Abstract
Light damage to the retina accelerates retinal degeneration in human diseases and rodent models. Recently, the polyphenolic phytoalexin resveratrol has been shown to exert various bioactivities in addition to its classical antioxidant property. In the present study, we investigated the effect of resveratrol on light-induced retinal degeneration together with its underlying molecular mechanisms. BALB/c mice with light exposure (5000-lux white light for 3 hours) were orally pretreated with resveratrol at a dose of 50 mg/kg for 5 days. Retinal damage was evaluated by TdT-mediated dUTP nick-end labeling, outer nuclear layer morphometry, and electroretinography. Administration of resveratrol to mice with light exposure led to a significant suppression of light-induced pathological parameters, including TdT-mediated dUTP nick-end labeling-positive retinal cells, outer nuclear layer thinning, and electroretinography changes. To clarify the underlying molecular mechanisms, the nuclear translocation of activator protein−1 subunit c-fos was evaluated by enzyme-linked immunosorbent assay, and the retinal activity of sirtuin 1 was measured by deacetylase fluorometric assay. Retinal activator protein-1 activation, up-regulated following light exposure, was significantly reduced by application of resveratrol. In parallel, retinal sirtuin 1 activity, reduced in animals with light damage, was significantly augmented by resveratrol treatment. Our data suggest the potential use of resveratrol as a therapeutic agent to prevent retinal degeneration related to light damage.
Resveratrol (3,5,4′-trihydroxystilbene), one of dietary polyphenols found in red wine and grape skin, is known to have an antioxidant effect1 for reducing cardiovascular events. This mechanism may contribute to the “French paradox,” which refers to a phenomenon that the French suffer a relatively low incidence of cardiovascular diseases, despite taking high-caloric and high-fat diet.2 Moreover, resveratrol has been reported to exhibit various bioactivities including anti-tumorigenic,3 anti-angiogenic,4 and neuroprotective5 effects. Recently, surtuin (SIRT) 1, a known regulator of aging,6 has proven to be activated by resveratrol.7 Indeed, administration of resveratrol extended life spans of yeast,7 Caenorhabditis elegans,8 Drosophila melanogaster,8,9 short-lived fish Nothobranchius furzeri10 and mice fed with a high-fat diet.11
We have shown that resveratrol is anti-inflammatory in the eye.12 In the murine model of endotoxin-induced retinal inflammation, resveratrol functioned dually as a SIRT1 activator and an antioxidant agent, both of which led to the deactivation of a nuclear factor-κB, the major redox-sensitive transcription factor that promotes the expression of various inflammation-related genes. Also in recent ex vivo data,13 resveratrol exerted vasodilative effects on the retina through the activation of nitric oxide synthase and potassium channels. Although these reports on the eye focused mainly on vascular changes, it remains to be determined if resveratrol is protective of retinal neurons.
Retinal neurodegenerative diseases such as retinitis pigmentosa and age-related macular degeneration are significant causes of severe vision loss and blindness. Increasing evidence has suggested that light damage to the retina accelerates human retinal degeneration, which leads to the concept of photoaging of the eye. Apoptotic cell death of photoreceptors is an essential feature shared by both human diseases and rodent models of light-induced retinal degeneration. Clinically, no therapeutic strategy has been established so far that hampers the development of retinal degeneration or restores the visual function. Therefore, investigation into photoreceptor apoptosis may provide a clue to prevent blindness due to retinal degeneration. Recently, in addition to the classically known pathway involving caspases, various chemical mediators are shown to play key roles in apoptotic cell death. These apoptosis-related molecules include apoptosis inducing factor,14 poly-(ADP-ribose) polymerase-1,15 endonuclease G16 and activator protein (AP)−1.17 Light-induced retinal degeneration is an established animal model for studying visual cell death by apoptosis. Herein, we report the preventive effect of resveratrol on the pathogenesis of light-induced retinal degeneration together with underlying molecular mechanisms.
Materials and Methods
Animals
BALB/c male mice (Clea, Tokyo, Japan) at the age of 6 weeks were used in the present study. Animals were housed in plastic cages in a climate-controlled animal facility and kept under dim cyclic light (5 lux, 12 hours on/off) in our institution, except where otherwise indicated. All animal experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Treatment with Resveratrol
Mice were orally administered with vehicle (6.67% dimethyl sulfoxide in PBS) or resveratrol (Sigma-Aldrich, St. Louis, MO) at the dose of 50 mg/kg body weight (BW) by using a gastric intubation daily for 5 days until light exposure. The dose of 50 mg/kg BW is equivalent to that applied to protect against neuronal injury associated with cerebral ischemia-reperfusion in mice.18
Light Exposure
Light exposure experiments were performed as described previously19 with slight modification. Mice were exposed to 5000 lux of white light for 3 hours from 9:00 AM in a dedicated exposure box having stainless mirrors at the lateral side and floors (Tinker-N, Kyoto, Japan). The box contained a white fluorescent lamp (FHD100ECW; Panasonic, Osaka, Japan) and an air conditioner to maintain the temperature inside as 23°C. Before light exposure, mice were dark adapted for 12 hours. The pupils were dilated with a mixed solution of 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P; Santen, Osaka, Japan) just before light exposure. Immediately after 3-hour exposure to light (at 12:00), the mice were carried under dim cyclic light (5 lux, 12 hours on/off).
TdT-Mediated dUTP Nick-End Labeling
Mice were anesthetized with pentobarbital sodium (70 mg/kg BW) and perfused with 10 ml of PBS 48 hours after the start of light exposure. Subsequently, eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4°C. After fixation, tissues were processed and embedded in an optimal cutting temperature compound (Tissue-Tek; Sakura Finetek, Torrance, CA) for cryosections. Six 10-μm cryosections from the optic nerve were prepared, and TdT-mediated dUTP nick-end labeling (TUNEL) was performed using the ApopTag Red apoptosis detection kit (Chemicon, Temecula, CA) according to the manufacturer's protocol. Nuclei were stained with 10-μg/ml Hoechst bisbenzimide 33258 (Sigma-Aldrich). Fluorescence images were obtained using Axio Imager (Carl Zeiss, Oberkochen, Germany), and TUNEL-positive cells were counted in the outer nuclear layer (ONL), composed exclusively of photoreceptor cell bodies.
Measurement of ONL Thickness
Mice were anesthetized with pentobarbital sodium (70 mg/kg BW), perfused with 10 ml of PBS 4 days, 1 week or 2 weeks after the start of light exposure, and the eyes were then enucleated. Paraffin-embedded retinal sections (3 μm) were prepared and stained with hematoxylin and eosin. ONL thickness was measured at each 0.2–0.3 mm point from the optic nerve head to the most peripheral area using ImageJ software (National Institutes of Health, Bethesda, MD).
Electroretinography (ERG)
ERG analysis was performed as previously described,19–23 4 days, 1 week or 2 weeks after light exposure. Mice were anesthetized with pentobarbital sodium (70 mg/kg BW) and placed on a heating pad that maintained their body temperature at 35–36°C throughout the experiment. The pupils were dilated with a mixed solution of 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P; Santen). The ground electrode was a subcutaneous needle in the tail and the reference electrode was placed subcutaneously between the eyes. The active contact lens electrodes (Mayo, Inazawa, Japan) were placed on the cornea. Recordings were performed with PowerLab system 2/25 (AD Instruments, New South Wales, Australia). Responses were differentially amplified and filtered through a digital bandpass filter ranging from 0.313 to 1000 Hz to yield a- and b-waves. Light pulses of 800 cds/m2 and 4 ms duration were delivered via a commercial Ganzfeld stimulator (Ganzfeld System SG-2002; LKC Technologies, Gaithersburg, MD). The amplitude of the a-wave was measured from the baseline to the trough of the a-wave, and the amplitude of the b-wave was determined from the trough of the a-wave to the peak of the b-wave. The implicit time of the a- and b-waves was measured from the onset of stimuli to the peak of each wave.
Enzyme-Linked Immunosorbent Assay for c-fos after Nuclear Extraction
Three hours after light exposure, mice were sacrificed with an overdose of anesthesia and the eyes were immediately enucleated. The retina was carefully isolated and homogenized in 50 μl of hypotonic buffer (10 mmol/L HEPES-KCl, 1 mmol/L β-mercapto-ethanol, 1 mmol/L dithiothreitol). After incubation on ice for 10 minutes, the homogenate was vortexed for 10 seconds and centrifuged. The supernatant was discarded and the pellet was resuspended in 100 μl lysis buffer in the presence of protease inhibitors, and incubated on ice for 10 minutes. Cellular debris was removed by centrifugation at 15,000 rpm for 15 minutes at 4°C, and 10 μg protein was subjected to enzyme-linked immunosorbent assay for the c-fos subunit of AP-1. Activation of c-fos was determined by measuring the c-fos protein level in the nuclear extracts with the AP-1 c-fos Transcription Factor Assay kit (Active Motif, Carlsbad, CA) according to the manufacturer's instruction. The tissue sample concentration was calculated from a standard curve and corrected for protein concentration evaluated by the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Deacetylase Fluorometric Assay for SIRT1
Three hours after light exposure, the animals were sacrificed with an overdose of anesthesia and the eyes were immediately enucleated. The retina was carefully isolated and placed into 100 μl of lysis buffer and then sonicated. The lysate was centrifuged at 15,000 rpm for 15 minutes at 4°C. The activity of SIRT1 in the supernatant was determined with SIRT1/Sir2 Deacetylase Fluorometric Assay kits (CycLex, Ina, Japan) according to the manufacturer's protocols.
Statistical Analysis
All results were expressed as mean ± SD. The values were processed for statistical analyses (Mann-Whitney test), and differences were considered statistically significant at P < 0.05.
Results
Suppression of Light-Induced Apoptotic Cell Death with Resveratrol
To unravel the effect of resveratrol on light-induced apoptotic cell death of photoreceptors, TUNEL assay was performed (Figure 1). Under dim cyclic light (5 lux, 12 hours on/off), the number of TUNEL-positive cells in ONL was negligible (only a few cells, if any).19 Light exposure to vehicle-treated mice substantially induced apoptotic cell death in ONL (Figure 1A) with the number of 863.4 ± 201.0 cells/section, which was significantly (P < 0.05, Figure 1B) reduced to 680.5 ± 98.0 cells/section by application with resveratrol.
Figure 1.
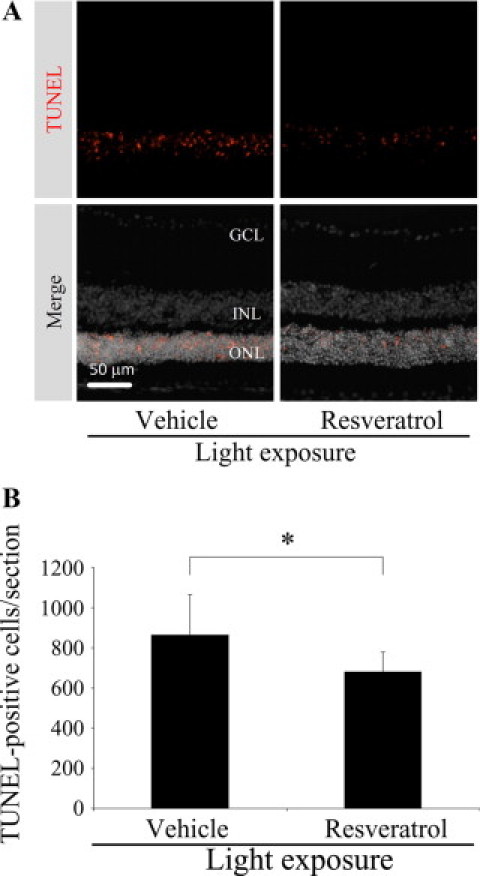
Suppression of light-induced apoptotic cell death with resveratrol. A: Representative images of TUNEL staining for retinal sections. B: Quantification of the number of TUNEL-positive cells. Light exposure to vehicle-treated mice induced apoptotic cell death in the ONL, which was significantly reduced by application with resveratrol. n = 9 to 11. *P < 0.05.
Suppression of Light-Induced ONL Thinning with Resveratrol
To evaluate the effect of resveratrol on light-induced histological damage to the retina, ONL thickness was analyzed at three time points, ie, 4 days (Figure 2, A–E), 1 week (data not shown) and 2 weeks (Figure 2, F–J) after light exposure. Light exposure to vehicle-treated mice (Figure 2, C and H) led to significant (red versus green in Figure 2, E and J) reduction of ONL thickness as compared to vehicle-treated mice receiving no light exposure (Figure 2, A and F). Systemic administration of resveratrol to light-exposed mice (Figure 2, D and I) significantly (green versus navy in Figure 2, E and J) suppressed the thinning of ONL thickness as compared to vehicle treatment to light-exposed animals (Figure 2, C and H). ONL thickness in mice with no light exposure (Figure 2, A and F) was not significantly (red versus blue in Figure 2, E and J) altered with resveratrol application (Figure 2, B and G). Accordingly, the protective effect of resveratrol pretreatment on light-induced ONL thinning (Figure 2D, blue in Figure 2E) proved to be narrowly maintained at least until 2 weeks (Figure 2I, blue in Figure 2J), although the light-damaged ONL at 2 weeks (Figure 2H, green in Figure 2J) substantially and progressively became thinner than that at 4 days (Figure 2C, green in Figure 2E) following light exposure.
Figure 2.
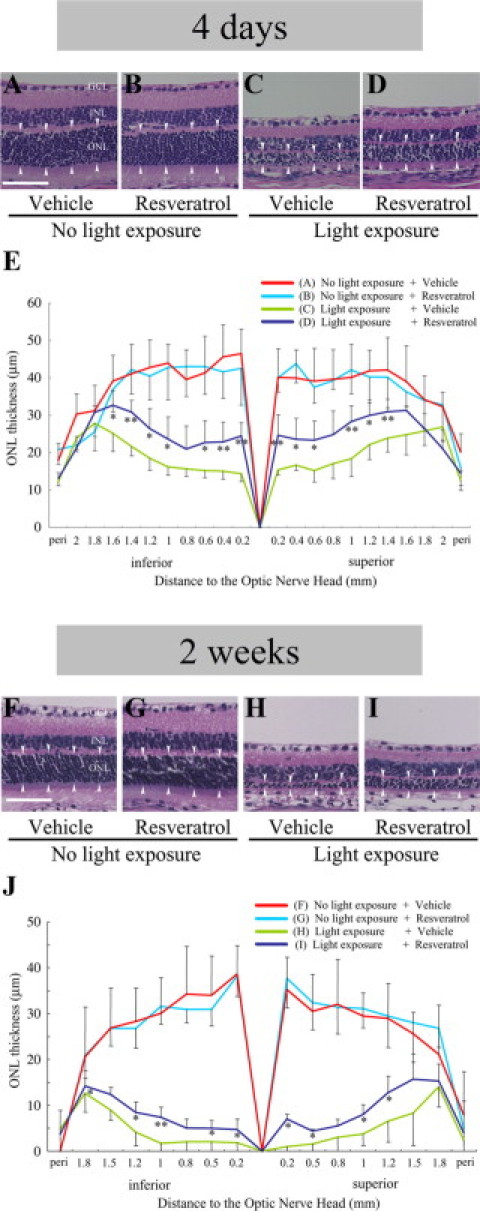
Suppression of light-induced ONL thinning with resveratrol. Data obtained at four days (A–E) and two weeks (F–J) after light exposure. A–D and F–I: Representative images of H&E staining for retinal sections. E and J: Quantification of ONL thickness (arrowheads in A–D and F–I). Compared to vehicle-treated mice receiving no light exposure (A and F), light exposure to vehicle-treated mice (C and H) led to significant (red versus green in E and J) reduction of ONL thickness, which was significantly (green versus navy in E and J) recovered by application with resveratrol (D and I). Scale bars = 50 μm. n = 6 to 8. *P < 0.05; **P < 0.01.
Suppression of Light-Induced Retinal Dysfunction with Resveratrol
To investigate the effect of resveratrol on light-induced retinal dysfunction, ERG analysis was performed at three evaluation points of 4 days (Figure 3, A,C, and E), 1 week (data not shown), and 2 weeks (Figure 3, B,D, and F) after light exposure. Light exposure to vehicle-treated mice at 4 days led to significant (P < 0.01 for both, Figure 3, A,C, and E) reduction of the amplitude of a-wave (88.4 ± 17.6 μV, Figure 3C) and b-wave (190.2 ± 63.0 μV, Figure 3E) as compared to vehicle-treated mice receiving no light exposure (301.6 ± 76.1 μV and 644.4 ± 234.0 μV for a-wave and b-wave, respectively). Systemic administration of resveratrol to light-exposed mice significantly (P < 0.01 for both, Figure 3, A,C, and E) recovered the reduction of the amplitude of a-wave (166.1 ± 39.9 μV, Figure 3C) and b-wave (435.9 ± 157.4 μV, Figure 3E) as compared to vehicle treatment to light-exposed animals (88.4 ± 17.6 μV and 190.2 ± 63.0 μV for a-wave and b-wave, respectively).
Figure 3.
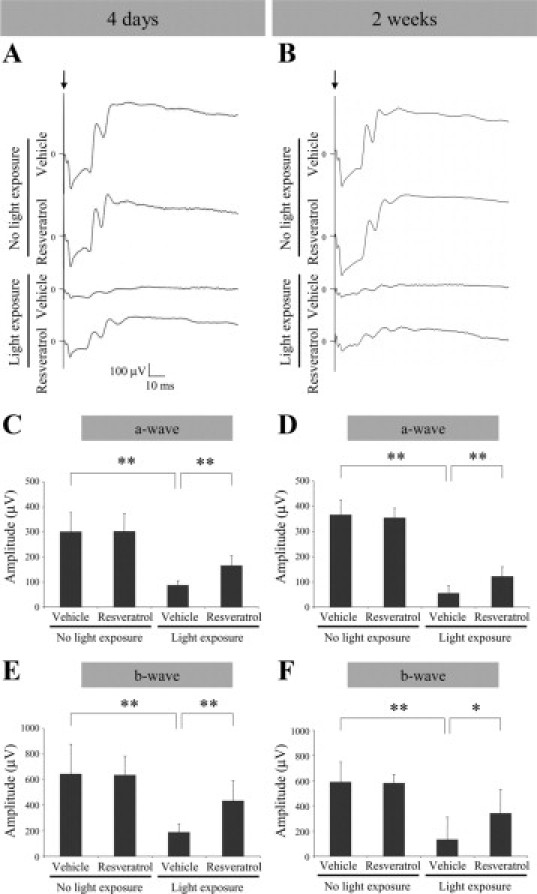
Suppression of light-induced retinal dysfunction with resveratrol. Data obtained at four days (A, C, and E) and two weeks (B, D, and F) after light exposure. A and B: Representative ERG wave responses. Quantification of the amplitude of a-wave (C and D) and b-wave (E and F). Light exposure to vehicle-treated mice led to significant reduction of the amplitude of a-wave (C and D) and b-wave (E and F), both of which were significantly recovered by application with resveratrol. n = 6 to 12. *P < 0.05; **P < 0.01.
Similarly, light exposure to vehicle-treated mice at 2 weeks led to significant (P < 0.01 for both, Figure 3, B,D, and F) reduction of the amplitude of a-wave (56.6 ± 28.9 μV, Figure 3D) and b-wave (134.6 ± 175.4 μV, Figure 3F) as compared to vehicle-treated mice receiving no light exposure (366.6 ± 58.1 μV and 590.5 ± 157.6 μV for a-wave and b-wave, respectively). Systemic administration of resveratrol to light-exposed mice significantly (P < 0.01 for a-wave and P < 0.05 for b-wave, Figure 3, B,D, and F) recovered the reduction of the amplitude of a-wave (122.7 ± 37.5 μV, Figure 3D) and b-wave (342.0 ± 188.7 μV, Figure 3F), as compared to vehicle treatment to light-exposed animals (56.6 ± 28.9 μV and 134.6 ± 175.4 μV for a-wave and b-wave, respectively).
No significant (P > 0.05 for each) differences between vehicle and resveratrol were detected in implicit time of a-wave or b-wave at each time point (4 days, 1 week or 2 weeks; data not shown).
Suppression of Light-Induced AP-1 Activation with Resveratrol
To clarify pro-apoptotic signal transduction involved in resveratrol-induced suppression of photoreceptor apoptosis (Figure 1) followed by retinal degeneration (Figure 2) and dysfunction (Figure 3), AP-1 activation was examined by measuring the c-fos level in nuclear extracts from the retina (Figure 4). Light exposure to vehicle-treated mice significantly (P < 0.01) induced retinal AP-1 activation (16.8 ± 3.2 ng/mg) as compared to vehicle-treated mice receiving no light exposure (6.2 ± 2.8 ng/mg). Systemic application of resveratrol to light-exposed mice significantly (P < 0.01) suppressed retinal AP-1 activation (10.2 ± 2.2 ng/mg) as compared to vehicle treatment to light-exposed animals (16.8 ± 3.2 ng/mg). In contrast, administration of resveratrol to mice with no light exposure (6.3 ± 3.6 ng/mg) did not alter (P > 0.05) physiological baseline levels of c-fos (6.2 ± 2.8 ng/mg).
Figure 4.
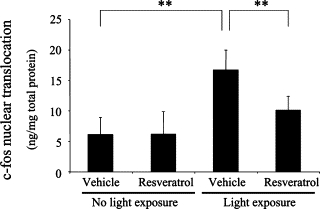
Suppression of light-induced AP-1 activation with resveratrol. Levels of nuclear translocation of c-fos, the subunit of AP-1, were quantified. Light exposure to vehicle-treated mice significantly induced retinal AP-1 activation, which was significantly suppressed by application with resveratrol. n = 6 to 10. **P < 0.01.
Suppression of Light-Induced SIRT1 Deactivation with Resveratrol
To reveal a possible role played by SIRT1 in resveratrol-induced suppression of the apoptotic signaling pathway (Figure 4), retinal SIRT1 activity was analyzed by deacetylase fluorometric assay (Figure 5). Light exposure to vehicle-treated mice led to significant (P < 0.01) reduction of retinal SIRT1 activity (83.9 ± 9.4% of control) as compared to vehicle-treated mice receiving no light exposure (100 ± 6.7% serving as control). Systemic administration of resveratrol to light-exposed mice significantly (P < 0.05) reversed retinal SIRT1 activity (99.4 ± 5.7% of control) as compared to vehicle treatment to light-exposed animals (83.9 ± 9.4% of control). In contrast, administration of resveratrol to mice with no light exposure (99.8 ± 14.3% of control) did not change (P > 0.05) physiological baseline levels of SIRT1 (100 ± 6.7%).
Figure 5.
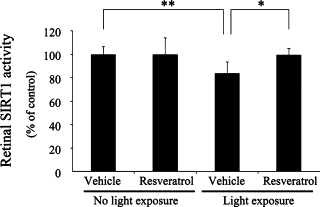
Suppression of light-induced SIRT1 deactivation with resveratrol. Light exposure to vehicle-treated mice led to significant reduction of retinal SIRT1 activity, which was significantly reversed by application with resveratrol. n = 5 to 7. *P < 0.05; **P < 0.01.
Discussion
The present study reveals, for the first time to our knowledge, the protective effect of resveratrol on light-induced retinal degeneration together with underlying molecular mechanisms. Resveratrol application to light-exposed mice ameliorated several retinal parameters including apoptotic cell death (Figure 1), anatomical structure (Figure 2), and functional change (Figure 3) induced by light damage. As possible molecular mechanisms, retinal AP-1 activation (Figure 4) and SIRT1 deactivation (Figure 5), both of which resulted from light exposure, were inhibited by pretreatment with resveratrol. Resveratrol is one of polyphenolic phytoalexins contained abundantly in red wine, grape skin, and peanut skin.24 Resveratrol has been shown to have various bioactivities including antioxidative,1 anti-inflammatory,25 antitumorigenic,3 anti-angiogenic,4 neuroprotective26 and vasodilative13,27 effects. Notably, it was reported that resveratrol exerts an anti-aging or lifespan-extending action7,28 through the activation of SIRT1. In addition to its anti-aging property, SIRT1 has been shown to suppress AP-1 bioactivity,29 suggesting the validity of the currently shown molecular pathway through which resveratrol protected from light-induced retinal degeneration.
In the present study, resveratrol application led to significant suppression of TUNEL-positive cells in the ONL 2 days after light exposure (Figure 1). The increase in the number of TUNEL-positive cells in the ONL following light exposure is a well-known landmark of photoreceptor apoptosis in light-induced retinal degeneration.30 The current finding that resveratrol reduced light-induced apoptotic cell death in the retina is supported in part by previous data31 showing the anti-apoptotic activity of resveratrol in vitro under oxidative stress. To investigate the sequential events following photoreceptor apoptosis, we measured ONL thickness 4 days, 1 week, and 2 weeks after light exposure. The ONL, composed exclusively of photoreceptor cell bodies, is a known target of light-induced retinal degeneration.17 In accordance with the data on apoptotic cell death (Figure 1), resveratrol pretreatment to light-exposed mice resulted in significant suppression of ONL thinning (Figure 2). To confirm these histological data (Figures 1 and 2), we analyzed retinal function by using ERG (Figure 3). Light exposure is known to cause damage to retinal function represented by ERG.32 Light-induced suppression of both a-wave and b-wave in ERG was reversed by application with resveratrol (Figure 3), suggesting that the amelioration of retinal dysfunction is attributed to resveratrol-mediated suppression of ONL injury (Figures 1 and 2). The long-term protection of resveratrol from light-induced retinal damages (Figures 2 and 3) was confirmed to last at least until 2 weeks following light exposure. The present study is the first to show the neuroprotective effect of resveratrol on retinal degeneration.
To further confirm the currently observed inhibitory effect of resveratrol on histological and functional damage to the retina (Figures 1–3), we investigated underlying molecular mechanisms (Figures 4 and 5). Of several important pro-apoptotic pathways,14–17 we focused on AP-1, the major transcription factor that regulates the intracellular signals for cell cycle, differentiation, and apoptosis.33 AP-1 is typically a heterodimer that consists of the c-fos and c-jun subunit proteins. It has been previously shown that c-fos is essential for light-induced apoptosis of photoreceptors.17 Mice lacking c-fos were resistant to light damage and exhibited a significant decrease in light-induced apoptotic cell death and subsequent ONL thinning as compared to wild-type controls.17 In our present data (Figure 4), c-fos levels were elevated in the nuclear extracts from the retina of light-exposed mice and significantly reduced by application with resveratrol. This is consistent with a previous report showing the inhibitory effect of resveratrol on c-fos expression in the murine model of skin cancer.34,35 Accordingly, the neuroprotective effect of resveratrol on light-induced retinal degeneration and dysfunction (Figures 1–3) is attributable at least in part to the modulation of AP-1 that is causally linked to light-induced apoptosis of photoreceptors.17
In addition to its suppressive effect on AP-1 activation, resveratrol is a known activator of the histone deacetylase SIRT1,7 a key modulator for extending the lifespan of several species including rodents.6 It has been recently revealed that in murine fibroblasts SIRT1 plays an inhibitory role in the transcriptional activity of AP-1 by targeting (directly binding to) c-jun.29 In the present study, resveratrol application to light-exposed mice significantly recovered retinal SIRT1 activity, which was decreased due to light damage (Figure 5). The reverse relationship between AP-1 (Figure 4) and SIRT1 (Figure 5) are comparable with and explained by the recent in vitro data29 showing the involvement of SIRT1 in AP-1 deactivation. Taken together, light exposure caused retinal SIRT1 deactivation (Figure 5) together with AP-1 activation (Figure 4), leading to photoreceptor apoptosis (Figure 1) and subsequent retinal degeneration (Figure 2) and dysfunction (Figure 3), all of which were reversed by the SIRT1 activator resveratrol (Figures 1–5).
Clinically, resveratrol is now under phase-II investigations for cancer and diabetes. Moreover, resveratrol has received orphan-drug designation for MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, stroke-like episodes) syndrome by the U.S. Food and Drug Administration. So far, no major safety concern has been reported.36 At present, there is no established treatment for retinal neurodegenerative diseases, to which light damage is causally linked, including retinitis pigmentosa and age-related macular degeneration. Our present data provide molecular evidence of the potential validity of resveratrol supplementation as a therapeutic strategy to prevent retinal degeneration related to light damage.
Footnotes
Supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (Global COE Program at Keio University) and the Japanese Ministry of Education, Culture, Sports, Science, and Technology (Grant-in-Aid for Scientific Research No. 22791686 to S.K.).
S.K. and T.K. contributed equally to this work.
References
- 1.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 2.Creasy EHSaLL Concentration of the Phytoalexin Resveratrol in Wine. American Journal of Enology and Viticulture. 1992;43:49–52. [Google Scholar]
- 3.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 4.Brakenhielm E, Cao R, Cao Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 6.Bordone L, Guarente L. Calorie restriction. SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 7.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 8.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 9.Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 11.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- 13.Nagaoka T, Hein TW, Yoshida A, Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci. 2007;48:4232–4239. doi: 10.1167/iovs.07-0094. [DOI] [PubMed] [Google Scholar]
- 14.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 15.Pieper AA, Verma A, Zhang J, Snyder SH. Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci. 1999;20:171–181. doi: 10.1016/s0165-6147(99)01292-4. [DOI] [PubMed] [Google Scholar]
- 16.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 17.Hafezi F, Steinbach JP, Marti A, Munz K, Wang ZQ, Wagner EF, Aguzzi A, Reme CE. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997;3:346–349. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- 18.Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara T, Omoto M, Noda K, Ebinuma M, Kubota S, Koizumi H, Yoshida S, Ozawa Y, Shimmura S, Ishida S, Tsubota K. Retinal phototoxicity in a novel murine model of intraocular lens implantation. Mol Vis. 2009;15:2751–2761. [PMC free article] [PubMed] [Google Scholar]
- 20.Kurihara T, Ozawa Y, Shinoda K, Nagai N, Inoue M, Oike Y, Tsubota K, Ishida S, Okano H. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest Ophthalmol Vis Sci. 2006;47:5545–5552. doi: 10.1167/iovs.06-0478. [DOI] [PubMed] [Google Scholar]
- 21.Kurihara T, Ozawa Y, Nagai N, Shinoda K, Noda K, Imamura Y, Tsubota K, Okano H, Oike Y, Ishida S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–2198. doi: 10.2337/db07-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki M, Ozawa Y, Kurihara T, Noda K, Imamura Y, Kobayashi S, Ishida S, Tsubota K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:1433–1439. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of diabetic mice. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Issuree PD, Pushparaj PN, Pervaiz S, Melendez AJ. Resveratrol attenuates C5a-induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009;23:2412–2424. doi: 10.1096/fj.09-130542. [DOI] [PubMed] [Google Scholar]
- 26.Sun AY, Simonyi A, Sun GY. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002;32:314–318. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 27.Li HF, Chen SA, Wu SN. Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc Res. 2000;45:1035–1045. doi: 10.1016/s0008-6363(99)00397-1. [DOI] [PubMed] [Google Scholar]
- 28.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence., Nature reviews. Drug discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, Ye J. Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem Biophys Res Commun. 2008;376:793–796. doi: 10.1016/j.bbrc.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 31.MacCarrone M, Lorenzon T, Guerrieri P, Agro AF. Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur J Biochem. 1999;265:27–34. doi: 10.1046/j.1432-1327.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 32.Lansel N, Hafezi F, Marti A, Hegi M, Reme C, Niemeyer G. The mouse ERG before and after light damage is independent of p53. Doc Ophthalmol. 1998;96:311–320. doi: 10.1023/a:1001795526628. [DOI] [PubMed] [Google Scholar]
- 33.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 34.Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetradecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998;134:81–89. doi: 10.1016/s0304-3835(98)00250-x. [DOI] [PubMed] [Google Scholar]
- 35.Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: nF-kappaB and AP-1 as prime targets. Biochem Pharmacol. 2006;72:1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev. 2008;66:591–596. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]


