Abstract
Exogenous bone marrow-derived cells (BMDCs) are promising therapeutic agents for the treatment of tissue ischemia and traumatic injury. However, until we identify the molecular mechanisms that underlie their actions, there can be no rational basis for the design of therapeutic strategies using BMDCs. The pro-healing effects of BMDCs are apparent very shortly after treatment, which suggests that they may exert their effects by the modulation of acute inflammation. We investigated this hypothesis by taking advantage of the fact that BMDCs from healthy, young, but not obese, diabetic mice stimulate vascular growth. By comparing both in vitro secretion and in vivo local induction of acute phase inflammatory cytokines by these cells, we identified monocyte chemoattractant factor 1 and tumor necrosis factor α as potential mediators of BMDC-induced tissue repair. In vivo analysis of BMDC-treated ischemic limbs and cutaneous wounds revealed that the production of monocyte chemoattractant factor 1 by exogenous and endogenous BMDCs is essential for BMDC-mediated vascular growth and tissue healing, while the inability of BMDCs to produce tumor necrosis factor α appears to play a lesser but still meaningful role. Thus, measurements of the secretion of cytokines by BMDCs may allow us to identify a priori individuals who would or would not be good candidates for BMDC-based therapies.
Exogenous bone marrow-derived cells (BMDCs), including peripheral blood mononuclear cells (PBMCs) promote tissue vascularization and show promise as therapeutic tools for treatment of tissue ischemia and traumatic injury. Although they may be a source of endothelial or other progenitor cells, they probably act principally through paracrine mechanisms.1,2 BMDCs are currently being used to treat ischemic conditions in clinical trials.3–5 However, given our rudimentary knowledge of how BMDCs act as agents of vascular growth and tissue repair, it is not surprising that results of clinical trials to date are mixed. The ability of the BMDCs to potentiate healing depends on the physiological status of both the BMDC donor and recipient, and while many subpopulations of BMDCs can potentiate vascular growth and may be of therapeutic value, their relative potency is not well characterized.6–8 At the same time, we do not know why exogenous BMDCs promote tissue vascularization and repair in many, but not all, animal models of injury and disease (See for example,9–13). Until we identify the molecular mechanisms underlying the action of BMDCs, there can be no rational basis for determining which cells delivered when, and at what dose might be most appropriate in a particular clinical situation. In light of this, our current study examines the molecular mechanism through which BMDCs exert their therapeutic effects.
BMDC therapy can lead to remarkably rapid improvements in blood flow. As early as 48 hours after local injection of human CD34+ PBMCs into ischemic murine hind limbs, there is a significant increase in limb blood flow compared to untreated controls.14 However, maximal effects are not observed until many days later, well after the injected CD34+ cells have been essentially cleared.14 That is, the PBMCs appear to act early to initiate a pro-angiogenic cascade that persists even after the exogenous PBMCs are no longer present. Thus, BMDCs may act by modulating early inflammatory responses, responses that initiate tissue repair.
In support of this hypothesis, treatment with BMDCs induces neovascularization in ischemic muscle of wild-type mice, but not in interleukin (IL)-1β knockout (Il-1β−/−) mice.15 However, Il-1β −/− mononuclear cells increase expression of IL-1β and pro-angiogenic factors in ischemic muscle and can stimulate vascular growth as effectively as wild-type cells.15 This suggests that exogenous BMDCs may act by regulating the acute phase inflammatory cytokine IL-1β through an as yet unidentified molecular stimulus though they need not to secrete it themselves.
Based on these findings we tested the hypothesis that BMDCs potentiate tissue repair by modulating the acute inflammatory response. We took advantage of the fact that lineage depleted (lin−) BMDCs from healthy young wild-type mice stimulate vascular growth while those from mice lacking the leptin receptor gene (Leprdb) mice do not.11 Differences in the secretory profiles and abilities to alter tissue levels of acute phase inflammatory cytokines between lin− cells derived from these two sources were compared. We identified monocyte chemoattractant factor 1 (MCP-1) and tumor necrosis factor α (TNF-α) as potential mediators of BMDC-potentiated neovascularization and tissue repair. Studies with BMDCs from knockout mice demonstrated that production of MCP-1, but not TNF-α, by exogenous BMDCs is essential for lin− BMDC-mediated vascular growth and tissue healing. We also showed that endogenous BMDCs must be able to express MCP-1 in order for exogenous BMDCs to be therapeutically beneficial.
Materials and Methods
Mice
All animal procedures were approved by the University of Iowa Institutional Animal Care and Use Committee. Male (8 to 12 weeks of age) C57Bl/6 (referred to wild-type in this manuscript), B6. Cg-Dock7mLeprdb/db(Leprdb),16 B6;129S6-TNFtm1Gkl(TNF-α−/−),17 B6.129S4-Ccl2tm1Rol(MCP-1−/−)18 mice (Jackson Laboratories, Bar Harbor, ME) were used. Chimeric mice were generated by whole-body irradiation of 6–8 weeks Leprdb mice at 6 Gy and 4 hours later at 5 Gy to destroy their bone marrow (BM), and the BM was reconstituted via tail vein injection of 1 × 107 freshly harvested whole TNF-α−/− or MCP-1−/− BM cells.19 Chimerism was verified by Y chromosome in situ hybridization of blood smears using biotinylated Y chromosome paint (Open Biosystems, Huntsville, AL) according to manufacturer's instructions. Only mice with greater than 90% donor-derived BM were used. Surgical procedures were performed 6 weeks after generating the chimeras. Blood glucose of Leprdb and chimeric mice was measured by glucometer (One Touch Ultra LifeScan, Inc. Milpitas, CA), and mice with a glucose level >270 mg/dL were considered diabetic.
Surgical Procedures
Hind limb ischemia was induced in anesthetized Leprdb or chimeric mice via left femoral artery ligation and confirmed by measuring limb blood flow using scanning LASER Doppler analysis as previously described.14 Vehicle or 5 × 105 wild-type, Leprdb, TNF-α−/−, or MCP-1−/− lin− BMDCs in PBS were injected i.m. into the medial thigh of the ischemic limb 2 to 4 hours after surgery (n = 4 to 9 per group) as described.8 Two 6-mm diameter full-thickness punch cutaneous wounds were made on the dorsorostral back skin of Leprdb or chimeric mice (n = 4 per group) at the level of the forelimbs and 3 days later, vehicle or 2.5 × 105 freshly isolated wild-type, Leprdb, TNF-α−/− or MCP-1−/− lin− cells in 25 μl 0.9% NaCl were injected under each wound as described.11
Mice were sacrificed by i.p. injection of sodium pentobarbital (150 mg/kg) followed by cervical dislocation after deep anesthesia had been achieved. Anesthesia was induced and maintained with 4% and 0.8% to 1.0% isoflurane at 1 L/min O2, respectively.
Isolation and Culture of lin− BMDCs
Wildtype, Leprdb, TNF-α−/− or MCP-1−/− mice were sacrificed and BM cells flushed from femurs and tibias as previously described.20 Enrichment for lin− cells was done by magnetic bead lineage depletion with an autoMACS (Miltenyi Biotech Inc., Auburn, CA) according to manufacturer's instructions. Freshly isolated cells were used immediately for in vivo studies. For in vitro studies, wild-type or Leprdb lin− cells from single mice or pools of 3 to 4 mice were plated in M199 with 20% heat-inactivated fetal bovine serum and 12 μg/ml bovine brain extract (Cambrex Biosciences Inc, Rockland, ME) on 5 μg/cm2 pronectin (Deepwater, Woodward, OK) coated 96-welltrays at 5 × 105 cells per well. Twenty-four hours after plating, the medium was replaced with M199 with 10% heat-inactivated fetal bovine serum and 10 U/ml of erythropoietin (Amgen Inc, Thousand Oaks, CA).21 Two days later, conditioned medium was collected (3 days after plating) frozen, aliquoted, and stored at −80°C.
Protein Measurements
Protein concentrations of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, interferon-γ, granulocyte macrophage–colony-stimulating factor, MCP-1, and TNF-α were measured in conditioned medium using a multiplex assay (Beadlyte, Upstate Biotech, Lake Placid, NY) per manufacturer's instructions. Conditioned medium were incubated with multiplex beads 2 hours, washed, incubated with biotinylated anti-mouse antibodies 1 hour, washed, incubated with streptavidin–phycoerythritin 30 minutes, washed, and then analyzed using a Luminex 100 plate reader (BioRad, Hercules, CA). All assays were performed in duplicate or triplicate on 4 to 6 individual samples or pools using incubated serum and erythropoietin containing medium as a control. Standard curves were generated to convert fluorescence units to cytokine concentration (pg/ml). Minimum detection levels were 6.9 pg/ml for IL-1β and 2.3 pg/ml for all other cytokines. Additionally, some conditioned medium samples were assayed by enzyme-linked immunosorbent assay at the Cytokine Core Laboratory (University of Maryland, Baltimore).
Contralateral control and ischemic limb biceps femoris, semitendinosus, and semimembranosus (hamstrings) muscles were harvested one or 5 days after surgery (n = 4 to 6 per group), pulverized in liquid nitrogen then resuspended in lysis buffer (50 mmol/L Tris, pH 7.5, 1 mmol/L EDTA, 1% Triton, 0.9% NaCl, 1 mmol/L phenylmethylsulfonyl fluoride) and clarified at 12,000 × g for 30 minutes at 4°C. Clarified lysates were aliquoted and stored at −80°C until use. Protein concentrations were determined via modified Bradford assay with Protein Assay Reagent (BioRad, Hercules, CA).22 Protein concentrations of TNF-α, IL-1β, and MCP-1 in muscle lysates were determined at the Cytokine Core Laboratory in triplicate multiplex assays.
Relative vascular endothelial growth factor-A (VEGF-A) levels in the lysates were analyzed by Western blot of two to four 150 μg protein samples per mouse (n = 4 to 6 mice per group). After separation by SDS-polyacrylamide gel electrophoresis and transfer to nitrocellulose membranes (BioRad, Hercules, CA), membranes were probed with 0.1 μg/ml anti-VEGF-A (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) followed by 0.4 μg/ml anti-rabbit IgG IRDy conjugated antibody (Rockland Immunochemicals Inc., Gilbertsville, PA) as described.8 Blots were re-probed with anti-glyceraldehyde-3-phosphate dehydrogenase (Chemicon, Temecula, CA). Bands were visualized using Odyssey infrared imaging (LI-COR, Lincoln, NE) and data were normalized to glyceraldehyde-3-phosphate dehydrogenase levels.
Histology, Immunodetection, and Morphometry
For all morphometric measurements, observers were blinded to treatment. Lower limb muscles were harvested 5 days after ischemia and fixed in methanol. Samples were then embedded in paraffin and serially sectioned at 7 μm moving proximally from the level of the distal end of the tibialis posterior. Five cross-sections at 700 μm intervals were examined. Sections were incubated with mouse anti-α smooth muscle actin 8.5 μg/ml (Sigma, St. Louis, MO), followed by incubation with a biotinylated anti-mouse (Jackson ImmunoResearch, West Grove, PA), then reacted with streptavidin alkaline phosphatase (Vector Laboratories) and revealed by Vector Red (Vector) to visualize arterioles, and counterstained with hematoxylin to visualize nuclei as previously described.8 Images were digitized (Nikon E600 microscope and DXM1200 camera). Microvessels (excluding capillaries) and normal or healthy (non-degenerating) muscle areas were traced using MetaMorph (Molecular Devices, Downingtown, PA). Muscle containing only peripherally localized nuclei, tightly abutted fibers, and minimal inflammatory infiltrate was considered healthy. Total noncapillary microvessel volume in healthy muscle and healthy muscle volume were determined according to the formula:
V = 4 × 700 μm × mean measured area × S; with:
S = a/b
a = short axis of the soleus fibers in the middle section
b = long axis of the soleus fibers in the middle section
S corrects for deviations of the mounted muscle from the longitudinal axis.
To visualize capillaries, additional sections were incubated with 0.5 mg/ml biotinylated Bandeira simplicifolia lectin (BSLB4) (Vector, Burlingame, CA). They were then incubated with alkaline phosphatase conjugated streptavidin (1:400 Vector) and reacted with Vector Red or treated with fluorescein isothiocyanate-conjugated streptavidin (1:200 Vector). The former were hematoxylin and eosin stained. For each animal, capillary/fiber ratio was determined in healthy muscle areas from five equally spaced sections (700 μm intervals). Three muscle groups were examined, and at least two 40× fields from each muscle group were examined in each section. Capillary length was computed in the same anatomically defined region. Total capillary length was determined using the formula:
Vascular parameters in cutaneous wounds were analyzed as previously described.11 Wounds were harvested 14 days post-wounding, methanol fixed, and paraffin-embedded. One wound from each mouse was serially sectioned (7 μm) and sections were immunolabeled every 350 μm with anti-CD31 to visualize endothelial cells and counterstained with hematoxylin. Images were digitized, wound margins determined, and vessels traced and measured with MetaMorph software.23 Vessel volume density (vessel area/wound area), numerical density (vessel number/wound area), and mean vessel size in the central two thirds of the wounds were computed.23
To analyze monocytes, hamstrings muscles from ischemic limbs were harvested 3 days after therapy, frozen in optimal cutting temperature compound, and sectioned at 5 μm. Procedure was performed as previously described.24 Sections were permeabilized in acetone, washed, blocked in 5% rat serum with 1% bovine serum albumin in PBS, and incubated with 1:200 Alexa 488-Ly6C (AbD Serotec, Raleigh, NC) for 1 hour. After washing in PBS, immunopositive cells were counted in five random fields from five sections of three mice per treatment group.
Data Analysis
Between group comparisons were performed by one way analysis of variance followed by Tukey's honestly significant difference post hoc tests. For analysis of correlations, linear regression analyses were performed by comparing the means (with standard deviations) for different treatment groups. P < 0.05 was considered statistically significant. All data are presented as mean with error bars indicating the SEM.
Results
In the remainder of the text we refer to lin−BMDCs as BMDCs to simplify reading. If we are referring to total BMDCs we will so indicate.
Wildtype but Not Leprdb lin− BMDCs Stimulate Vascular Growth and Healing of Ischemic Limbs
Leprdb mice are obese diabetic mice that exhibit impaired healing.16,25 Previous studies showed that treatment of Leprdb murine cutaneous wounds with wild-type, but not Leprdb BMDCs, markedly stimulates vascularization of cutaneous wounds.11 To determine whether the differential therapeutic capacity of wild-type and Leprdb BMDCs is a general property of the cells and not specific to cutaneous wounds, we focused on the ischemic hind limb model. We compared the ability of the two cell types to promote tissue vascularization and muscle salvage in ischemic hind limbs of Leprdb mice. After femoral artery ligation,26 ischemic muscles were injected intramuscularly with wild-type or Leprdb BMDCs or vehicle. Five days later, ischemic muscles were harvested and assessed for vascularity and muscle salvage in an anatomically defined region of the lower limb. Morphometric assessment of the lower limb muscles showed that wild-type BMDCs increased arteriolar volume, total capillary length, and muscle salvage relative to vehicle and Leprdb BMDC treated limbs. More muscle was salvaged in limbs treated with wild-type BMDCs than in those injected with Leprdb cells (Figure 1, A–H).
Figure 1.
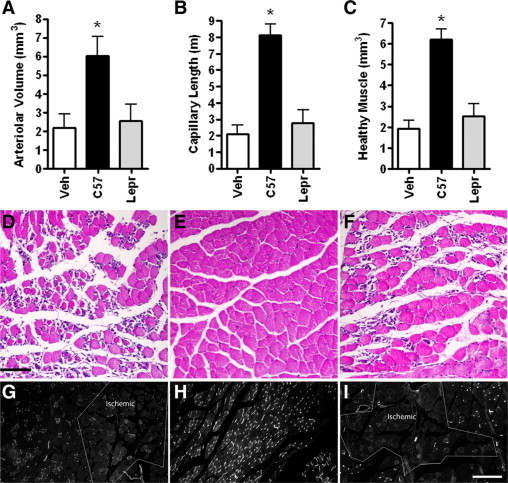
Wild-type, but not Leprdb, BMDCs increase healthy muscle and vascular volume. Morphometric analysis of cross-sections of ischemic Leprdb murine calf muscle treated on the day of femoral artery ligation with vehicle (Veh) or wild-type (C57) or Leprdb (Lepr) BMDCs and harvested at day 5. A–C: Volumes and length computed in a 3.5-mm thick cross section of an anatomically defined region. Measurements were made in five sections at 700-μm intervals. A: Total arteriolar volume of anti-smooth muscle actin labeled vessels. B: Total capillary length of BSLB4 labeled vessels. C: Total volume of healthy muscle. *P < 0.05 relative to all groups. N = 5 to 9 for each group. Bars = SEM. D–F: H&E-labeled sections from ischemic muscle treated with vehicle (D), wild-type BMDCs (E), or Leprdb BMDCs (F). Note the necrosis in D and F. G–I: Fluorescein isothiocyanate-BSLB4-labeled muscle treated with vehicle (G), wild-type BMDCs (H), or Leprdb BMDCs (I). Ischemic indicates regions with low capillary density and necrotic muscle. Black bar = 100 μm; white bar = 200 μm.
TNF-α and MCP-1 Are Differentially Secreted by Cultured Wildtype and Leprdb BMDCs
Because our data indicate, that Leprdb and wild-type cells have intrinsically distinct therapeutic properties, comparison of their behaviors and effects may help elucidate the mechanisms by which BMDCs potentiate vascular growth and healing. We compared the secretory profile of cultured wild-type and Leprdb BMDCs. Protein concentrations of eleven inflammatory cytokines thought to be involved in the regulation of angiogenesis were measured by multiplex assay in 3-day conditioned medium. Among the tested cytokines, only TNF-α and MCP-1 were differentially secreted (Table 1). Findings were confirmed by enzyme-linked immunosorbent assay. Culturing the cells under hypoxic conditions did not significantly alter the secretion of these two cytokines (data not shown).
Table 1.
Cytokines in Medium of BMDCs Cultured for 3 Days
| Cytokine | Wildtype (pg/ml) | Leprdb (pg/ml) | P value |
|---|---|---|---|
| TNF-α | 116 ± 20 | 69.9 ± 6.6 | 0.04 |
| MCP-1 | 726 ± 180 | 260 ± 35.8 | 0.04 |
| Il-1β | 12.5 ± 4.6 | 13.4 ± 3.2 | 0.88 |
| Il-2 | ND | ND | |
| Il-4 | 10 ± 1.6 | 20 ± 4.1 | 0.08 |
| Il-5 | ND | ND | |
| Il-6 | 563 ± 99.7 | 826 ± 186 | 0.16 |
| Il-10 | ND | ND | |
| Il-12 | 18.1 ± 3.3 | 25.5 ± 5.7 | 0.25 |
| IFN-γ | ND | ND | |
| GM-CSF | ND | ND |
ND = not detectable. Mean ± SEM N = 7 mice/group.
Wildtype but Not Leprdb BMDCs Recruit Monocytes to the Site of Ischemia
We tested whether differential secretion of TNF-α or MCP-1 translated to local changes in TNF-α or MCP-1 in vivo. Because up-regulation of IL-1β in muscle tissue is essential for BMDC-mediated vascular growth in nondiabetic mice,15 we also measured IL-1β concentrations in ischemic limbs. One day after femoral artery ligation and treatment, MCP-1 and IL-1β were significantly up-regulated in limbs treated with wild-type BMDCs relative to healthy control muscle and vehicle and Leprdb BMDC-treated ischemic muscle (P < 0.05) (Figure 2, A and B). Wild-type murine-derived cells also appeared to induce TNF-α relative to nonischemic muscle and vehicle treated limbs (P < 0.01), and they tended (P = 0.10) to increase induction of TNF-α relative to Leprdb BMDC-treated muscle (Figure 2C). However, tissues levels of TNF-α are near the limits of detection.
Figure 2.
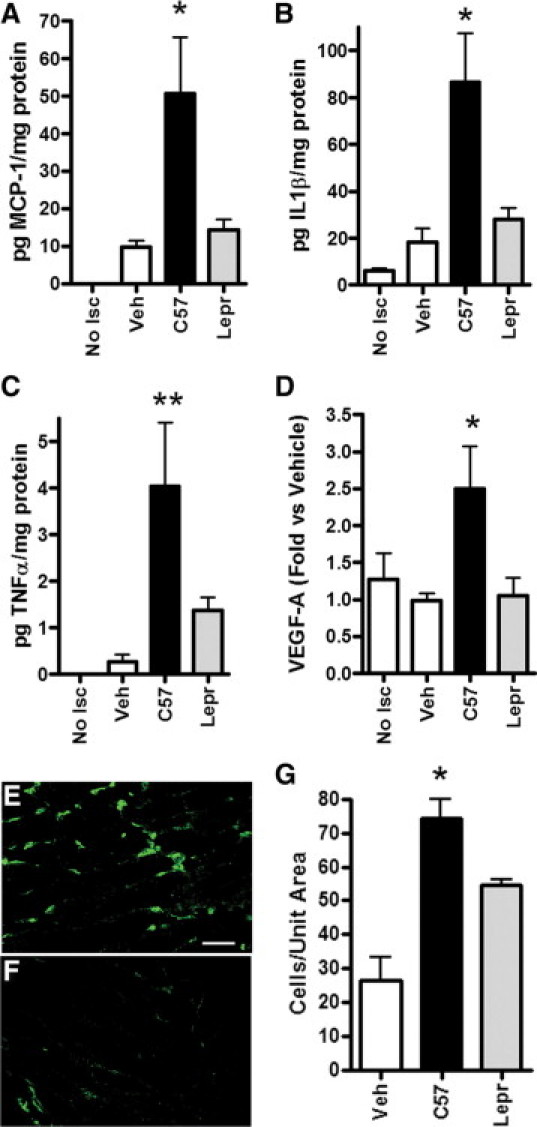
Differential effects of wild-type and Leprdb BMDCs on pro-inflammatory/angiogenic molecules and monocyte recruitment in ischemic limbs. Protein concentrations measured by multiplex assay (A–C) or Western blot (D) in lysates of ischemic Leprdb murine hamstrings muscle harvested one day after treatment with vehicle (Veh), wild-type (C57) or Leprdb BMDCs, as indicated on the x axis. Nonischemic muscle (No Isc). *P < 0.05 relative to all other groups. **P < 0.05 relative to Veh and No Isc. N = 4 to 6 per group. Bars = SEM. E–G: MOMA-2 macrophage/monocyte marker (green)-immunolabeled histological sections of ischemic Leprdb limb muscle treated with wild-type (E) or Leprdb (F) BMDCs and harvested 3 days after treatment. Scale bar = 40 μm. G: Quantitation of Ly6C-labeled cells in ischemic limb muscle three days after surgery and treatment with the indicated BMDCs. *P < 0.05 relative to all other groups. N = 3 to 4 per group. Bars = SEM.
VEGF-A, one of the most potent stimulators of angiogenesis, can be up-regulated by TNF-α, IL-1β, and MCP-1.27–29 Western blot analysis of tissue lysates from ischemic muscle treated with BMDCs showed that only wild-type BMDCs induced VEGF-A, and its concentration was approximately two-fold higher in these mice than in vehicle or Leprdb cell treated mice (Figure 2D). We did not detect induction of VEGF-A by muscle ischemia in the healing impaired Leprdb mice at this early time point.
MCP-1 is produced by endothelial cells, fibroblasts, smooth muscle and monocytic cells, among others though monocytes are the major source of MCP-1.30–32 Thus, lower tissue levels of MCP-1 would be expected to be associated with reduced monocyte accumulation in injured muscle and indeed, three days after injury, fewer monocytes were observed in Leprdb BMDC treated muscle relative to wild-type BMDC treated limbs (Figure 2, E–G).
TNF-α−/− and MCP-1−/− BMDCs Have Altered Abilities to Induce Inflammatory Cytokines and Promote Healing
To examine whether BMDCs alter tissue levels of TNF-α, IL-1β, and MCP-1 by themselves secreting the molecules, by inducing local cells to produce them, or both, we measured the levels of cytokines in ischemic muscles of Leprdb mice one day after being treated with TNF-α−/− or MCP-1−/− BMDCs. We did not test Il-1β−/− BMDCs since IL-1β was not differentially secreted by wild-type and Leprdb BMDCs (Table 1).
MCP-1−/− BMDCs failed to induce either IL-1β or MCP-1 while TNF-α−/− BMDCs induced IL-1β and MCP-1 albeit at tissue levels that appeared to be intermediate between those of muscle treated with wild-type and MCP-1−/− cells (Figure 3, A and B). Concentrations of TNF-α were at the limit of detection and too variable to determine potential differential effects of the cells (Figure 3C).
Figure 3.
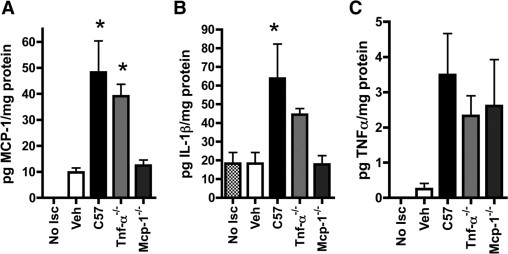
MCP-1−/− BMDCs do not induce IL-1β or MCP-1 in ischemic muscle. Leprdb murine ischemic muscle treated on the day of femoral artery ligation with vehicle (Veh) or wild-type (C57) (A), MCP-1−/− (B), or TNF-α−/− (C) BMDCs. Protein concentrations measured by multiplex bead assay in lysates of ischemic hamstrings muscle harvested 1 day after treatment. Nonischemic muscle (No Isc) served as a control *P < 0.05 relative to No Isc, Veh, and MCP-1−/−. N = 4 to 6 for per group. Bars = SEM.
After induction of hindlimb ischemia in diabetic Leprdb mice, ischemic muscles were injected with TNF-α−/− or MCP-1−/− BMDCs or vehicle as described above to examine the effects of the cells on tissue repair. Five days later, ischemic muscles were harvested and assessed for capillarity and muscle salvage. As predicted, TNF-α−/−, but not MCP-1−/− BMDCs, increased capillary length and healthy muscle volume (Figure 4, A and B).
Figure 4.
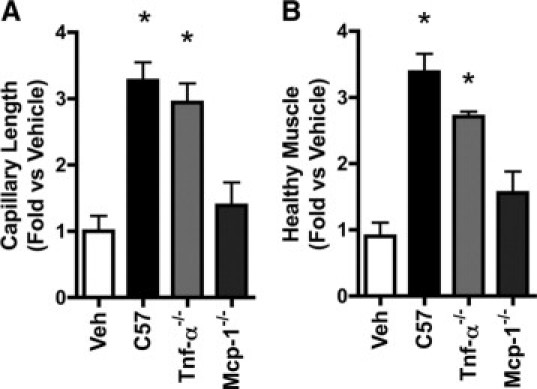
MCP-1−/− BMDCs do not stimulate healing in ischemic limbs. Morphometric analysis of cross-sections of ischemic Leprdb murine calf muscle treated on the day of femoral artery ligation with vehicle (Veh) or wild-type (C57), TNF-α−/−, or MCP-1−/− BMDCs and harvested at day five. Capillary length and muscle volume were computed in a 3.5-mm thick cross section of an anatomically defined region. Measurements were made on five sections at 700-μm intervals. A: Total capillary length of BSLB4-labeled vessels. B: Total volume of healthy muscle. *P < 0.05 relative to Veh and MCP-1−/−. N = 5 to 9 for each group. Bars = SEM.
In addition, full-thickness cutaneous wounds on the back of Leprdb mice were injected with vehicle or BMDCs from wild-type, Leprdb, TNF-α−/−, or MCP-1−/− animals 3 days after wounding, when wound revascularization begins.11 By 14 days after wounding, wounds treated with wild-type cells consistently exhibited a well-organized stratified and differentiated neo-formed epidermis (Figure 5B). In contrast, the epidermis of wounds treated with saline or Leprdb or TNF-α−/− BMDCs was thin and lacked organization of the suprabasal layers yet maintained columnar basal keratinocytes (Figure 5, A and C, and data not shown.). The epidermis of wounds treated with MCP-1−/− BMDCs was typically very poorly formed and was often almost acellular and lacked long continuous stretches of basal cells (Figure 5D).
Figure 5.
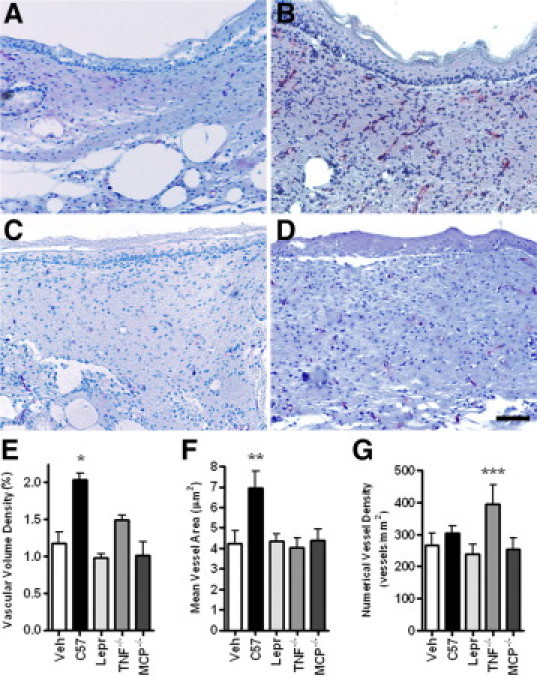
MCP-1−/− BMDCs do not induce vascularization of cutaneous wounds. Morphometric analysis of cutaneous wounds of Leprdb mice treated with vehicle (Veh), wild-type (C57), Leprdb (Lepr), TNF-α−/−, or MCP-1−/− BMDCs three days after the wound and harvested 14 days after wounding. Cutaneous wounds were serially sectioned, anti-CD31 immunolabeled, and examined at 140-μm intervals. A–D: Immunodetection of CD31 (red staining) in cutaneous wounds injected with vehicle (A), wild-type BMDCs (B), Leprdb BMDCs (C), or MCP-1−/− BMDCs (D). E: Vascular volume density expressed as percentage of wound volume. F: Mean vessel size (cross-sectional area) in wounded tissue. G: Numerical vessel density (number of blood vessels per area of injured skin). Note the correlation between the poor epidermal morphology and the lack of vascularization. *P < 0.05 relative to all groups except TNF-α−/−. **P < 0.05 relative to all groups. ***P < 0.05 relative to Leprdb. N = 4 to 8 per group. Error bars = SEM; Scale bar = 100 μm.
When compared to vehicle controls, wild-type-, but not Leprdb-derived cells, increased the vascular volume density (vessel volume/wound volume) and mean vessel size but not the number of vessels in cutaneous wounds (P < 0.001) (Figure 5, E and F). Mean vascular volume density of wounds treated with TNF-α−/− BMDCs was intermediate between that of wounds treated with wild-type and Leprdb cells, and was most likely due to an increase in vessel number rather than size (Figure 5, E and G). Neither Leprdb nor MCP-1−/− BMDCs induced any significant increase in vascularity relative to vehicle treated controls (Figure 5G).
Secretion of MCP-1 by Endogenous BMDCs Is Essential for Induction of Inflammatory Cytokines and Healing
Exogenous BMDCs must secrete MCP-1 to induce vascular growth and regression analyses of tissue levels of MCP-1 at 1 day after injury versus healing at 5 days (as assessed by vascularity and muscle salvage) suggest that the two may be correlated (Figure 6, A and B). Note that although the r2 values are excellent, the number of data points is small and the data are clustered.
Figure 6.
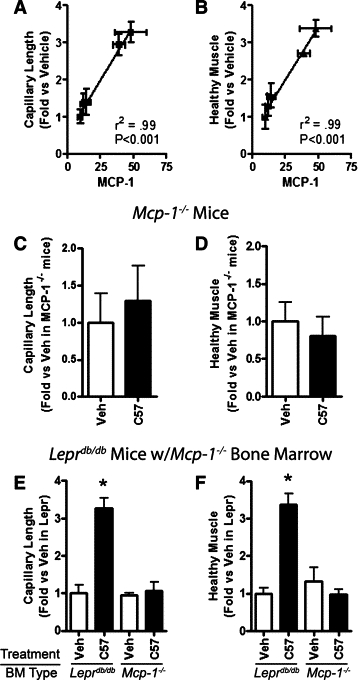
BMDC-mediated tissue repair is attenuated in mice whose endogenous cells lack MCP-1. A and B: Regression analysis of tissue concentrations of MCP-1 at one day after injury versus capillary length (A) and healthy muscle volume (B) at day five in Leprdb mice treated with vehicle or wild-type (C57), Leprdb, TNF-α−/−, or MCP-1−/− BMDCs. C–F: Morphometric analysis of ischemic calf muscle in mice treated on the day of arterial ligation with vehicle (Veh) or wild-type (C57) BMDCs and harvested at day five. Total capillary length of BSLB4-labeled vessels (C and E) and total healthy muscle volume (D and F) in an anatomically defined region. Measurements were made on five sections at 700 μm intervals. Recipients were MCP-1−/− mice (C and D) and Leprdb mice whose BM of was replaced with BM from MCP-1−/− mice (E and F). *P < 0.05 relative to all groups. N = 3 to 9 per group. Bars = SEM.
To determine whether endogenous MCP-1 production is also necessary for exogenous BMDC mediated healing, we treated ischemic limbs of MCP-1−/− mice with vehicle or wild-type BMDCs as above. The BMDCs did not induce capillary growth or enhance muscle salvage, indicating that endogenous MCP-1 is also essential for BMDCs to exert their effects (Figure 6, C and D). To determine whether endogenous BMDCs in particular must produce MCP-1, we replaced the BM of Leprdb mice with BM from MCP-1−/− mice, and 6 weeks later hind limb ischemia was induced.19 Ischemic limbs were injected with vehicle or wild-type BMDCs and harvested 5 days later. Again, wild-type BMDCs failed to stimulate capillary growth or improve muscle salvage in the chimeric limbs (P < 0.01) (Figure 6, E and F). Together, these data suggest that MCP-1 is necessary for BMDC-mediated angiogenesis and tissue repair.
Discussion
BMDCs from obese diabetic Leprdb mice do not stimulate tissue repair and so provided a physiologically relevant system to investigate the molecular mechanism by which wild-type BMDCs promote vascular growth and tissue repair. We focused on BMDC modulation of the acute inflammatory response because BMDCs act rapidly, the majority of them are cleared within a few days after treatment, and induction of the acute inflammatory cytokine IL-1β is essential for BMDC mediated healing.15
Our data show that TNF-α and MCP-1, but not IL-1β are differentially secreted by wild-type and Leprdb BMDCs although tissue levels of both MCP-1 and IL-1β are higher in limbs treated with wild-type than in those injected with and Leprdb BMDCs (tissue levels of TNFα were too low to be measured reliably). These data are consistent with a previous report that induction of IL-1β in the tissue but not secretion of IL-1β is required for BMDC-mediated healing.15
We also found that MCP-1−/− BMDCs do not induce IL-1β and MCP-1 in ischemic tissue or improve healing of ischemic limbs or skin wounds but that these abilities are only somewhat attenuated in TNF-α−/− BMDCs treated mice. Whether the small loss of healing potential by TNF-α−/− BMDCs is related to small reductions in tissue MCP-1 remains to be determined.
It is perhaps not surprising that our data implicate roles for TNF-α, IL-1β, and MCP-1 as a group since their regulation is intertwined. TNF-α and IL-1β regulate and are regulated by many of the same signaling pathways33 and they can directly and indirectly influence tissue levels of MCP-1. MCP-1 recruits monocytes and neutrophils through the CCR2 receptor which is constitutively expressed at high levels on monocytes and is up-regulated on neutrophils in response to injury.34,35 IL-1β activates monocytes and neutrophils secrete TNF-α, IL-1β, and MCP-1.36,37 CCR2 has also been detected on primitive multipotent hematopoietic stem and progenitor cells and so may recruit them as well.38
Our data are consistent with the establishment of such a positive feedback loop.39 It would predict that fewer monocytes would be recruited to ischemic tissue treated Leprdb BMDCs than that treated with wild-type cells. This is what we observed. It also predicts that endogenous BMDCs must secrete MCP-1 if BMDCs are to potentiate healing. Again, our data showing that wild-type BMDCs have no therapeutic effects in chimeric mice with MCP-1−/− bone marrow are consistent with this expectation.
Activated monocytes are key regulators of vascular remodeling and secrete pro-angiogenic molecules including VEGF-A.40 VEGF-A in turn is chemoattractive for monocytes.41 Our VEGF-A data are consistent with a key role for MCP-1 and monocytes.
The findings reported here suggest that MCP-1 therapy might potentiate tissue repair, though administration of MCP-1 at appropriate times and dosages might prove difficult. Nevertheless, levels of MCP-1 in BMDCs of patients may identify a priori the ones who would or would not be good candidates for BMDC based therapies. This is a significant consideration because BMDC dysfunction is associated with increasing age and risk factors for cardiovascular disease.9–12
Acknowledgements
We acknowledge the staff of and equipment in the Central Microscopy Research Facility and the Flow Cytometry Facility (University of Iowa) for facilitating this work. We thank Michael Rebagliati for critical reading of the manuscript.
Footnotes
Supported by NIH grants DK55965 (G.C.S., M.D.), DK59223 (G.C.S.), and AR055313 (M.D.).
None of the authors disclosed any relevant financial relationships.
References
- 1.Heil M, Clauss M, Suzuki K, Buschmann IR, Willuweit A, Fischer S, Schaper W. Vascular endothelial growth factor (VEGF) stimulates monocyte migration through endothelial monolayers via increased integrin expression. Eur J Cell Biol. 2000;79:850–857. doi: 10.1078/0171-9335-00113. [DOI] [PubMed] [Google Scholar]
- 2.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, Gregory CA, Spees JL. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 2010;103:696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- 4.Nesselmann C, Li W, Ma N, Steinhoff G. Stem cell-mediated neovascularization in heart repair. Ther Adv Cardiovasc Dis. 2010;4:27–42. doi: 10.1177/1753944709353338. [DOI] [PubMed] [Google Scholar]
- 5.Yeo C, Mathur A. Autologous bone marrow-derived stem cells for ischemic heart failure: REGENERATE-IHD trial. Regen Med. 2009;4:119–127. doi: 10.2217/17460751.4.1.119. [DOI] [PubMed] [Google Scholar]
- 6.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Droetto S, Viale A, Primo L, Jordaney N, Bruno S, Pagano M, Piacibello W, Bussolino F, Aglietta M. Vasculogenic potential of long term repopulating cord blood progenitors. FASEB J. 2004;18:1273–1275. doi: 10.1096/fj.03-1444fje. [DOI] [PubMed] [Google Scholar]
- 8.Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol. 2006;26:758–764. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- 9.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 10.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 11.Stepanovic V, Awad O, Jiao C, Dunnwald M, Schatteman G. Leprdb diabetic mouse bone marrow cells inhibit skin wound vascularization but promote wound healing. Circ Res. 2003;92:1247–1253. doi: 10.1161/01.RES.0000074906.98021.55. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Fazel S, Tian H, Mickle DA, Weisel RD, Fujii T, Li RK. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005;289:H2089–H2096. doi: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- 13.Sander AL, Jakob H, Henrich D, Powerski M, Witt H, Dimmeler S, Barker J, Marzi I, Frank J. Systemic transplantation of progenitor cells accelerates wound epithelialization and neovascularization in the hairless mouse ear wound model. J Surg Res. 2009 doi: 10.1016/j.jss.2009.07.003. S0022–4804(0009)00369–00362 [pii] 00310.01016/j.jss.02009.00307.00003. [DOI] [PubMed] [Google Scholar]
- 14.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tateno K, Minamino T, Toko H, Akazawa H, Shimizu N, Takeda S, Kunieda T, Miyauchi H, Oyama T, Matsuura K, Nishi J, Kobayashi Y, Nagai T, Kuwabara Y, Iwakura Y, Nomura F, Saito Y, Komuro I. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res. 2006;98:1194–1202. doi: 10.1161/01.RES.0000219901.13974.15. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 17.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crosby JR, Kaminski WE, Schatteman GC, Martin JC, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 20.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 21.Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. Cd34(−) blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–312. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 22.Bradford A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–377. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 24.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 25.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 26.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 27.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 28.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 29.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 30.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Standiford TJ, Kunkel SL, Phan SH, Rollins BJ, Strieter RM. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–9918. [PubMed] [Google Scholar]
- 32.Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard EJ. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989;142:1956–1962. [PubMed] [Google Scholar]
- 33.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 34.Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–1276. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichel CA, Rehberg M, Lerchenberger M, Berberich N, Bihari P, Khandoga AG, Zahler S, Krombach F. Ccl2 and Ccl3 mediate neutrophil recruitment via induction of protein synthesis and generation of lipid mediators. Arterioscler Thromb Vasc Biol. 2009;29:1787–1793. doi: 10.1161/ATVBAHA.109.193268. [DOI] [PubMed] [Google Scholar]
- 36.Abrahams VM, Cambridge G, Lydyard PM, Edwards JC. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000;43:608–616. doi: 10.1002/1529-0131(200003)43:3<608::AID-ANR18>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Iwamoto S, Iwai S, Tsujiyama K, Kurahashi C, Takeshita K, Naoe M, Masunaga A, Ogawa Y, Oguchi K, Miyazaki A. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J Immunol. 2007;179:1449–1457. doi: 10.4049/jimmunol.179.3.1449. [DOI] [PubMed] [Google Scholar]
- 38.Si Y, Tsou CL, Croft K, Charo IF: CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 120:1192–1203 [DOI] [PMC free article] [PubMed]
- 39.Sozzani S, Locati M, Zhou D, Rieppi M, Luini W, Lamorte G, Bianchi G, Polentarutti N, Allavena P, Mantovani A. Receptors, signal transduction, and spectrum of action of monocyte chemotactic protein-1 and related chemokines. J Leukoc Biol. 1995;57:788–794. doi: 10.1002/jlb.57.5.788. [DOI] [PubMed] [Google Scholar]
- 40.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 41.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]


