | 3 | |
| Tumor depth* | 0.175 | ||
| 1:2:3:4 | 7:7:1:8 | 5:2:0:9 | |
| Node metastasis | 0.557 | ||
| No:yes | 13:30 | 5:11 | |
| UICC staging | 0.194 | ||
| I:II:III:IV | 11:4:10:18 | 6:1:5:4 | |
| Recurrence | 0.441 | ||
| No | 25 | 10 | |
| Yes | 18 | 6 |
FAK-positive, overexpressed FAK; FAK-negative, low or nonexpressed FAK; M, male; F, female.
Tumor depth: 1, mucosa or submucosa; 2, muscularis propria; 3, subserosa; 4, serosa or extra-serosal involvement.
pY397 FAK Is a Prognostic Factor Associated with the Recurrence of Gastric Carcinoma After Surgical Resection
The prognostic significance of FAK and pY397 FAK expression was determined using the Cox regression multivariate analysis and taking into account the clinical follow-up records of each patient. Multiple clinical factors, including age, sex, tumor location, surgical procedure, histological types (Lauren's classification), tumor size, depth of invasion, lymph node metastasis, TNM staging (UICC), as well as FAK and pY397 FAK overexpression levels were correlated with the recurrence of gastric cancer after surgery. This analysis revealed that pY397 FAK, but not FAK expression, was an independent prognostic factor for the recurrence of gastric cancer (P = 0.023; Table 3). These results suggest that pY397 FAK overexpression is intimately associated with higher recurrence rates of gastric carcinoma after surgical resection.
Table 3.
Multivariate Analysis of Predictive Factors for Recurrence in Gastric Cancer
| Odds ratio (95% CI) | P Value | |
|---|---|---|
| Age | 0.932–1.041 | 0.590 |
| Sex | 0.104–3.003 | 0.399 |
| Tumor site | 0.337–1019 | 0.058 |
| Procedure | 0.440–15.902 | 0.602 |
| Lauren | 0.137–2.212 | 0.913 |
| Tumor Size | 0.899–4.531 | 0.089 |
| Tumor depth | 0.351–30.476 | 0.277 |
| Lymph node metastasis | 0.350–2.051 | 0.879 |
| FAK | 0.212–2.416 | 0.299 |
| pY397 | 1.06–5.276 | 0.023* |
| UICC staging | 1.314–2.514 | 0.001* |
P values of <0.05 were considered to be statistically significant.
Phosphorylation of FAK at Tyr397 Is Required for Cell Migration, Invasion, and Proliferation in Gastric Carcinoma Cells
To elucidate potential mechanisms by which pY397 FAK contributes to malignancy in gastric carcinomas, an AGS human gastric carcinoma cell line was used to assess various cellular functions, such as cell migration, proliferation, and invasion. Adenoviruses were used to overexpress FAK, Y397F mutant (mutation of Tyr 397 to Phe results in an inactive form of FAK to generally act as a dominant negative mutant by reducing FAK activation), or FRNK (FAK-related nonkinase, a truncated mutant harbors a carboxyl terminus of FAK to serve as a dominant negative mutant capable of competing endogenous FAK out from focal adhesion sites),4 in AGS cells, as shown in Figure 3A. Western blot analyses demonstrated that the expression levels of exogenous wild-type FAK, Y397F mutant, and FRNK were fivefold higher than the amount of endogenous FAK. As expected, the amount of pY397 FAK in AGS cells overexpressing wild-type FAK was dramatically elevated. In contrast, the total pY397 FAK levels in AGS cells expressing Y397F mutant or FRNK were negligible due to their dominantly negative effects through affecting endogeneous FAK for intermolecular activation or competing endogenous FAK localization out from focal contacts,4 respectively.
Figure 3.
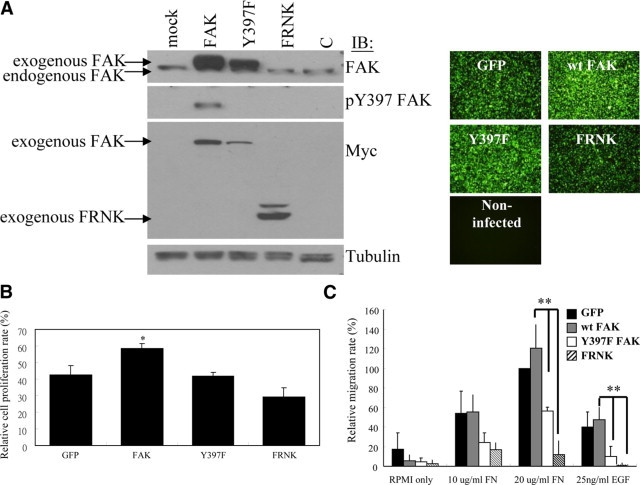
Requirement of FAK Tyr397 phosphorylation for FAK-mediated cell proliferation and migration in gastric carcinoma cells. A: A fivefold excess of exogenous FAK or mutant FAK relative to endogenous FAK was expressed in AGS cells via an adenovirus-based gene expression system harboring a GFP reporter, as described in the Materials and Methods section. The amount of pY397 was dramatically elevated in cells in which wild-type FAK was overexpressed, whereas it was absent in cells overexpressing either the Y397F mutant or FRNK. B: AGS cells overexpressing wild-type FAK, the Y397F mutant, or FRNK were subjected to BrdU incorporation assays as described in the Materials and Methods section. Results show the mean ± SD of the relative percentage of BrdU-positive cells from at least three independent experiments. C: Cell migration assays were performed with AGS cells overexpressing wild-type FAK, the Y397F mutant, or FRNK. The capability of cells to migrate toward fibronectin (10 and 20 μg/ml) or EGF (25 ng/ml) was measured using a modified Boyden chamber as described in the Materials and Methods section. Compared to cells expressing wild-type FAK or GFP alone, cell migration was impaired in Y397F- and FRNK-overexpressing cells. Cell migration was quantified by counting the number of cells that migrated toward the chemoattractants in the bottom wells. Mean cell counts from at least nine fields of polycarbonate membranes (under an Olympus IX71 20X objective microscopic lens) and three experiments are shown. Error bars represent ± SD. *P ≤ 0.05, as compared with GFP controls; **P < 0.01 as compared with wild-type FAK.
BrdU incorporation assays revealed that FAK overexpressing AGS cells prominently promoted cell proliferation compared with virus controls and both Y397F and FRNK expressing cells (Figure 3B). In addition, we used a modified Boyden chamber to measure migration of cells toward fibronectin or EGF in AGS cells (Figure 3C). We found that AGS cells overexpressing either Y397F or FRNK showed a prominent inhibition of cell migration to both chemoattractants compared to cells overexpressing wild-type FAK or virus controls. In invasion assays, overexpression of wild-type FAK led to increased invasiveness of AGS cells into Matrigel relative to Y397F or FRNK (Figure 4A). Moreover, matrix metalloproteinase-2 (MMP-2) has been reported to play a key role in tumor invasion22 and its expression and/activity could be regulated by FAK.23 Using in-gel zymography assays, we observed that the activity of MMP-2 was up-regulated on overexpression of wild-type FAK compared with that of the virus alone in AGS cells. Conversely, both Y397F- and FRNK-infected cells did not augment the activity of MMP-2 (Figure 4B), which is consistent with a less-invasive phenotype (Figure 4A). These results indicate that phosphorylation of FAK at Tyr397 is required for the induction of gastric carcinoma cell migration and invasion, which play key roles in tumor malignancy. Taken together, these results further support a strong link between phopshorylation of FAK at Tyr397 and malignancy of gastric cancer.
Figure 4.
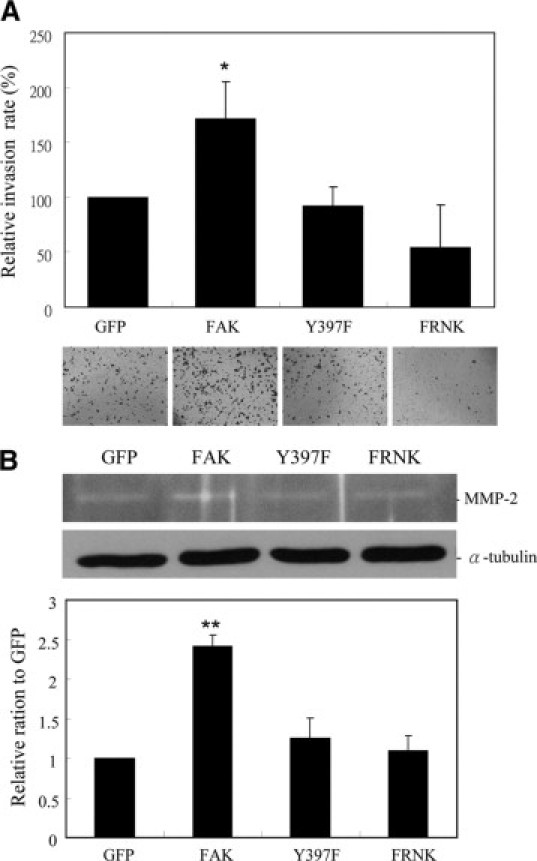
An essential role for FAK Tyr397 phosphorylation in tumor cell invasion. A: AGS cells were infected with adenoviruses harboring wild-type FAK, the Y397F mutant, or FRNK and then subjected to tumor invasion assays using matrigel transwells. B: In-gelatin zymography assays were conducted to detect the MMP2 activity of the cells as described in the Materials and Methods section. The mean ± SD from at least three experiments is shown. *P < 0.05; **P < 0.01 as compared with GFP controls.
Discussion
FAK is known to be a key mediator of cell migration, invasion, and proliferation. Given its essential role in FAK activation and signaling, autophosphorylation of FAK at Tyr397 has been shown to be a pivotal event in the activation of FAK-mediated cellular functions in response to a variety of extracellular stimuli. Increased expression and elevated activity of FAK has been linked with pathological relevance in different types of cancer; however, few studies have focused on the role of FAK and its activation/signaling in gastric cancer development and progression. In this study, we found that pY397 FAK, rather than FAK per se, is an independent prognostic factor for the recurrence of gastric cancer. Accordingly, we demonstrated the pY397 FAK is required for gastric cancer cell migration, invasion, and proliferation in vitro. Therefore, for patients with a high risk of recurrence, our results may justify the use of molecular markers such as pY397 FAK that could predict the recurrence of this disease. Furthermore, our study suggests that novel therapeutic approaches may be used to alter FAK phosphorylation and its resulting function in an effort to treat advanced gastric cancer.
FAK overexpression has been reported to be a prognostic factor in liver, lung, and cervical cancer.24–26 It is also reported that FAK has an insignificant impact on the outcome of different types of cancer.27,28 These contradictory results suggest a complicated regulatory role for FAK in cancer progression. Moreover, there is a lack of direct in situ evidence for FAK activation and signaling in relation to clinicopathological parameters and patient survival.29 Several studies have implied that FAK activation and its related signaling might be involved in tumor progression. For example, Yu et al showed that cholecystokinin-2 activation of FAK promotes colon cancer progression.30 The motility and invasiveness of EBV-expressing AGS gastric carcinoma cells are associated with an increase in FAK phosphorylation.31 Overexpression of lysyl oxidase-like 2 (LOXL2)-mediated gastric carcinoma invasion and metastasis require the Src/FAK pathway.32 In support of these studies, our current research provides additional in vivo evidence that FAK is intimately involved in gastric carcinoma development. The statistical analyses, however, show that the expression of FAK is only associated with the occurrence of the intestinal type gastric carcinoma, but not significantly related to the incidence of recurrence (Table 2). Conversely, pY397 FAK is a bona fide prognostic marker for recurrence (Table 1 and 3). Consistent with this, we also demonstrated that elevated levels of pY397 FAK result in enhanced cell migration, invasion, and proliferation in AGS human gastric cancer cells. In fact, phosphorylation of FAK at Tyr397 dictates its function in response to integrin-mediated cell adhesion, migration, and antiapoptosis, as well as growth factor–stimulated cell proliferation, as described in previous reports.33 Recently, two ATP-competing FAK inhibitors, PF-562271 (Pfizer) and TAE226 (Novartis), reduce its kinase activity and tyrosine autophosphorylation; these two inhibitors were also reported to impair tumor cell migration, proliferation, invasion, and metastasis in vitro and in vivo,9,34 thus supporting the importance of FAK activation and signaling in tumor development and progression. Our findings show a concomitant relationship between FAK activation (ie, pY397 FAK) and gastric cancer malignancy in vitro and in vivo, which may provide a better understanding of the diagnostic and prognostic relevance of FAK phosphorylation in gastric cancer.
The role of FAK in gastric cancer development is not well understood. Previous studies have only showed an association between enhanced FAK expression and poor differentiation, deep invasion, and lymph node metastasis of gastric carcinoma but have not provided any diagnostic and prognostic relevance.35 As shown by Giaginis et al,36 FAK is mildly, moderately, and intensely expressed in 47%, 35%, and 18% of gastric carcinomas, respectively. In this study, the authors found that, in diffuse type carcinomas, FAK was correlated with tumor size and disease stage. Enhanced FAK expression was also found to be positively associated with overall survival time in diffuse type gastric carcinomas, but no significant relevance was found in intestinal type carcinomas. Although the authors concluded that FAK can be considered as a diagnostic and prognostic marker in gastric neoplasia, the association of FAK with patient survival was not significant. Consistently, in our study, we also found that FAK itself is not a significant predictor of patient survival. In contrast, we found a significant association between FAK overexpression and the development of the intestinal type of gastric cancer. More importantly, we observed that 35.6% of samples with pY397 FAK, compared to 69.5% of samples with FAK, were significantly associated with 5-year recurrence-free survival from gastric carcinomas. This result indicated that pY397 FAK is a bona fide prognostic factor for malignant development of gastric carcinomas. Indeed, more than 50% of the FAK-positive group contains more active FAK in their cancerous tissues in our study. Nevertheless, only few reports have addressed the in vivo function of FAK phosphorylation and its activation in tumorigenesis. By immunostaining in colorectal, esophageal, pancreatic, and mammary cancers, pY397 FAK was found in the nuclei, which has been attributed to the enhancement of the cell cycle in cancer cells.37 However, the authors could not recapitulate the similar localization of pY397 FAK in the corresponding cancer cell lines, suggesting that carrier proteins such as Hic-5 or Zyxin may be required for the observed translocation.37 Activation and autophosphorylation of FAK leads to its association with several signaling molecules, which triggers signal transduction to modulate a diverse array of signaling events that regulate cell adhesion, spreading, proliferation, survival, migration, and differentiation.38 Such functions of FAK, recapitulated in tumor cells, are believed to promote tumor growth, survival, migration, invasiveness, metastasis, and angiogenesis.39 Therefore, the future for targeting FAK for cancer therapy requires further studies to delineate the link between FAK and its phosphorylation/ activation.
Based on several in vivo studies, including ours, one potential question has arisen with respect to the disparate expression of FAK and pY397 autophosphorylated FAK. One possibility is likely due to the complicated regulation of pY397 FAK mediated by a coordinated content among up-regulation and/or down-regulation of certain phosphatases. For example, Shp2 (Src homology region 2, phosphatase 2),40 PTEN (phosphatase and tensin homolog),41 or low M(r) phosphotyrosine protein phosphatase42 as well as PTP-PEST (protein tyrosine phosphatase containing PEST motif)43 and PTP-1 α (protein tyrosine phosphatase 1 α)44 can suppress pY397 FAK and its mediated signaling and cellular functions in the context of FAK overexpressing cancer tissues. Alternatively, the impairment of kinase activity or mutagenesis of tyrosine residues, including Tyr-397, may occur during carcinogenesis. In this regard, the up-regulation of Pyk2 expression, the other member of the FAK family kinases, has been reported to rescue certain functions of FAK-deficient cells in vitro and in vivo.45,46 Accordingly, the contribution of Pyk2 and FAK to turmorigenesis and progression are currently emerging as targets for investigation.23,47 Therefore, a comprehensive in vivo study for FAK-mediated tumorigenesis and malignancy is needed before targeting FAK for cancer therapy.
To be noted, in Figure 1E, an unexpected observation for the higher pY397 in the normal than in the tumor sample in gastric cancer patient 149 with multiple liver metastasis is likely due to its rare and distinct progression type. Lymphatic metastasis is the most common metastatic pathway of gastric cancers, while hematogenous spread to liver happened in this patient case. Hence, these data implied a featured molecular characteristic for this patient in tumorigenesis and progression, at least with respect to FAK activity. Indeed, some emerging reports provide novel insights on the roles of FAK dephosphorylation (ie, Tyr397) and/or a dynamic regulation of FAK activation during tumorigenicity and metastasis. For example, reduced expression of FAK in liver metastases compared with matched primary human colorectal adenocarcinoma was observed.48 Moreover, Helicobacter pyroli CagA was demonstrated to enable reducing the level of FAK tyrosine phosphorylation and led to increased cell motility during the development of gastric adenocarcinoma.40 Furthermore, recent studies also reveal that oncogene-induced FAK dephosphorylation, such as Ras, EGFR, HER2/ErbB2, etc, could facilitate cancer invasion and metastasis,43,49 implicating a negative role of FAK activation in regulating cancer cell progression under certain oncogenic signaling in various tumors.50 Nevertheless, the significance for the involvement of pY397 FAK in tumor progression and cancer recurrence in most common types of gastric cancers is convinced based on our statistical data.
In conclusion, we found that the phosphorylation of FAK at Tyr397, rather than FAK expression per se, is strongly predictive of recurrence of gastric cancer after curative resection. Activation of FAK, by way of its autophosphorylation at Tyr397, may contribute to the progression of gastric carcinoma by enhancing cell migration, invasion, and proliferation. For patients with high risk of recurrence, our results may justify the use of molecular markers that are able to predict recurrence of diseases, such as phosphorylated FAK, to select patients for adjuvant therapy after surgical resection of gastric cancer. Furthermore, targeting FAK phosphorylation might represent a novel therapeutic approach to inhibit advanced gastric cancer.
Acknowledgements
We thank Dr. Shan-Li Wang for critically reading this manuscript.
Footnotes
Supported by the National Science Council of Taiwan (NSC-96-2314-B-002-030 to I.-R.L.; NSC-96-2311-B-002-023-MY3 to T.-L.S.) and the National Health Research Institute (NHRI-EX99–9723SC to T.-L.S.).
References
- 1.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Wu CW, Lo SS, Shen KH, Hsieh MC, Chen JH, Chiang JH, Lin HJ, Li AF, Lui WY. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 2003;27:153–158. doi: 10.1007/s00268-002-6279-7. [DOI] [PubMed] [Google Scholar]
- 3.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 4.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 5.Guan JL, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 6.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Schwock J, Dhani N, Hedley DW: Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets 14:77–94 [DOI] [PubMed]
- 10.Akasaka T, van Leeuwen RL, Yoshinaga IG, Mihm MC, Jr, Byers HR. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J Invest Dermatol. 1995;105:104–108. doi: 10.1111/1523-1747.ep12313396. [DOI] [PubMed] [Google Scholar]
- 11.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 12.McCormack SJ, Brazinski SE, Moore JL, Jr, Werness BA, Goldstein DJ. Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines and human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene. 1997;15:265–274. doi: 10.1038/sj.onc.1201186. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 15.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86:1551–1556. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance WG. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9:215–222. [PubMed] [Google Scholar]
- 17.Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 1998;20:745–752. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Staging system for breast cancer: revisions for the ed 6 of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 19.Jan YJ, Ko BS, Hsu C, Chang TC, Chen SC, Wang J, Liou JY. Overexpressed focal adhesion kinase predicts a higher incidence of extrahepatic metastasis and worse survival in hepatocellular carcinoma. Hum Pathol. 2009;40:1384–1390. doi: 10.1016/j.humpath.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu PY, Huang LY, Hsu CH, Liang CC, Guan JL, Hung TH, Shen TL. Tyrosine phosphorylation of growth factor receptor-bound protein-7 by focal adhesion kinase in the regulation of cell migration, proliferation, and tumorigenesis. J Biol Chem. 2009;284:20215–20226. doi: 10.1074/jbc.M109.018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overall CM, Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25:69–75. doi: 10.1007/s10555-006-7890-0. [DOI] [PubMed] [Google Scholar]
- 23.Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, Liu W, Xing F, Okuda H, Kobayashi A, Watabe K. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–7620. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh S, Maeda T, Shimada M, Aishima S, Shirabe K, Tanaka S, Maehara Y. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res. 2004;10:2812–2817. doi: 10.1158/1078-0432.ccr-1046-03. [DOI] [PubMed] [Google Scholar]
- 25.Imaizumi M, Nishimura M, Takeuchi S, Murase M, Hamaguchi M. Role of tyrosine specific phosphorylation of cellular proteins, especially EGF receptor and p125FAK in human lung cancer cells. Lung Cancer. 1997;17:69–84. doi: 10.1016/s0169-5002(97)00650-8. [DOI] [PubMed] [Google Scholar]
- 26.Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, Yatabe Y, Takeda S, Nakao A. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol. 2004;41:104–111. doi: 10.1016/j.jhep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Theocharis SE, Kouraklis GP, Kakisis JD, Kanelli HG, Apostolakou FE, Karatzas GM, Koutselinis AS. Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur J Surg Oncol. 2003;29:571–574. doi: 10.1016/s0748-7983(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 28.Gabriel B, zur Hausen A, Stickeler E, Dietz C, Gitsch G, Fischer DC, Bouda J, Tempfer C, Hasenburg A. Weak expression of focal adhesion kinase (pp125FAK) in patients with cervical cancer is associated with poor disease outcome. Clin Cancer Res. 2006;12:2476–2483. doi: 10.1158/1078-0432.CCR-05-1867. [DOI] [PubMed] [Google Scholar]
- 29.Chatzizacharias NA, Kouraklis GP, Theocharis SE. Clinical significance of FAK expression in human neoplasia. Histol Histopathol. 2008;23:629–650. doi: 10.14670/HH-23.629. [DOI] [PubMed] [Google Scholar]
- 30.Yu HG, Tong SL, Ding YM, Ding J, Fang XM, Zhang XF, Liu ZJ, Zhou YH, Liu QS, Luo HS, Yu JP. Enhanced expression of cholecystokinin-2 receptor promotes the progression of colon cancer through activation of focal adhesion kinase. Int J Cancer. 2006;119:2724–2732. doi: 10.1002/ijc.22207. [DOI] [PubMed] [Google Scholar]
- 31.Kassis J, Maeda A, Teramoto N, Takada K, Wu C, Klein G, Wells A. EBV-expressing AGS gastric carcinoma cell sublines present increased motility and invasiveness. Int J Cancer. 2002;99:644–651. doi: 10.1002/ijc.10382. [DOI] [PubMed] [Google Scholar]
- 32.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, Zhao P, Yang ZH. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 33.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 34.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Su JM, Gui L, Zhou YP, Zha XL. Expression of focal adhesion kinase and alpha5 and beta1 integrins in carcinomas and its clinical significance. World J Gastroenterol. 2002;8:613–618. doi: 10.3748/wjg.v8.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giaginis CT, Vgenopoulou S, Tsourouflis GS, Politi EN, Kouraklis GP, Theocharis SE. Expression and clinical significance of focal adhesion kinase in the two distinct histological types, intestinal and diffuse, of human gastric adenocarcinoma. Pathol Oncol Res. 2009;15:173–181. doi: 10.1007/s12253-008-9120-2. [DOI] [PubMed] [Google Scholar]
- 37.Murata T, Naomoto Y, Yamatsuji T, Okawa T, Shirakawa Y, Gunduz M, Nobuhisa T, Takaoka M, Sirmali M, Nakajima M, Ohno Y, Tanaka N. Localization of FAK is related with colorectal carcinogenesis. Int J Oncol. 2008;32:791–796. [PubMed] [Google Scholar]
- 38.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006;26:261–276. doi: 10.1128/MCB.26.1.261-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura M, Gu J, Tran H, Yamada KM. PTEN gene and integrin signaling in cancer. J Natl Cancer Inst. 1999;91:1820–1828. doi: 10.1093/jnci/91.21.1820. [DOI] [PubMed] [Google Scholar]
- 42.Rigacci S, Rovida E, Sbarba PD, Berti A. Low Mr phosphotyrosine protein phosphatase associates and dephosphorylates p125 focal adhesion kinase, interfering with cell motility and spreading. J Biol Chem. 2002;277:41631–41636. doi: 10.1074/jbc.M201709200. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y, Xia Y, Hawke D, Halle M, Tremblay ML, Gao X, Zhou XZ, Aldape K, Cobb MH, Xie K, He J, Lu Z. FAK phosphorylation by ERK primes ras-induced tyrosine dephosphorylation of FAK mediated by PIN1 and PTP-PEST. Mol Cell. 2009;35:11–25. doi: 10.1016/j.molcel.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng L, Si X, Yu WP, Le HT, Ng KP, Teng RM, Ryan K, Wang DZ, Ponniah S, Pallen CJ. PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J Cell Biol. 2003;160:137–146. doi: 10.1083/jcb.200206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behmoaram E, Bijian K, Jie S, Xu Y, Darnel A, Bismar TA, Alaoui-Jamali MA. Focal adhesion kinase-related proline-rich tyrosine kinase 2 and focal adhesion kinase are co-overexpressed in early-stage and invasive ErbB-2-positive breast cancer and cooperate for breast cancer cell tumorigenesis and invasiveness. Am J Pathol. 2008;173:1540–1550. doi: 10.2353/ajpath.2008.080292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayaki M, Komatsu K, Mukai M, Murata K, Kameyama M, Ishiguro S, Miyoshi J, Tatsuta M, Nakamura H. Reduced expression of focal adhesion kinase in liver metastases compared with matched primary human colorectal adenocarcinomas. Clin Cancer Res. 2001;7:3106–3112. [PubMed] [Google Scholar]
- 49.Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y, Lu Z. Paradoxical roles of FAK in tumor cell migration and metastasis. Cell Cycle. 2009;8:3474–3479. doi: 10.4161/cc.8.21.9846. [DOI] [PubMed] [Google Scholar]


