Abstract
Acute kidney injury (AKI) is a serious problem in critically ill patients of intensive care units. It has been reported previously that AKI can induce acute lung injury (ALI), as well as cause injuries to other remote organs, including the lungs. Patients with AKI complicated by ALI show remarkably high mortality. ALI is characterized by neutrophil infiltration into the lung. Neutrophil elastase (NE) is a key enzyme for tissue injury caused by activated neutrophils, such as occurs in ALI. Therefore, this study investigated the role of NE in AKI-induced ALI using a specific NE inhibitor, sivelestat sodium hydrate (ONO-5046), in a mouse bilateral nephrectomy model. Bilateral nephrectomy showed not only a remarkable increase in blood urea nitrogen levels, but also demonstrated neutrophil infiltration into the lung, increased pulmonary inflammatory cytokine expression [interleukin-6, neutrophil chemokine keratinocyte-derived chemokine, and tumor necrosis factor-α], and protein leakage with early increases in both systemic and pulmonary NE activity. ONO-5046 treatment reduced NE activity and improved these pulmonary inflammatory responses. Additionally, ONO-5046-treated animals had longer survival times. These data demonstrate that increasing NE activity induces pulmonary inflammatory damage in a bilateral nephrectomy model. Blockade of NE activity will be a useful therapeutic strategy for ALI complications in AKI patients.
Acute lung injury (ALI) is a life-threatening condition that is frequently complicated with acute kidney injury (AKI), which is a serious condition in intensive care units. The ALI mortality is higher than 50% and increases with the development of other organ failure including AKI.1 One report described a mortality rate higher than 80% for patients with AKI complicated with ALI.2 Current prevention and treatment strategies for AKI cannot sufficiently improve the mortality of these severely ill patients. Although many basic and clinical researchers are investigating novel therapies for AKI,3 targeting of other organ damages caused by AKI is also necessary for improving AKI outcomes.
Recently, it has been suggested that AKI influences other remote organs including the lungs.4–6 Experimental evidence indicates that AKI-induced ALI occurs not only by volume overload, but also through deleterious kidney–lung interactions. Mechanisms of AKI-induced ALI include dysregulation of inflammatory reaction, innate immune response, oxidative stress, apoptosis, and soluble mediator metabolism.7 Rodent models of bilateral nephrectomy (BNx) and renal ischemia reperfusion have been used to investigate the role of AKI in the pathogenesis of ALI.8–11 Ischemic AKI engenders increased pulmonary vascular permeability by down-regulating pulmonary epithelial sodium channel, Na,K-ATPase, and aquaporin-5.10 Ischemic AKI induced leukocyte accumulation and activation of inflammatory transcription factors such as nuclear factor-κB and p38 mitogen-activated protein kinase in the lung, which was attenuated by an anti-inflammatory cytokine α-melanocyte stimulating hormone.11 Faubel and colleagues5 demonstrated that BNx induced increases of multiple serum cytokines including interleukin (IL)-6 and lung injury characterized by neutrophil infiltration. Anti-inflammatory cytokine IL-10 attenuated pulmonary histological damage, bronchoalveolar lavage fluid (BALF) protein levels, and pulmonary myeloperoxidase activity.8
Neutrophils play a central role in the pathogenesis of ALI by releasing several proteases and reactive oxygen species.12 Among them, neutrophil elastase (NE) is recognized as a potential target of therapeutic intervention. NE is a serine protease secreted by neutrophils during inflammation; it destroys not only bacteria but also host tissue.13,14 A specific NE inhibitor, sivelestat sodium hydrate (ONO-5046), is now approved for clinical use on ALI complicated with systemic inflammatory response syndrome in Japan. Although several clinical reports describe the protective effects of ONO-5046 on human ALI associated with systemic inflammatory response syndrome,15–19 the literature includes no report of a clinical study evaluating the effect of ONO-5046 on ALI complicated with AKI. The present study is designed to elucidate the possible therapeutic effects of a specific NE inhibitor ONO-5046 on ALI induced by AKI using mouse BNx model. In addition to systemic and pulmonary NE activity and histological analysis of the lung, inflammatory cytokine expression in the lung was evaluated to clarify the role of NE in AKI-induced ALI.
Materials and Methods
Animals and Surgical Protocol
Eight- to ten-week-old male C57BL/6 mice, weighing 20 to 25 g, were obtained from Japan SLC (Hamamatsu, Japan). Those mice were kept on a 12-hour light/dark cycle with free access to diet and water. All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services, Public Health Services, National Institutes of Health, NIH publication no. 86-23, 1985).
Animals were anesthetized by injection (i.p.) with a combination of ketamine hydrochloride and xylazine hydrochloride. The kidneys were exposed from the flank, dissected, and removed after the pedicles were ligated using 5-0 nylon sutures; alternatively, kidneys were isolated in sham surgeries. ONO-5046, provided by Ono Pharmaceutical Co., Ltd. (Osaka, Japan), was dissolved in normal saline and adjusted to pH 7.8 with Na2CO3. In the BNx+ONO-5046 group, ONO-5046 was administered intraperitoneally at the dose of 50 mg/kg 11 hours before the surgery and every 11 hours after (Figure 1). In the previous study on a mouse model of acute colitis, treatment of ONO-5046 at the dose of 50 mg/kg twice a day improved histological damages and suppressed the NE activities.20 The same amount of saline was injected to the BNx+saline and the sham group. Animals were killed 6 hours or 24 hours after the surgery and blood and lung specimens were harvested for analyses, except for survival analysis. For survival analysis, animals were treated with ONO-5046 or saline every 11 hours until they died. Survival was assessed every 6 to 12 hours after the surgery.
Figure 1.
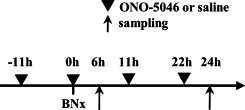
Protocol of ONO-5046 treatment. Acute lung injury was induced by bilateral nephrectomy. The experimental design of the BNx model and ONO-5046 treatment is shown. ONO-5046 treatment was performed 11 hours before surgery and every 11 hours after until death. Animals were killed 6 hours and 24 hours for blood and lung tissue sampling except survival analysis.
Measurement of Blood Urea Nitrogen, Aspartate Aminotransferase, and Alanine Aminotransferase
The blood urea nitrogen was measured using the urease–indophenol method with Urea NB (Wako Pure Chemical Industries Ltd., Osaka, Japan). The aspartate aminotransferase and alanine aminotransferase was measured using the POP-TOOS [pyruvate oxidase-N-ethyl-N-(2-hydroxy-3-sulfopropyl)-m-toluidine] method with Transaminase CII-test Wako (Wako Pure Chemical Industries Ltd.). An absorbance 96-well plate reader (SpectraMAX Plus; Molecular Devices Corp., Sunnyvale, CA) was used with a wavelength of 570 nm (blood urea nitrogen) and 555 nm (aspartate aminotransferase and alanine aminotransferase).
Histological Examination of Lung Tissue
The lungs were resected from the mice after perfusion with PBS. The lungs were fixed with formalin and embedded in paraffin. Sections (3 μm thick) were stained with Giemsa (Wako Pure Chemical Industries Ltd.). The number of neutrophils was determined in 10 randomly selected nonoverlapping fields at ×400 magnification in each section of the individual mouse lung. Scores of respective lungs were averaged; scores of each animal were also averaged.
Analysis of BALF
The lungs were lavaged with 4 × 0.5 ml of PBS. The BALF was centrifuged 1500 rpm at 4°C for 10 minutes, and the level of protein in the supernatant was measured using Bradford total protein assay (Bio-Rad Laboratories Inc., Hercules, CA).21 The cells were resuspended in 1 ml of saline with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and the total cell numbers were counted with a hemocytometer. Cytospin samples were prepared by centrifuging the suspensions at 350 rpm for 10 minutes and cell differentials were determined by counting at least 300 leukocytes on Giemsa Stain (Wako Pure Chemical Industries, Ltd.).22,23 For neutrophil elastase activity measurement and Western blot analysis, BALF samples were condensed by spin condensing columns (Amicon Ultra; Millipore Corp., Billerica, MA).
Wet/Dry Lung Weight Ratios
Lung wet/dry (W/D) weight ratios were measured as described previously.24 The lungs were surgically dissected, with removal of the trachea and main bronchi. Lungs were weighed and dried in an oven at 60°C for 48 hours. The W/D weight ratio was calculated to evaluate pulmonary edema.
Measurement of NE Activity
NE activity was determined as described previously.25 Briefly, plasma, supernatant of the lung homogenate, and BALF samples were incubated with 0.1 mol/L Tris-HCl buffer (pH 8.0) containing 0.5 mol/L NaCl and 1 mmol/L N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide, a highly specific synthetic substrate for NE, at 37°C for 24 hours and the liberated amount of p-nitroanilide was measured spectrophotometrically at 405 nm. NE activity was calculated as the concentration of liberated p-nitroanilide. NE activity of lung homogenate and BALF was corrected with protein concentration.
Western Blot Analysis
Western blot analysis was performed as described previously.26 Briefly, 10 μg proteins extracted from the lung or contained in BALF were separated on a 10 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Amersham Biosciences Corp., Uppsala, Sweden). As a positive control, human leukocytes extracted from peripheral blood were used.27 Western blot analysis was performed using 1:150 diluted neutrophil elastase antibody (H-57; Santa Cruz Biotechnology Inc., Santa Cruz, CA) overnight at 4°C. Subsequently, the chemiluminescent signal labeled using ECL Plus (Amersham Biosciences Corp.) was detected using a CCD camera system (LAS-4000 Mini; Fuji Photo Film Co. Ltd., Tokyo, Japan). The membrane was incubated at 50°C for 30 minutes in a stripping buffer to remove all probes. The reprobing procedure was performed further with the antibody to β-actin (Chemicon Co. Ltd., Temecula, CA). Densitometric analysis of bands compared with β-actin was performed using image software (Multi Gauge Ver. 3.0; Fuji Photo Film Co. Ltd.).
Real-Time PCR Assay for IL-6, Keratinocyte-Derived Chemokine, and Tumor Necrosis Factor-α Expression in the Lung
Total RNA was extracted from whole lung homogenates using Trizol (Invitrogen Corp., Carlsbad, CA). A High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) was used according to the manufacturer's protocol to synthesize cDNA from total RNA with random primers. Lung transcription levels were assessed using real-time quantitative PCR with LightCycler Faststart DNA Master SYBR Green I (F. Hoffman La Roche Ltd./Roche, Basel, Switzerland) and a PCR system (Prism 7000; Applied Biosystems), according to the manufacturer's instructions. The following primer-specific nucleotide sequences of IL-6 (sense 5′-TTCCATCCAGTTGCCTTCTT-3′ and antisense 5′-ATTTCCACGATTTCCCAGAG-3′), keratinocyte-derived chemokine (KC) (sense 5′-GGCTGGGATTCACCTCAAGAAC-3′, antisense 5′-TGTGGCTATGACTTCGGTTTGG-3′), tumor necrosis factor (TNF)-α (sense 5′-ATCCGCGACGTGGAACTG-3′ and antisense 5′-ACCGCCTGGAGTTCTGGAA-3′), and β-actin (sense 5′-CGCACCACTGGCATTGTCAT-3′ and antisense 5′-TTCTCCTTGATGTCACGCAC-3′) were used. The PCR reaction conditions of IL-6, KC, and TNF-α were 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 30 seconds. Condition of β-actin was 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 s, 53.5°C for 10 s, and 72°C for 30 seconds. Quantification of gene expressions were calculated relative to β-actin. Amplification data were analyzed using software (Prism sequence detection ver. 2.1; Applied Biosystems).
Statistical Analysis
The results of statistical analyses are expressed as means ± SEM. Differences between groups were analyzed for statistical significance using one-way analysis of variance followed by Tukey-Kramer test for multiple pairwise comparisons. P < 0.05 was inferred as statistically significant. Survival was compared using a log-rank test. These calculations were performed using software (JMP 8.0; SAS Institute Inc., Cary, NC).
Results
Effect of ONO-5046 on ALI Induced by BNx
The protocol of ALI induced by BNx and ONO-5046 treatment is presented in Figure 1. A significant increase of blood urea nitrogen was observed in the BNx+saline and BNx+ONO-5046 group; no significant difference was found between them at any measurement time point of 2, 4, 8, and 24 hours. No significant difference was found in any body weight change or lung W/D weight ratio between the groups, which suggests that this BNx model had no remarkable volume overload. The ONO-5046 treatment did not change the body weight and lung W/D weight ratio. Morphological analyses revealed no pulmonary interstitial edema in bilateral nephrectomized mice at 6 and 24 hours. Neutrophil infiltration evaluated by Giemsa stain was demonstrated in the BNx+saline group. Administration of ONO-5046 to sham-operated animals did not cause any neutrophil infiltration in the lung (Figure 2, A–C). The number of neutrophils was higher at 6 hours than at 24 hours after BNx and ONO-5046 treatment decreased neutrophil infiltration at 6 hours and 24 hours (Figure 2, D).
Figure 2.
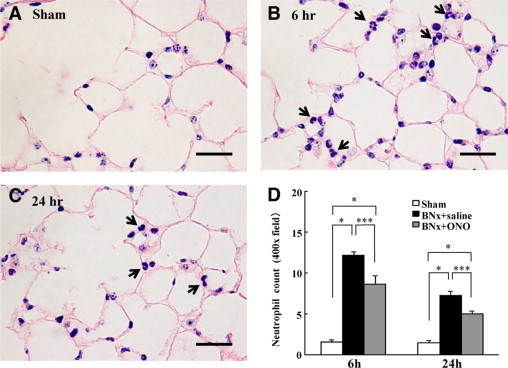
Neutrophil infiltration in ALI induced by BNx. Bilateral nephrectomy induced neutrophil infiltration in the lung. ONO-5046 treatment significantly reduced neutrophil numbers in the lung tissue. A–C: Neutrophil infiltration at 6 and 24 hours after the surgery was observed by Giemsa stain (arrows). Original magnification, ×400. Scale bar = 50 μm. D: The neutrophil count in the lung was decreased significantly by ONO-5046 (ONO) treatment (n = 5 to 6 in each group; the number of neutrophils was determined in 10 randomly selected nonoverlapping fields in each section of the individual mouse lung.). *P < 0.001 versus BNx+saline, ***P < 0.05 versus sham.
BALF cellular content and protein levels were measured. BNx did not increase BALF cell counts including neutrophil compared with sham-operated animals (Figure 3A). No increase of BALF protein levels was found 6 hours after surgery. The BALF protein concentration in the BNx+saline group was significantly higher than that of the sham group at 24 hours. The ONO-5046 treatment engendered significantly lower BALF protein levels at 24 hours (Figure 3B).
Figure 3.
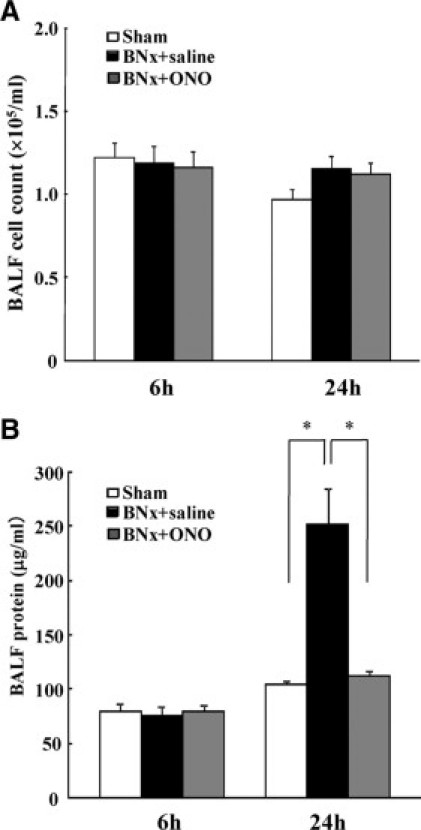
BALF cell count and protein concentration in ALI induced by BNx. BALF obtained at 6 hours and 24 hours after bilateral nephrectomy was analyzed for evaluating pulmonary inflammation and vascular leakage. A: No significant difference was found in BALF cell count among the groups at 6 hours and 24 hours (n = 5 to 6 in each group). B: BALF protein concentration was increased at 24 hours and ONO-5046 (ONO) treatment significantly decreased BALF protein at 24 hours (n = 5 to 6 in each group). *P < 0.001 versus BNx+saline.
Survival and Liver Injury in ALI Induced by BNx
The animals started to die 24 hours after the surgery of BNx. The ONO-5046 treatment significantly improved survival compared with saline injection (Figure 4). In accordance with the previous report,28 BNx significantly increased plasma aspartate aminotransferase and alanine aminotransferase levels. The ONO-5046 treatment did not attenuate these liver enzymes (Figure 5, A and B).
Figure 4.
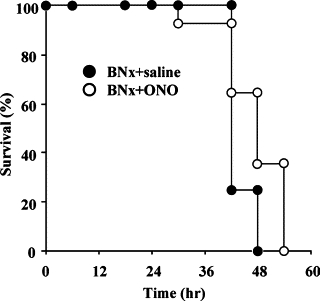
Effect of ONO-5046 on survival. Animals started to die 24 hours after bilateral nephrectomy and ONO-5046 (ONO) treatment showed a significant improvement of the survival after BNx compared with saline injection (P < 0.05, by log-rank test). Closed circles indicate the BNx+saline group (n = 16) and open circles ONO-5046 treatment group (n = 14).
Figure 5.
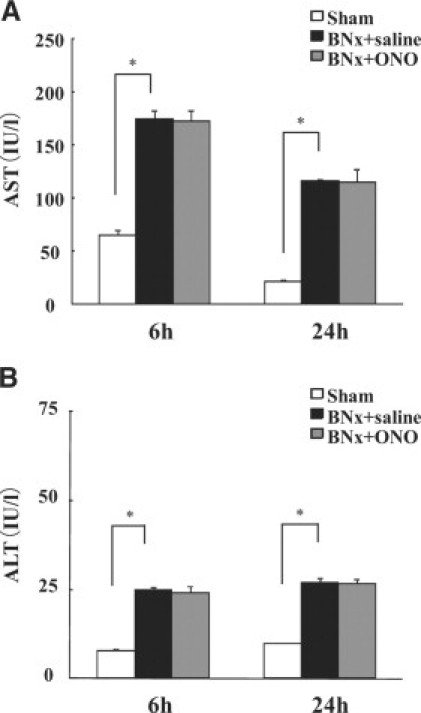
Liver injury in ALI induced by BNx. Liver injury was observed after bilateral nephrectomy. Plasma aspartate aminotransferase (A) and alanine aminotransferase (B) levels were significantly increased after the surgery. ONO-5046 (ONO) treatment did not improve liver injury (n = 7 in each group). *P < 0.001 versus sham.
Neutrophil Elastase in ALI Induced by BNx
Pulmonary and plasma NE activity increased at 6 hours after BNx and decreased to an equivalent level of sham after 24 hours. The BNx+ONO-5046 group showed significantly lower pulmonary (Figure 6A) and plasma (Figure 6B) NE activity at 6 hours. The NE activity in the BALF was not detected in all of the groups even when the BALF was condensed by spin condensing column. The amount of NE in the lung tissue was examined using Western blot analysis. Immunoreactive bands at approximately 30 kDa were detected which corresponds to the molecular size of NE. NE protein in the lung was increased at 6 hours and 24 hours after BNx. The ONO-5046 treatment attenuated NE protein levels in the lung (Figure 7, A and B). No NE protein was detected in BALF by Western blot analysis (Figure 7A).
Figure 6.
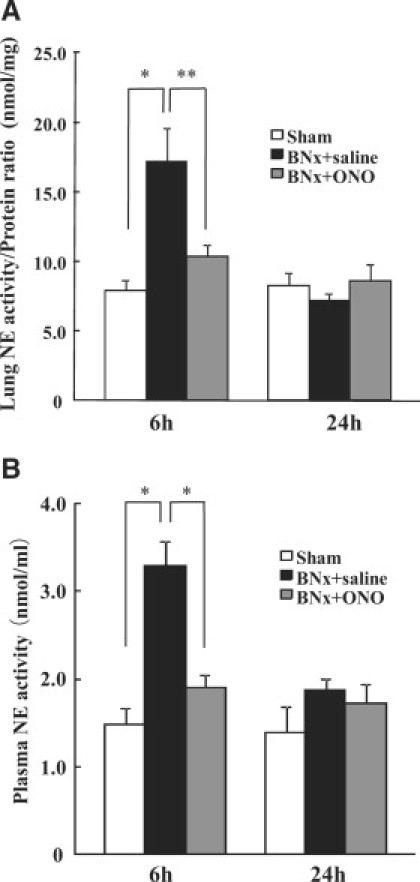
NE activity after AKI by BNx. To demonstrate the mechanism of protective effect of ONO-5046, lung (A) and plasma (B) NE activity after BNx were examined. BNx increased pulmonary and plasma NE activity at 6 hours and ONO-5046 (ONO) treatment significantly decreased it (n = 5 to 6 in each group). *P < 0.001, **P < 0.01 versus BNx+saline.
Figure 7.
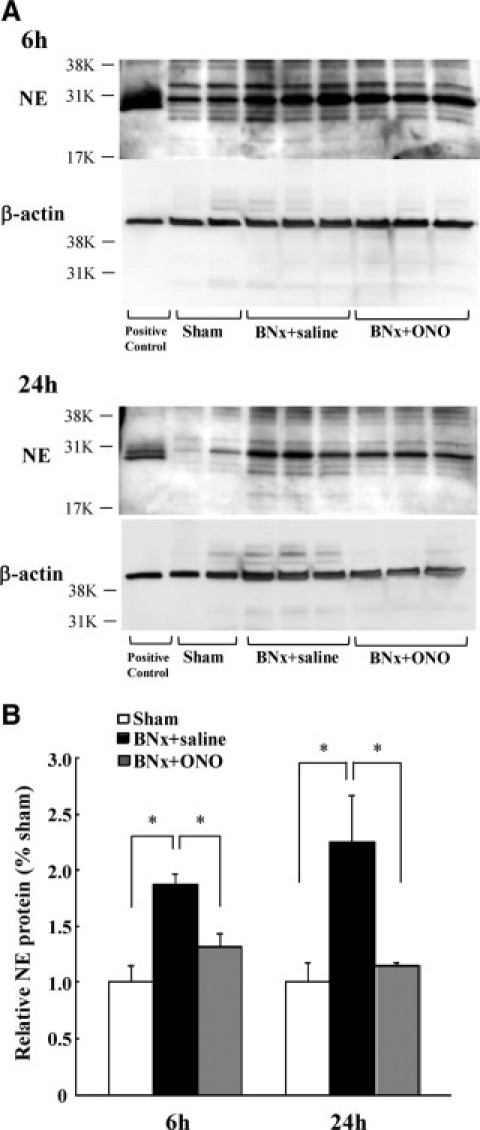
Pulmonary NE expression in ALI induced by BNx. A: The amount of NE protein was measured by Western blot analysis of lung homogenates. B: Densitometric analysis of immunoreactive bands compared with the density of β-actin demonstrated NE protein amount was significantly increased in the BNx+saline group, and attenuated by ONO-5046 (ONO) treatment (n = 6 in each group). *P < 0.05 versus BNx+saline.
Inflammatory Cytokine Expression in ALI Induced by BNx
To evaluate pulmonary inflammatory responses in ALI induced by BNx, IL-6, KC, and TNF-α mRNA expressions in the lung were examined. Increased expressions of IL-6 (Figure 8A), KC (Figure 8B), and TNF-α (Figure 8C) were found in the BNx+saline group at 6 hours and 24 hours after BNx. These cytokine expressions were attenuated significantly by the ONO-5046 treatment (Figure 8, A–C).
Figure 8.
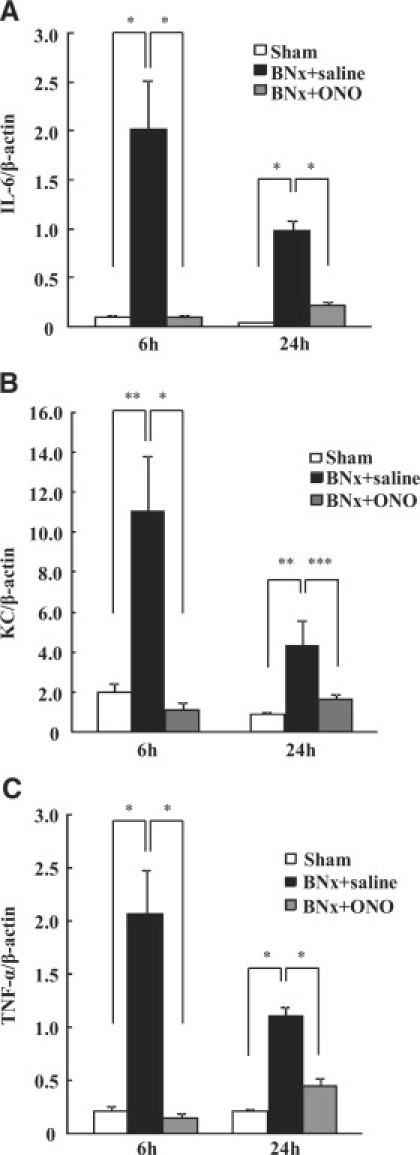
Inflammatory cytokine mRNA expression in ALI induced by BNx. Inflammatory responses induced by BNx were evaluated by several inflammatory cytokine expressions. mRNA expressions of IL-6 (A), KC (B), and TNF-α (C) in the lung were increased by BNx and ONO-5046 (ONO) treatment remarkably reduced these inflammatory cytokine expressions (n = 5 to 7 in each group). *P < 0.001, **P < 0.01, ***P < 0.05 versus BNx+saline.
Discussion
Acute lung injury complicated with AKI is highlighted by an unacceptably high mortality. Novel therapeutic strategies targeting this severe condition will improve critical care in intensive care units remarkably. This study demonstrated a specific NE inhibitor ONO-5046, which is now available for human ALI induced by systemic inflammatory response syndrome in Japan, improved ALI induced by AKI in a mouse model of BNx. Our data indicated that NE plays a crucial role in the pathogenesis of ALI induced by AKI.
NE is a serine protease located in neutrophil lysosome. Its role as a bactericidal agent has been established by generating mice lacking NE.29 The NE is secreted from activated neutrophils and contributes to tissue destruction in inflammatory diseases such as acute respiratory distress syndrome,30 lung emphysema,31 and rheumatoid arthritis.32 In normal conditions, NE is inactivated systemically and tightly by endogenous protease inhibitors such as α1-antitrypsin (AT) and α2-macroglobulin. However, inflammatory conditions inactivate these protease inhibitors by neutrophil-derived reactive oxygen species12 and enable NE to have strong protease activity of destroying extracellular matrix such as elastin, collagen type I–IV, and proteoglycan.33 In the present study, systemic and pulmonary NE activity increased early after BNx (6 hours), which suggests that the acute decline of kidney function triggers inactivation of endogenous NE inhibitors. Reportedly, plasma elastase protein levels in dialyzed patients were increased even before dialysis treatment.34 Liver injury evaluated by blood aspartate aminotransferase and alanine aminotransferase levels was also found in the bilateral nephrectomized animals. α1-AT and α2-macroglobulin are produced by the liver and reduced synthesis of these endogenous NE inhibitors by the liver injury may partly contribute to NE activation in this model. Further investigations are necessary to clarify the regulatory mechanism of NE activation.
ONO-5046 attenuates lipopolysaccharide-induced pulmonary inflammation by inhibiting neutrophil infiltration and reducing lung vascular permeability in several animal models.35–37 In contrast to endogenous protease inhibitors, ONO-5046 assumed to be able to inhibit NE effectively in inflammatory conditions because it is not inactivated by reactive oxygen species.38,39 The present study is the first report that shows the protective effect of ONO-5046 on AKI-induced ALI. Protective effects of ONO-5046 have also been demonstrated on neutrophil-mediated other organ injury models such as hippocampal neuronal damage after transient forebrain ischemia,40 neurological damage after spinal cord injury,41 collagen-induced arthritis,42 and acute colitis.20 Although one randomized controlled trial including 492 patients demonstrated no improvement of ALI by ONO-5046,18 several small trials showed beneficial effects of ONO-5046 on ALI in critically ill patients.17,19 No clinical trial evaluating ONO-5046 on ALI complicated with AKI has been reported in the relevant literature. Our data may indicate the potential of NE inactivation for ALI treatment and induce new drug development for ALI complicated with AKI, which has an unacceptably high mortality rate. In addition, the therapeutic window of NE inactivation therapy should be considered for future clinical trials, because our data suggested the early intervention to NE activity by ONO-5046 is necessary to reduce AKI-induced ALI.
Results of this study showed a remarkable pulmonary neutrophil infiltration in ALI induced by BNx. Increased expression of KC, which is a potent chemoattractant for neutrophils, was found in the lung tissue. NE induced IL-8 (human analogue of mouse KC) expression in human bronchial epithelial cells.43 The KC production in zymosan-stimulated cremaster muscles of NE knockout mice was lower than in wild-type mice.44 We found attenuation of KC expression in the lung using ONO-5046 treatment. These data suggested that a vicious circle of NE-induced KC expression and subsequent neutrophil infiltration at least partly contribute to AKI-induced ALI; NE increased KC expression in the lung, increased KC, a potent chemoattractant, allowed neutrophil to infiltrate to the lung, and infiltrated neutrophil released NE and further increased KC levels.
We found a significant increase of NE protein in the lung, which was attenuated by ONO-5046 treatment. Reportedly, NE-α1-AT complex formation reduces elastase activity and NE-α1-AT complexes are cleared via serpin-enzyme complex receptors.45,46 If the clearance of NE-α1-AT complexes were blocked by ONO-5046, ONO-5046 treatment would increase NE protein amount. However, α1-AT is larger and has higher potency compared with ONO-5046 (α1-AT; 55 kDa, Ki 0.033 pM versus ONO-5046; 0.5 kDa, Ki 29 nM).47,48 NE inhibition by α1-AT is irreversible, whereas ONO-5046 inhibits NE activity reversibly.49 Therefore, it might be reasonable to discuss that α1-AT traps NE immediately and irreversibly in the injured lung, and ONO-5046 inhibited the excess NE that escaped from the natural defense system of α1-AT. In the situation that BNx induces NE production, ONO-5046 may not influence on the NE-α1-AT complex formation and clearance. It is true that there is no evidence whether ONO-5046 influences NE clearance and further investigations should be performed to clarify the influence of ONO-5046 on NE-α1-AT complex. It is of note that Endo et al50 reported ONO-5046 treatment on human septic ALI patients significantly reduced blood NE concentration.
Although one report showed that increased pulmonary vascular permeability in a rat ischemia reperfusion model was attenuated by an inhibitor of macrophage activation,51 BNx did not induce any macrophage infiltration in the lung (data not shown). It can be speculated that activated macrophage might play a role in ALI in our model by releasing some humoral factors from other distant organs such as the liver.
In this study, we induced AKI not by ischemia reperfusion, but using BNx. As with renal ischemia, ischemia reperfusion models of other organs such as the liver,52 gut53 and hind limb54 also cause ALI. Intestinal ischemia reperfusion injury induced tremendous lung injury, as characterized by lung edema, histopathological changes (alveolar congestion, hemorrhage, and infiltration of inflammatory cells), increased myeloperoxidase activity, and pro-inflammatory cytokines (IL-6 and TNF-α) levels in the lung.55 In addition, renal ischemia reperfusion degrades other organ functions of the heart and brain.56,57 Therefore, it is difficult to distinguish the contribution of renal dysfunction itself on lung injury from the effects derived from non-renal organ injury or increased systemic oxidative stress. Because the BNx model induces abrupt and complete decline of renal function, it enables us to investigate the direct relation of renal dysfunction with ALI without considering the additive issues described above.5
In accordance with results described in a previous report,8 the mouse model of AKI-induced ALI using BNx in the present study showed no volume overload or pulmonary edema. No significant difference was found between bilateral nephrectomized and sham-operated animals in terms of total body weight change or the lung W/D weight ratio. Severely ill patients of AKI-induced ALI frequently show lung edema possibly because they need fluid resuscitation to maintain their hemodynamics. However, we administered no fluid after the surgery to the animals. Although remarkable inflammatory responses including neutrophil infiltration, increased vascular permeability, and inflammatory cytokine expression were demonstrated, adding fluid as resuscitation will produce this AKI-induced ALI animal model closer to the clinical settings. Moreover, cell counts including neutrophil in BALF did not increase in this model. We could not detect any NE protein and activity in BALF. We observed neutrophil infiltration in the pulmonary interstitium but not in the alveolar cavities. This indicates the abrupt loss of renal function by BNx caused insufficient pulmonary inflammation to induce neutrophil migration into the alveolar cavity when compared with severe ALI models such as using oleic acid or LPS.58 Further investigation is necessary to evaluate whether ONO-5046 can protect AKI-induced ALI in more clinically relevant animal models.
In conclusion, we demonstrated that AKI induced by BNx caused lung injury highlighted by neutrophil infiltration; it also increased NE activity. Treatment with a specific NE inhibitor, ONO-5046, attenuated systemic and pulmonary NE activity and decreased neutrophil infiltration and inflammatory cytokine expression in the lung. These data indicate that NE plays a crucial role in AKI-induced ALI and that it can be a potential drug targeted to severe ALI complicated with AKI.
Acknowledgements
We are very grateful to Dr. Fumihiko Nakamura, Mami Haba, Kahoru Amitani (University of Tokyo), and Ryoji Matsumoto (Ono Pharmaceutical Co., Ltd.) for their skilled assistance.
Footnotes
Supported by the BioBank Japan Project on the Implementation of Personalized Medicine #3023168, Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (E.N.), KAKEN-HI #19590935, MEXT, Japan (E.N.), the Special Coordination Funds for Promoting Science and Technologies #1200015, MEXT, Japan (E.N.), and KAKEN-HI #21790795, MEXT, Japan (K.D.).
References
- 1.Liu KD, Matthay MA. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol. 2008;3:578–586. doi: 10.2215/CJN.01630407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 4.Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74:849–851. doi: 10.1038/ki.2008.390. [DOI] [PubMed] [Google Scholar]
- 5.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15:284–296. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Feltes CM, Van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron Physiol. 2008;109:80–84. doi: 10.1159/000142940. [DOI] [PubMed] [Google Scholar]
- 7.Ko GJ, Rabb H, Hassoun HT. Kidney-lung crosstalk in the critically ill patient. Blood Purif. 2009;28:75–83. doi: 10.1159/000218087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 9.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 10.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63:600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 11.Deng J, Hu X, Yuen PS, Star RA. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]
- 12.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 13.Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188. doi: 10.1126/science.289.5482.1185. [DOI] [PubMed] [Google Scholar]
- 14.Chua F, Laurent GJ. Neutrophil elastase: mediator of extracellular matrix destruction and accumulation. Proc Am Thorac Soc. 2006;3:424–427. doi: 10.1513/pats.200603-078AW. [DOI] [PubMed] [Google Scholar]
- 15.Tamakuma S, Ogawa M, Aikawa N, Kubota T, Hirasawa H, Ishizaka A, Taenaka N, Hamada C, Matsuoka S, Abiru T. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther. 2004;17:271–279. doi: 10.1016/j.pupt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Okayama N, Kakihana Y, Setoguchi D, Imabayashi T, Omae T, Matsunaga A, Kanmura Y. Clinical effects of a neutrophil elastase inhibitor, sivelestat, in patients with acute respiratory distress syndrome. J Anesth. 2006;20:6–10. doi: 10.1007/s00540-005-0362-9. [DOI] [PubMed] [Google Scholar]
- 17.Inoue Y, Tanaka H, Ogura H, Ukai I, Fujita K, Hosotsubo H, Shimazu T, Sugimoto H. A neutrophil elastase inhibitor, sivelestat, improves leukocyte deformability in patients with acute lung injury. J Trauma. 2006;60:936–943. doi: 10.1097/01.ta.0000217271.25809.a0. discussion 943. [DOI] [PubMed] [Google Scholar]
- 18.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, Bernard G. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 19.Akamoto S, Okano K, Sano T, Yachida S, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Neutrophil elastase inhibitor (sivelestat) preserves antitumor immunity and reduces the inflammatory mediators associated with major surgery. Surg Today. 2007;37:359–365. doi: 10.1007/s00595-006-3409-0. [DOI] [PubMed] [Google Scholar]
- 20.Morohoshi Y, Matsuoka K, Chinen H, Kamada N, Sato T, Hisamatsu T, Okamoto S, Inoue N, Takaishi H, Ogata H, Iwao Y, Hibi T. Inhibition of neutrophil elastase prevents the development of murine dextran sulfate sodium-induced colitis. J Gastroenterol. 2006;41:318–324. doi: 10.1007/s00535-005-1768-8. [DOI] [PubMed] [Google Scholar]
- 21.Nagatani K, Dohi M, To Y, Tanaka R, Okunishi K, Nakagome K, Sagawa K, Tanno Y, Komagata Y, Yamamoto K. Splenic dendritic cells induced by oral antigen administration are important for the transfer of oral tolerance in an experimental model of asthma. J Immunol. 2006;176:1481–1489. doi: 10.4049/jimmunol.176.3.1481. [DOI] [PubMed] [Google Scholar]
- 22.To Y, Dohi M, Tanaka R, Sato A, Nakagome K, Yamamoto K. Early interleukin 4-dependent response can induce airway hyperreactivity before development of airway inflammation in a mouse model of asthma. Lab Invest. 2001;81:1385–1396. doi: 10.1038/labinvest.3780352. [DOI] [PubMed] [Google Scholar]
- 23.Harada H, Imamura M, Okunishi K, Nakagome K, Matsumoto T, Sasaki O, Tanaka R, Yamamoto K, Dohi M. Upregulation of lung dendritic cell functions in elastase-induced emphysema. Int Arch Allergy Immunol. 2009;149(Suppl 1):25–30. doi: 10.1159/000210650. [DOI] [PubMed] [Google Scholar]
- 24.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimura K, Nakagawa S, Koyama S, Kobayashi T, Homma T. Roles of neutrophil elastase and superoxide anion in leukotriene B4-induced lung injury in rabbit. J Appl Physiol. 1994;76:91–96. doi: 10.1152/jappl.1994.76.1.91. [DOI] [PubMed] [Google Scholar]
- 26.Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73:1374–1384. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- 27.Doi K, Noiri E, Nakao A, Fujita T, Kobayashi S, Tokunaga K. Functional polymorphisms in the vascular endothelial growth factor gene are associated with development of end-stage renal disease in males. J Am Soc Nephrol. 2006;17:823–830. doi: 10.1681/ASN.2005010094. [DOI] [PubMed] [Google Scholar]
- 28.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 30.McGuire WW, Spragg RG, Cohen AB, Cochrane CG. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982;69:543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blue ML, Janoff A. Possible mechanisms of emphysema in cigarette smokers. Release of elastase from human polymorphonuclear leukocytes by cigarette smoke condensate in vitro. Am Rev Respir Dis. 1978;117:317–325. doi: 10.1164/arrd.1978.117.2.317. [DOI] [PubMed] [Google Scholar]
- 32.Schnebli HP, Christen P, Jochum M, Mallya RK, Pepys MB. Plasma levels of inhibitor-bound leukocytic elastase in rheumatoid arthritis patients. Adv Exp Med Biol. 1984;167:355–362. doi: 10.1007/978-1-4615-9355-3_30. [DOI] [PubMed] [Google Scholar]
- 33.Havemann K, Gramse M. Physiology and pathophysiology of neutral proteinases of human granulocytes. Adv Exp Med Biol. 1984;167:1–20. doi: 10.1007/978-1-4615-9355-3_1. [DOI] [PubMed] [Google Scholar]
- 34.Caimi G, Carollo C, Montana M, Iatrino R, Bondi B, Lo Presti R. Nitric oxide metabolites, leukocyte activation markers and oxidative status in dialyzed subjects. Blood Purif. 2009;27:194–198. doi: 10.1159/000193218. [DOI] [PubMed] [Google Scholar]
- 35.Kubo K, Kobayashi T, Hayano T, Koizumi T, Honda T, Sekiguchi M, Sakai A. Effects of ONO-5046, a specific neutrophil elastase inhibitor, on endotoxin-induced lung injury in sheep. J Appl Physiol. 1994;77:1333–1340. doi: 10.1152/jappl.1994.77.3.1333. [DOI] [PubMed] [Google Scholar]
- 36.Yasui S, Nagai A, Aoshiba K, Ozawa Y, Kakuta Y, Konno K. A specific neutrophil elastase inhibitor (ONO-5046.Na) attenuates LPS-induced acute lung inflammation in the hamster. Eur Respir J. 1995;8:1293–1299. doi: 10.1183/09031936.95.08081293. [DOI] [PubMed] [Google Scholar]
- 37.Sakamaki F, Ishizaka A, Urano T, Sayama K, Nakamura H, Terashima T, Waki Y, Tasaka S, Hasegawa N, Sato K, Nakagawa N, Obata T, Kanazawa M. Effect of a specific neutrophil elastase inhibitor. ONO-5046, on endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1996;153:391–397. doi: 10.1164/ajrccm.153.1.8542148. [DOI] [PubMed] [Google Scholar]
- 38.Iwamura H, Moore AR, Willoughby DA. Interaction between neutrophil-derived elastase and reactive oxygen species in cartilage degradation. Biochim Biophys Acta. 1993;1156:295–301. doi: 10.1016/0304-4165(93)90046-b. [DOI] [PubMed] [Google Scholar]
- 39.Zeiher BG, Matsuoka S, Kawabata K, Repine JE. Neutrophil elastase and acute lung injury: prospects for sivelestat and other neutrophil elastase inhibitors as therapeutics. Crit Care Med. 2002;30:S281–S287. doi: 10.1097/00003246-200205001-00018. [DOI] [PubMed] [Google Scholar]
- 40.Matayoshi H, Hirata T, Yamashita S, Ishida K, Mizukami Y, Gondo T, Matsumoto M, Sakabe T. Neutrophil elastase inhibitor attenuates hippocampal neuronal damage after transient forebrain ischemia in rats. Brain Res. 2009;1259:98–106. doi: 10.1016/j.brainres.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 41.Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 42.Kakimoto K, Matsukawa A, Yoshinaga M, Nakamura H. Suppressive effect of a neutrophil elastase inhibitor on the development of collagen-induced arthritis. Cell Immunol. 1995;165:26–32. doi: 10.1006/cimm.1995.1183. [DOI] [PubMed] [Google Scholar]
- 43.McElvaney NG, Nakamura H, Birrer P, Hebert CA, Wong WL, Alphonso M, Baker JB, Catalano MA, Crystal RG. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992;90:1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, Perretti M, Nourshargh S. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol. 2004;172:4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 45.Perlmutter DH, Glover GI, Rivetna M, Schasteen CS, Fallon RJ. Identification of a serpin-enzyme complex receptor on human hepatoma cells and human monocytes. Proc Natl Acad Sci USA. 1990;87:3753–3757. doi: 10.1073/pnas.87.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlmutter DH, Joslin G, Nelson P, Schasteen C, Adams SP, Fallon RJ. Endocytosis and degradation of alpha 1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (SEC) receptor. J Biol Chem. 1990;265:16713–16716. [PubMed] [Google Scholar]
- 47.Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 48.Gardiner PJ. Neutrophil elastase inhibitors. Eur Resp Rev. 2002;12:373–374. [Google Scholar]
- 49.Nakayama Y, Odagaki Y, Fujita S, Matsuoka S, Hamanaka N, Nakai H, Toda M. Clarification of mechanism of human sputum elastase inhibition by a new inhibitor. ONO-5046, using electrospray ionization mass spectrometry. Bioorg Med Chem Lett. 2002;12:2349–2353. doi: 10.1016/s0960-894x(02)00393-1. [DOI] [PubMed] [Google Scholar]
- 50.Endo S, Sato N, Yaegashi Y, Suzuki Y, Kojika M, Yamada Y, Yoshida Y, Nakadate T, Aoki H, Inoue Y. Sivelestat sodium hydrate improves septic acute lung injury by reducing alveolar dysfunction. Res Commun Mol Pathol Pharmacol. 2006;119:53–65. [PubMed] [Google Scholar]
- 51.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 52.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caty MG, Guice KS, Oldham KT, Remick DG, Kunkel SI. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg. 1990;212:694–700. doi: 10.1097/00000658-199012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seekamp A, Mulligan MS, Till GO, Smith CW, Miyasaka M, Tamatani T, Todd RF, 3rd, Ward PA. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993;143:464–472. [PMC free article] [PubMed] [Google Scholar]
- 55.Dwivedi AJ, Wu R, Nguyen E, Higuchi S, Wang H, Krishnasastry K, Marini CP, Ravikumar TS, Wang P. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. J Am Coll Surg. 2007;205:284–293. doi: 10.1016/j.jamcollsurg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


