Abstract
Stem cells isolated from human amniotic fluid are gaining attention with regard to their therapeutic potential. In this work, we investigated whether these cells contribute to tubular regeneration after experimental acute kidney injury. Cells expressing stem cell markers with multidifferentiative potential were isolated from human amniotic fluid. The regenerative potential of human amniotic fluid stem cells was compared with that of bone marrow-derived human mesenchymal stem cells. We found that the intravenous injection of 3.5 × 105 human amniotic fluid stem cells into nonimmune-competent mice with glycerol-induced acute kidney injury was followed by rapid normalization of renal function compared with injection of mesenchymal stem cells. Both stem cell types showed enhanced tubular cell proliferation and reduced apoptosis. Mesenchymal stem cells were more efficient in inducing proliferation than amniotic fluid-derived stem cells, which, in contrast, were more antiapoptotic. Both cell types were found to accumulate within the peritubular capillaries and the interstitium, but amniotic fluid stem cells were more persistent than mesenchymal stem cells. In vitro experiments demonstrated that the two cell types produced different cytokines and growth factors, suggesting that a combination of different mediators is involved in their biological actions. These results suggest that the amniotic fluid-derived stem cells may improve renal regeneration in acute kidney injury, but they are not more effective than mesenchymal stem cells.
Stem cell-based therapy is a promising and plausible option for organ repair.1 Several groups have been successful in demonstrating the use of different stem cell types in the treatment of acute kidney injury (AKI) in different experimental animal models. Ex vivo expanded mesenchymal stem cells (MSCs) or resident renal stem cells were used in these studies.1 The advantage of MSC use in therapy is their pluripotency, the relative ease of isolation, and the possible ex vivo expansion of the cells.2–5 It has been shown that MSCs may improve the recovery from cytotoxic6,7 and ischemic AKI.8,9 However, the exact mechanisms that promote kidney regeneration after stem cell injection are mostly unknown. The process might involve recruitment of stem cells to the site of injury, fusion of stem cells with injured cells, or most likely paracrine/endocrine stimulation.10 In addition, the best source of stem cells for therapeutic use remains to be defined.
A number of studies focused on alternative sources of stem cells with multipotential differentiating capabilities and accessibility.11–13 Mesenchymal stem cells obtained from the adipose tissue, the umbilical cord vein, and the dental pulp have been investigated as a potential source for stem cells to be used in tissue regeneration.14–17 In search for alternative sources of stem cells, several groups have reported the isolation of stem cells from human amniotic fluids (hAFSCs) and their subsequent differentiation into all three types of germ layer cells.18–22 Amniotic fluid (AF) supplies the developing embryo with nutrients and provides mechanical protection. AF contains cells of embryonic origin, and it is indeed used in routine prenatal diagnosis to test for chromosomal aberrations of the embryo.
Perin et al23 injected hAFSCs into murine embryonic kidneys and tested their contribution to the early renal development. They found that hAFSCs differentiated into renal vesicles and comma- and s-shaped bodies.
Because the retrieval of hAFSCs is considered ethically acceptable and does not involve the destruction of human embryos, stem cells from amniotic fluid present an attractive alternative to embryonic stem cells.24–27 A further finding that is making stem cells from AF a very attractive source for therapeutic use is the absence of teratoma formation when these cells are injected in vivo.23
The aim of the present study was to investigate whether hAFSCs may contribute to tubular regeneration in AKI induced by glycerol injection in non-immune-competent SCID mice. In addition, we aimed to compare the regenerative potential of hAFSCs with that of human bone marrow-derived MSCs.
Materials and Methods
Isolation and Culture of hAFSCs
hAFSCs were isolated as reported recently.28 AF samples were collected from 38 women (median age: 37 years) undergoing amniocentesis for routine prenatal diagnosis at 14 to 17 weeks of pregnancy with informed written consent and approval of the local ethics committee. The cellular content of the AF samples (5–10 ml) was pelleted by centrifugation (1200 rpm, room temperature) and washed in PBS. The resulting pellet was resuspended in α-minimal essential medium (Lonza, Basel, Switzerland) containing 20% Amniomax (Gibco, Carlsbad, CA) + 10% fetal calf serum (Euroclone, Wetherby, UK) + 100 μg/ml glutamine + 100 IU/ml penicillin/streptomycin (all from Sigma-Aldrich, St. Louis, MO), plated in 25-cm2 T-flasks and maintained at 37°C with a 5% CO2 atmosphere. After 5 to 7 days, nonadherent cells were removed, and the adherent cells were refed every 3 to 4 days and grown until they reached confluence. To expand the isolated cells, the adherent semiconfluent monolayer was detached with nonenzymatic solution (Sigma-Aldrich) and expanded for several passages until they no longer reached confluence.
Isolation and Culture of Bone Marrow-Derived Mesenchymal Stem Cells
Bone marrow cells were harvested from healthy donors with informed consent obtained in accordance with the Declaration of Helsinki. Bone marrow-derived MSCs were isolated, cultured, and characterized as described earlier.29 In brief, bone marrow cells were layered on a Ficoll gradient (density 1022 g/ml; Sigma-Aldrich) and centrifuged at 1500 rpm for 30 minutes. The mononucleated cells were cultured in the presence of mesenchymal stem cell basal medium (Lonza). After 5 days of culture, the medium was changed. To expand the isolated cells, the adherent monolayer was detached by trypsin treatment for 5 minutes at 37°C, after 15 days for the first passage and every 7 days for successive passages. Cells were seeded at a density of 1 × 104 cells/cm2 and used within passage 6.
At each passage, cells were counted and analyzed for immunophenotype by cytofluorimetric analysis and immunofluorescence. All of the MSC preparations at different passages of culture expressed the typical MSC markers: CD105, CD73, CD44, CD90, and CD166. They also expressed HLA class I. MSC preparations did not express hematopoietic markers (CD45, CD14, and CD34) and the costimulatory molecules (CD80, CD86, and CD40). The adipogenic, osteogenic, and chondrogenic differentiation abilities of MSCs were determined.
Cytofluorimetric Analysis of hAFSCs
Cytofluorimetric analysis was performed as described previously.30,31 Fluorescein isothiocyanate- and phycoerythrin-conjugated antibodies with specificities against CD44, CD45, CD90, CD105 (AbD Serotec, Raleigh, NC), CD73, CD166 (BD Pharmingen, San Diego, CA), Oct3/4, and SSEA4 (R&D Systems, Minneapolis, MN) were used. Nonimmune isotypic IgG antibodies conjugated to fluorescein isothiocyanate and phycoerythrin were used as controls (Dako Cytomation, Copenhagen, Denmark). For each staining, approximately 100,000 cells were incubated for 20 minutes with a particular antibody (as listed above), washed with PBS, and analyzed on FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) using CellQuest Pro 5.2.1 software.
PCR
To confirm the cytofluorimetric analysis of nuclear markers (Oct4, SSEA4, and vimentin), we performed real-time PCR. In brief, total RNA was isolated with the TRIzol method (Invitrogen, Carlsbad, CA) and transcribed into cDNA using a High-Capacity cDNA RT Kit (Applied Biosystems, Foster City, CA). Primers for Oct4, SSEA4, and vimentin were designed using Primer Express (Applied Biosystems) and synthesized from Invitrogen (Carlsbad, CA) as previously described.28 Quantitative real-time PCR was performed using a Step One Sequence Detection system according to TaqMan protocols (Applied Biosystems). The SYBR ® Green detection method was used to detect the amplification rate of the targets. Reactions were set up using the TaqMan Universal PCR MasterMix (2×) (Applied Biosystems). Each target was measured in triplicate using 48-well plates. Relative expression of mRNAs was compared using the relative Ct quantification method.
Differentiation Potential Assay
To assess the differentiation potential, AF-derived adherent cells were cultured in osteogenic, adipogenic, chondrogenic, and neurogenic medium according to the manufacturer's instructions (Lonza). Osteogenic differentiation was demonstrated by detection of the intracellular hydroxyapatite accumulation by Von Kossa staining, after incubation of the cells over a period of 21 days in osteogenic differentiation media. Adipogenic differentiation was performed by incubation in adipogenic induction and maintenance medium (alternated every 3 to 4 days). Oil Red O staining was used to assess the presence of intracellular lipid vesicles, which were visible after 2 to 3 weeks in culture. For chondrogenic differentiation, cells were grown as freely floating pelleted aggregate kept in suspension culture for 2 to 3 weeks in the presence of transforming growth factor-β3. The pellet was included in paraffin and stained with Alcian Blue to identify the presence of hyaluronic acid and sialomucin.
Cytokine Assay
To measure the cytokines produced by hAFSCs and MSCs, we performed a multiplex cytokine array, based on fluorescently dyed microspheres (Bio-Plex, Bio-Rad Laboratories, Hercules, CA). hAFSCs and MSCs were cultured to a confluence of 90%. Cells were then exposed to α-minimal essential medium with 0.5% albumin (Sigma-Aldrich) for 12 hours. The superantant (conditioned medium) was collected, centrifuged (1200rpm, 4°C, 5 minutes) to remove cellular debris and frozen at −20°C. The cytokine assay was performed for five different isolated hAFSCs and MSCs.
Cell Proliferation Assay
Tubular epithelial cells (TECs) were prepared and characterized as described previously.29 The cells were seeded at 4000 cells/well into 96-well plates in Dulbecco's modified Eagle's medium (Sigma-Aldrich), deprived of fetal calf serum incubated with or without 3 μg/ml of human leukemia inhibitory factor (LIF) blocking antibody (R&D Systems) in the presence of 30% conditioned medium from hAFSCs or MSCs. DNA synthesis was detected as incorporation of 5-bromo-2′-deoxyuridine (BrdU) into the cellular DNA after 48 hours of culture. Cells were then fixed with 0.5 mol/L ethanol-HCl and incubated with nuclease to digest the DNA. BrdU incorporated into the DNA was detected using an anti-BrdU peroxidase-conjugated antibody and visualized with a soluble chromogenic substrate (Roche Applied Science, Mannheim, Germany). Optical density was measured with an ELISA reader at 405 nm.
Acute Renal Injury in SCID Mice and Treatment
Rhabdomyolyses was induced in a total of 75 male SCID BALB/c mice (Charles River, Milano, Italy) as described before.6,32,33 In brief, animals (body weight 23 ± 6 g) were anesthetized and injected with glycerol into the left and right hind femoral muscles. Animals received 8 μl of 50% glycerol/g b.wt. Three days after induction of experimental injury, animals were injected with 350,000 hAFSCs or MSCs (Figure 1). For in vivo detection of proliferating cells, mice were injected intraperitoneally with 2 mg of BrdU 24 and 48 hours before biopsy samples were harvested. Animals were sacrificed as follows: day 3 (n = 6), day 5 (CTRL n = 8, hAFSC n = 8, and MSC n = 8), day 8 (CTRL n = 6, hAFSC n = 9, and MSC n = 8), and day 21 (CTRL n = 8, hAFSC n = 8, and MSC n = 6). Kidney tissue was fixed in 10% formaldehyde or frozen in OCT (Tissue-Tek, Sakura FineTek, Torrance, CA). Studies were approved and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.34
Figure 1.
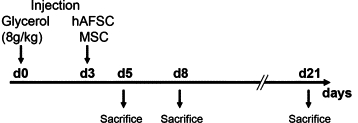
Experimental schedule. Schematic representation of the protocol of glycerol-induced AKI and treatment with either hAFSCs or MSCs. Glycerol was injected intramuscularly at time 0. The arrow at day three indicates the administration of 350,000 cells or vehicle alone; the subsequent arrows indicate the time of sacrifice.
Biochemical Data
To assess renal function, serum was collected at the time of sacrifice. Serum creatinine was measured using a colorimetric microplate assay based on the Jaffe reaction (Quantichrom Creatinine Assay, BioAssay Systems, Hayward, CA) from blood collected at days 0, 3, 5, 9, and 15. Serum was isolated from the supernatant by centrifugation of blood samples (3000 rpm, 5 minutes). Blood urea nitrogen (BUN) was measured by direct quantification of serum urea with a colorimetric assay kit according to the instruction protocol (Quantichrom Urea Assay, BioAssay Systems).
Renal Morphology
To evaluate renal histology, paraffin-fixed biopsy samples were sectioned (5 μm) and stained with H&E (Merck, Darmstadt, Germany). To compare the changes in renal histology, luminal hyaline casts and necrosis were assessed in >25 nonoverlapping high-power fields (×40). The quantification of casts and tubular profiles showing necrotic cells was recorded in a single-blinded fashion. To detect proliferating tubular cells, immunohistochemical analysis was performed as described earlier (T.W. Kirkman: Statistics to Use, http://www.physics.csbsju.edu/stats, last accessed September 2, 2009). In brief, kidney sections were boiled in citrate buffer for 10 minutes to unmask antigens and subsequently blocked with 5% bovine serum albumin in PBS. Kidney tissue was stained with a monoclonal anti-proliferating cell nuclear antigen (Pcna) antibody (1:400, Santa Cruz Biotechnology, Santa Cruz, CA) monoclonal anti-BrdU antibody (1:25, Dako Cytomation, Dako, Milano, Italy). An anti-mouse horseradish peroxidase-conjugated antibody (1:300, Pierce, Rockford, IL) was used for immunoperoxidase stainhigh-powering. Pcna- and BrdU-positive cell quantification was performed by counting positive nuclei per field (×40) in 20 kidney sections that were chosen randomly from the kidney cortex.
Tubular Apoptosis
To quantify tubular apoptosis at day 5 after injury, paraffin-fixed tissue from five animals per group (hAFSC- or MSC-injected and untreated controls) was stained by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) using the ApopTaq Apoptosis Detection Kit (Millipore Corporation, Billerica, MA) according the manufacturer's protocol.
Identification of CFSE-Labeled Human MSCs or hAFSCs
To study intrarenal localization and to quantify localized human MSCs or hAFSCs, cells were labeled with a Vybrant CFDA SE Cell Tracer Kit (CFSE) (Molecular Probes, Leiden, The Netherlands) and subsequently injected in glycerol-treated mice (n = 3 per experimental groups). After 1, 2, and 5 days from cell injection, mice were sacrificed, and kidney samples were embedded in OCT and snap-frozen in liquid nitrogen. Tissue sections (5 μm) were fixed in acetone (10 minutes) and double-stained with an anti-fluorescein/Oregon Green polyclonal antibody (Molecular Probes) (detects cells labeled with CFSE) and with anti-cytokeratin monoclonal antibody (Immunological Sciences, Rome, Italy), used to better identify tubular structures. Alexa Fluor 488 goat anti-rabbit IgG and Texas Red anti-goat IgG (all from Molecular Probes) were used as secondary antibody. Confocal microscopy analysis was performed using a Zeiss LSM 5 Pascal model confocal microscope (Carl Zeiss International, Oberkochen, Germany). Hoechst 33258 dye (Sigma-Aldrich) was added for nuclear staining. To confirm the human origin of MSCs or hAFSCs, kidney sections of mice injected with CFSE-labeled cells were stained with HLA class I polyclonal antibody (Santa Cruz Biotechnology). Scoring for CFSE-positive or HLA-positive cells was performed by counting the number of positive cells per field in 10 randomly chosen sections of kidney cortex of three mice per group using ×40 magnification. Data are expressed as number of CFSE-positive or HLA-positive cells per high-power field (hpf).
Statistical Analysis
Results are generally expressed as mean ± SEM. Statistical significance was defined as P < 0.05; tests were performed by using Microsoft Excel. Analysis of variance (P < 0.05) was performed to compare differences of the biochemical data, tubular proliferation, and renal morphology.35 One-way analysis of variance followed by Dunnett's or the Newman-Keuls multicomparison test or t-test was performed using XLSTAT 2009 (Addinsoft Inc., New York, NY).
Results
Isolation and Characterization of hAFSCs
hAFSCs were obtained as described previously28 and were characterized on the basis of their phenotype and their differentiating capabilities. Cytofluorimetric analysis showed that cells were uniformly positive for surface markers CD44, CD73, CD105, and CD166, typically expressed by mesenchymal stem cells. At early passages,4–7 cells were also positive for the surface marker SSEA4 (a human embryonic stage-specific marker) and partially for Oct4 (∼21%), a stem cell marker of pluripotency.36 hAFSCs were negative for CD45, a marker of the hematopoietic lineage cells and fibroblasts. At variance with bone marrow-derived MSCs, CD90 was negative. A representative fluorescence-activated cell sorter analysis of hAFSCs is shown in Figure 2. Expression of Nanog-1 and Oct-4 was detected in all cultures by real-time PCR (data not shown). hAFSCs were negative for SSEA1, SSEA3, CD133, and NCAM (data not shown). This phenotype was similar to that described previously.28
Figure 2.
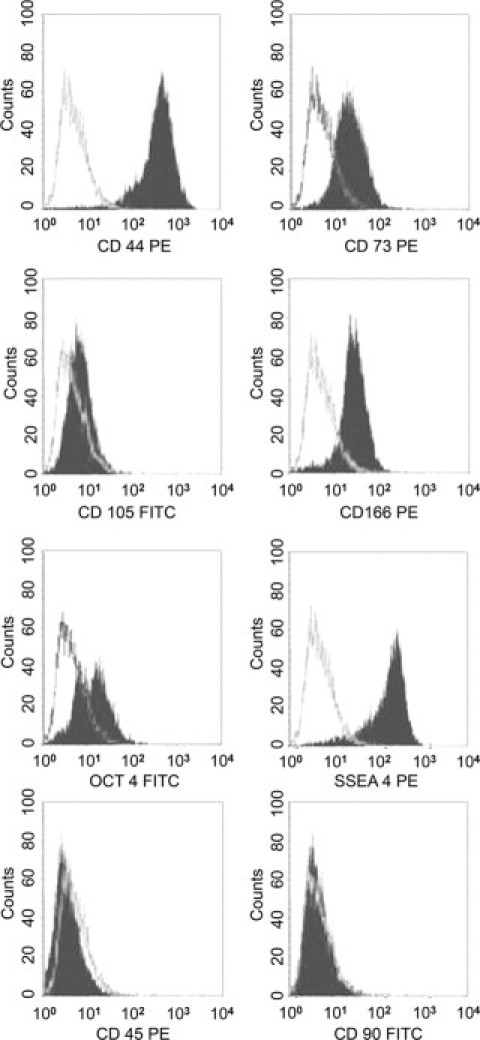
Fluorescence-activated cell sorter analysis of hAFSCs. Representative fluorescence-activated cell sorter analyses for the expression of surface markers CD44, CD45, CD73, CD90, CD 105, CD166, and SSEA4 and the intracellular transcription factor Oct4. The gray line indicates the isotypic controls. All analyzed hAFSCs (n = 12) showed similar results in passages 2 to 5. PE, phycoerythrin; FITC, fluorescein isothiocyanate.
Using appropriate differentiation media, we were able to differentiate hAFSCs into chondrocytes, adipocytes, and osteocytes. As shown in Figure 3, hAFSCs showed calcium oxalate precipitates after osteogenic differentiation (Figure 3A) and intracellular lipid vesicles by Oil Red O staining after adipogenic induction (Figure 3B). Chondrogenic differentiation was demonstrated by Alcian Blue staining of the pelleted hAFSCs, visualizing the expression of hyaluronic acid and sialomucin (Figure 3C).
Figure 3.
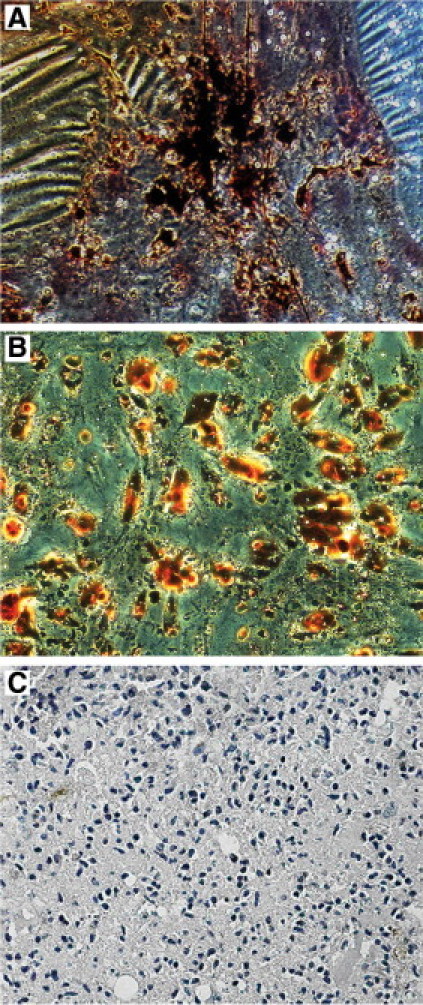
Differentiation characteristics of hAFSCs. A: Representative of osteogenic differentiation detected as calcium oxalate deposits detectable by Van Kossa staining. B: Representative of adipogenic differentiation visualized by Oil Red O staining of the intracellular lipid vesicles. C: Representative of chondrogenic differentiation detected by Alcian Blue staining. Micrographs are representative of 12 experiments.
Effect of hAFSC on the Recovery of Glycerol-Induced AKI in SCID Mice and Comparison with MSCs
To investigate whether hAFSCs have a beneficial effect on the regeneration after AKI, experimental AKI was induced by injection of glycerol, as described before.6 Intramuscular injection of glycerol causes rhabdomyolyses of the muscular tissue, thereby releasing large quantities of enzymes, hemoglobin, and myoglobin to the kidney. Exposure of the kidney to myoglobin and iron is followed by tubular injury. After the glycerol injection serum creatinine and BUN levels increased, peaking after 72 hours, and remained elevated over the course of 10 days and normalized on day 21. Three days after induction of AKI, mice received 3.5 × 105 hAFSCs or MSCs in 150 μl of PBS intravenously via the tail vein. Control animals received PBS instead. The experimental time line is shown in Figure 1. Animals were sacrificed at days 5, 8, and 21 after the induction of AKI.
Mice injected with hAFSCs exhibited significantly reduced BUN from day 5 (48 hours after treatment), throughout the experiment (Figure 4A), with respect to that in animals treated with vehicle alone. In addition, treatment with hAFSCs resulted in the normalization of the creatinine levels as soon as day 5 (Figure 4B). The functional recovery obtained with hAFSCs was similar to that obtained by infusion of MSCs (Figure 4, A and B).
Figure 4.
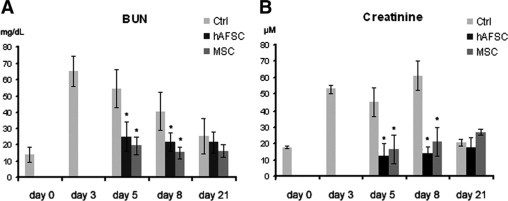
Evaluation of renal function. Changes in renal function were measured by BUN (A) and creatinine (B). Glycerol injection resulted in elevated BUN levels starting at day three. In animals injected with hAFSCs or MSCs, BUN normalized 48 hours after injection and remained close to baseline throughout the experiment (A). Similar results were obtained measuring creatinine levels (B). Analysis of variance was performed: *P < 0.05, stem cells treated versus untreated.
Histological analysis of the kidneys of glycerol-injected animals on day 3 after injury showed epithelial injury characterized by tubular necrosis and vacuolization of tubular epithelial cells. In addition, proximal tubular cells showed a loss of brush border and intratubular protein casts (Figure 5A). Mice treated with hAFSCs or MSCs showed a marked reduction of tissue damage with respect to that in untreated controls. On day 5 after glycerol injection (48 hours after stem cell injection) animals treated with hAFSCs (Figure 5C) or MSCs (Figure 5D) exhibited reduced tissue damage, compared with that in untreated control animals (Figure 5B). At day 8, an almost normal renal structure was observed in mice treated with hAFSCs or with MSCs (Figure 5, F and G) compared with that in untreated animals (Figure 5E). Normal histology was restored in all of the experimental groups on day 21 (Figure 5, H–J).
Figure 5.
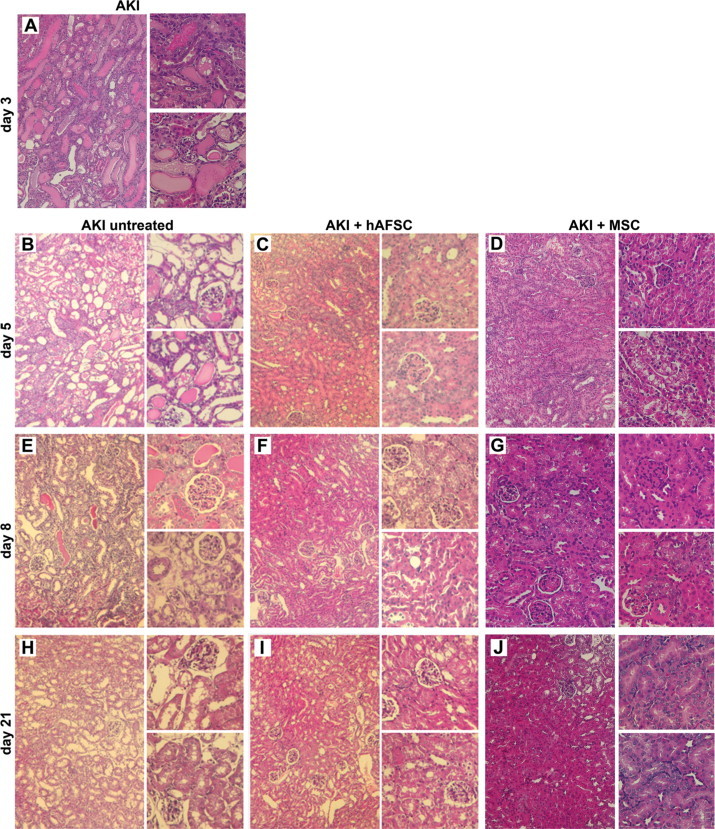
Histology of AKI in hAFSC- or MSC-treated and control untreated mice. A and B: Representative micrographs of renal tissue from mice on day 3 (A) and day 5 (B) after glycerol injection, showing tubular necrosis, tubular protein casts, and loss of brush border; vacuolization of the epithelial cells of the proximal tubules is visible in the high power images. C and D: Representative micrographs of renal tissue from mice on day 5 after glycerol injection treated with hAFSCs (C) or MSCs (D), showing signs of recovery of tissue injury. E–G: Representative micrographs of renal tissue from mice on day 8 after glycerol injection, showing the persistence of renal injury in untreated animals (E) and signs of recovery in hAFSC-treated (F) or MSC-treated (G) animals. H–J: Representative micrographs of renal tissue from mice on day 21 showing the normal morphology of tissue in animals untreated (H) or treated with hAFSCs (I) and MSCs (J). Original magnification of each panel: ×200 (left); ×400 (right).
In Table 1, the morphometric evaluation of injury in mice with AKI injected or not with hAFSCs or MSCs was compared. Stem cell-injected animals had a significantly lower number of cast-containing tubules and a lower number of necrotic tubules compared with untreated animals with AKI. No significant difference was observed between animals treated with hAFSCs or MSCs.
Table 1.
Effects of hAFSCs and MSCs on Renal Morphology and Tubular Apoptosis
| Control | AKI + hAFSCs | AKI + MSCs | AKI | |
|---|---|---|---|---|
| Casts (n/hpf) | 0 | 0.36 ± 1.01* | 0.54 ± 0.17† | 5.05 ± 7.54 |
| Necrosis (n/hpf) | 0 | 0.81 ± 1.38* | 0.3 ± 0.14† | 3.85 ± 4.44 |
| TUNEL-positive cells (n/hpf) | 0 | 3.2 ± 0.8* | 7.8 ± 2.2† | 21.7 ± 16.9 |
Comparison of hAFSC or MSC injection on tubular morphology and apoptosis at day 5 after AKI induction. Numbers in the table represent the mean ± SD of tubular casts and necrotic tubules and of apoptotic cells detected by TUNEL, observed under high power (magnification, ×400).
P < 0.05 for hAFSC injection versus untreated AKI.
P < 0.05 for MSC injection versus untreated AKI.
Effect of hAFSCs on Tubular Cell Proliferation and Apoptosis after AKI and Comparison with MSCs
To assess the regenerative response of kidney tissue in hAFSC-injected animals, we quantified the proliferation of tubular cells by counting BrdU- and Pcna-positive cells (Figure 6). Proliferation of tubular cells was significantly increased in the stem cell-treated animals with respect to that in untreated controls. In hAFSC-injected mice proliferation at days 5 was slightly increased but not significantly, whereas at day 8 proliferation was significantly increased compared with that in controls (Figure 6, A-C). MSC treatment induced significantly increased proliferation of tubular epithelial cells both at day 5 and 8. Comparing proliferation induced by hAFSCs and MSCs, we observed an overall greater proliferation in animals treated with MSCs than in those treated with hAFSCs, which was significant at day 5 (Figure 6, A and B). On day 21, animals treated with hAFSC showed sustained tubular proliferation, which was completely absent in animals treated with MSCs (Figure 6, A and B).
Figure 6.
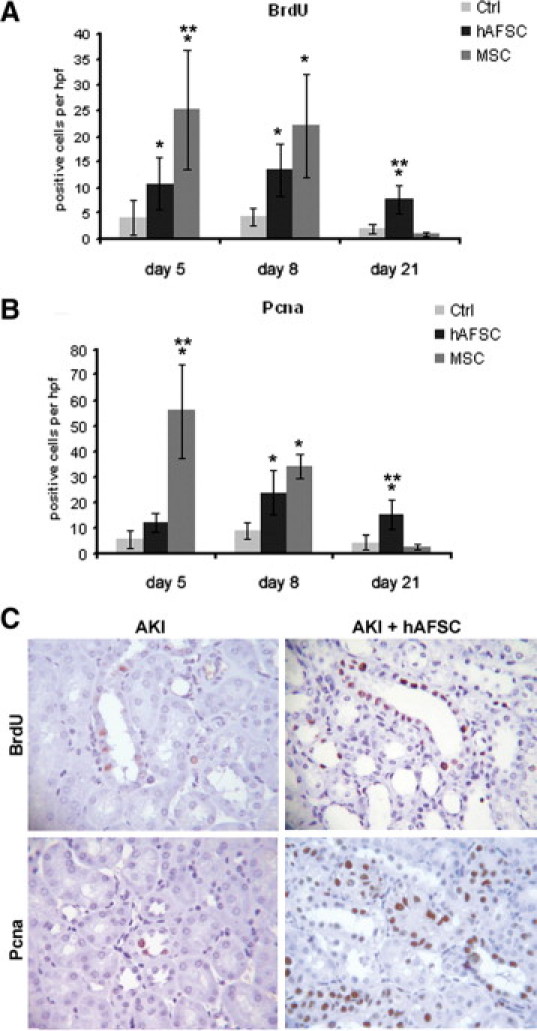
Tubular proliferation in AKI untreated or treated with hAFSCs or MSCs. A and B: Graphs illustrating the quantification of BrdU (A)- and Pcna (B)-positive cells at days 5, 8, and 21 in AKI mice treated with hAFSCs or MSCs or injected with saline as a control. Statistical significance was calculated using analysis of variance with the Newman-Keuls multicomparison test: *P < 0.05, stem cells in AKI-treated versus untreated mice; **P < 0.005, MSCs versus hAFSCs. C: Representative micrographs showing BrdU- and Pcna-positive cells in kidney tissue from AKI mice injected with saline or hAFSCs at day eight. Original magnification, ×400. Ctrl, control.
Quantification of apoptotic tubular cells by TUNEL in tissues retrieved from animals on day 5 after AKI showed a significant reduction of apoptosis in hAFSC- and MSC-injected animals compared with that in untreated control animals (Table 1). However, the antiapoptotic effect of hAFSCs was significantly greater than that of MSCs.
hAFSC and MSC Renal Localization in Mice with AKI
To evaluate the engraftment of hAFSCs and of MSCs in the kidneys of mice with AKI, stem cells were labeled with the CFSE Cell Tracer Kit before their in vivo infusion. In renal tissues of glycerol-treated mice injected with CFSE-positive cells, 7.9 ± 1.5 hAFSCs/hpf and 8.7 ± 1 MSCs/hpf were detected at day 4, 1 day after cell injection (Figure 7, A and C). CFSE-positive cells were seen mainly in peritubular capillaries (Figure 7C). At day 5 of AKI (2 days after cell injection), 8 ± 1.7 CFSE-positive hAFSCs were detected in peritubular capillaries and in the interstitium. In contrast, MSCs were significantly decreased (1.6 ± 0.25) at this time point (Figure 7A). Moreover, CFSE-positive hAFSCs persisted to be significantly elevated at day 8 in the renal interstitium of AKI mice (4.6 ± 0.95 CFSE-positive cells), whereas CFSE-positive MSCs were almost absent (Figure 7, A and C). The human origin of CFSE-positive cells was confirmed in kidney sections stained for human HLA-I antigen (Figure 7, B and C), and the amount of cells detected by HLA immunostaining was comparable with that detected by counting CFSE-positive cells. With use of HLA staining, hAFSCs were detectable also at day 21, whereas MSCs were completely absent (Figure 7, B and C). There were no signals detected in CFSE-labeled or HLA-positive cells within the kidneys of control mice (Figure 7D).
Figure 7.
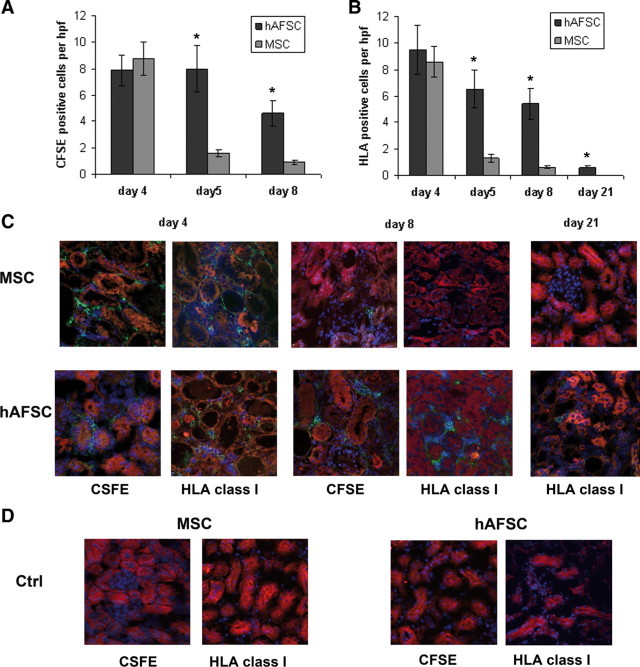
Detection of hAFSCs or MSCs within the kidneys of AKI mice by CFSE or HLA immunostaining. A and B: Graphs illustrating the quantification of CFSE-labeled cells (A) and of HLA-positive cells (B). Student's t-test was performed between hAFSC- and MSC-treated AKI mice at each time point. *P < 0.001. C: Representative confocal micrographs showing the presence of MSCs or hAFSCs within the kidneys of mice with AKI at days 4, 8, and 21. Tubular epithelial cells were stained for cytokeratin (red). The CFSE-labeled and HLA-positive cells were detected as green fluorescence. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (blue).D: Representative confocal micrographs showing the absence of detection of CFSE-labeled or HLA-positive cells within the kidneys of control mice (Ctrl). Original magnification, ×400.
Comparison of Grow Factors and Cytokines Produced by Cultured hAFSCs and MSCs
To investigate the possible differential mechanism of repair induced by hAFSCs and MSCs, we investigated the secretion of cytokines involved in tissue repair by a multiplex cytokine assay (Figure 8A). Both cell types released high levels of vascular endothelial growth factor. The main difference in cytokine/growth factors produced by hAFSCs and MSCs were a higher release of hepatocyte growth factor and stromal derived factor-1 by MSCs with respect to hAFSCs and a higher release of LIF by hAFSCs with respect to MSCs. To evaluate whether the enhanced production of LIF may account for the biological activity of hAFSCs, we tested the effect of LIF blockade on TEC proliferation induced by the conditioned medium. Incubation of TECs with hAFSC- or MSC-derived conditioned medium promoted cell proliferation compared with that of control cells incubated with vehicle only (Figure 8B). When TECs were incubated with conditioned medium in the presence of LIF-blocking antibody, we observed a ∼50% decrease in proliferation of cells stimulated with hAFSC-derived conditioned medium. No effect was found with cells stimulated with MSC-derived conditioned medium.
Figure 8.
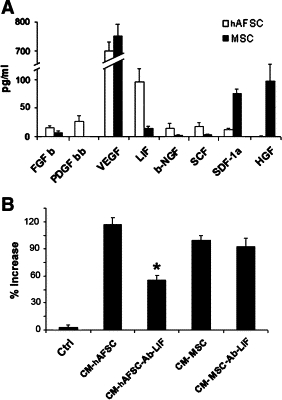
Cytokines/growth factors released into the conditioned medium of hAFSCs and MSCs and effects on tubular cell proliferation. A: Evaluation of cytokines/growth factors released into the conditioned medium of 1 × 106 hAFSCs and MSCs after a 12-hour incubation in RPMI 1640 plus 0.5% bovine serum albumin. Cytokines were measured using a multiplex cytokine array. Data represent the mean ± SD of five different cell lines. FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; LIF, leukemia inhibitory factor; NGF, nerve growth factor; SCF, stem cell factor; SDF, stromal derived factor; HGF, hepatocyte growth factor. B: Proliferation of TECs induced by the conditioned medium (CM) of hAFSCs and MSCs was evaluated by incorporation of BrdU and expressed as the percent increase over unstimulated control cells (Ctrl). The role of LIF in proliferation induced by the conditioned medium was evaluated using 3 μg/ml anti-LIF blocking antibodies (Ab-LIF). Data represent the mean ± SD of three different experiments performed in duplicate. Analysis of variance with Dunnett's comparison test: *P < 0.005, CM plus Ab-LIF versus CM alone.
Discussion
Amniotic fluid contains stem cells from the fetus, and it has been suggested that they could represent an ethically unproblematic alternative to embryonic stem cells for stem cell therapy.24,37 The results of the present study demonstrated that stem cells isolated from human amniotic fluid accelerate the recovery of murine experimental AKI similarly to MSCs. Treatment with hAFSCs improved renal function and increased the proliferative response in the tubular compartment of the kidney. However, when the effect of hAFSCs was compared with that of MSCs, despite a partial difference in growth factor production, a substantial overlap on the recovery from AKI was observed.
Extensive studies have been performed to investigate the role and potential of stem cells in AKI. Most of the research focused on bone marrow-derived stem cells (BMSCs) and in vitro expanded MSCs, which were applied to different mouse models of AKI.
By transplanting bone marrow to animals with subsequent induction of AKI, several groups were able to demonstrate the contribution of BMSCs in the regeneration after tubular injury.38–41 It has been suggested that BMSCs are recruited to the site of injury and contribute to regeneration.42 Whether BMSCs are able to trans-differentiate into tubular epithelial cells is still disputed.8,9
Much emphasis has been put on exogenous administration of MSCs, because of their greater therapeutic potential. MSCs can be differentiated into all renal cell types, as shown by injecting MSCs into a mouse whole-embryo culture.43 When injected into mice with cisplatin- or glycerol-induced AKI, MSC have been shown to accelerate the recovery.6,7,32 Moreover, MSCs also protected from ischemia-related AKI by differentiation-independent mechanisms.8,9,44 It has been suggested that the beneficial effect of BMSCs or exogenous MSCs is due to transient localization to the damaged renal structures. Lange et al44 injected MSCs labeled with iron-dextran particles into rats and detected MSCs in the kidney cortex of animals with ischemia-reperfusion injury and not in the healthy controls. In their study, labeled MSCs were predominantly found in the glomerular capillaries, whereas Tögel et al,9 using two-photon laser confocal microscopy, found MSCs to be localized in the glomerular and the peritubular capillaries of rats with ischemia-reperfusion injury.9 MSCs were also found to localize to the peritubular capillaries and further migrate to the tubular interstitium.32 The low number of MSCs that were found to engraft within the injured kidney suggests that MSCs exhibit a trophic effect on the injured tissue, thereby promoting the repopulation of the damaged tubular structures.
In the present study, we found that intravenous injection of hAFSCs accelerated the recovery of glycerol-induced AKI. Moreover, we compared this effect with that of MSCs. hAFSCs promoted histological and functional amelioration of renal damage comparable to that of MSCs. Moreover, both types of stem cells stimulated proliferation of tubular epithelial cells. However, the quantification of proliferating tubular cells by counting BrdU- or Pcna-positive cells revealed that the kinetics of proliferation induced by MSCs and hAFSCs was different. MSCs promoted an early tubular proliferation, whereas proliferation induced by hAFSCs was delayed and persisted up to 21 days after injury. In addition, hAFSCs exerted a pronounced antiapoptotic action on tubular cells, whereas MSCs were less effective.
We tried to explain the differences in the protective mechanisms observed for hAFSCs and MSCs by investigating the renal localization kinetics in vivo and the production of different soluble factors in vitro. hAFSCs persisted significantly longer within the renal tissue than MSCs. We found that neither hAFSCs or MSCs integrated into the renal tissue but rather remained localized into the interstitium. A similar result was reported previously by several authors for MSCs.35,42,44 The peritubular localization of stem cells suggests that the mechanism of tissue repair for both cell types is dependent on the local release of soluble factors rather than on tissue integration. These results differ from the recent study of Perin et al,45 who showed that 1 × 106 hAFSCs injected directly into the renal tissue of nu/nu mice with AKI are able to differentiate into cells expressing both adult proximal and distal tubular markers, thus contributing to renal regeneration. This discrepancy may depend on the amount of cells administered and on the modality of administration.
Analysis of cytokines and growth factors released by hAFSCs and MSCs into the conditioned medium revealed differences in the combination of the factors produced. In particular, hAFSCs produced less hepatocyte growth factor and stromal derived factor-1 than MSCs but more LIF. LIF has been shown to be an important factor in renal development as well as in the repair of the adult renal tissue after AKI.46 The different combination of growth factors may be the cause for the differences in proliferation and apoptosis of TEC after treatment with stem cells. Experiments of LIF blockade in the hAFSC conditioned medium indicate a potential critical contribution of this factor, as it significantly reduced proliferation of TECs stimulated with hAFSC conditioned medium.
Proliferation of resident epithelial cells is indeed considered the main repair mechanism, because it has been shown by genetic fate-mapping techniques in a model of ischemic tubular injury.47,48 The rapid postinjury onset of regenerative tubular growth could depend on the high number of proximal tubular cells found in the G1 phase in tubules.49
The paracrine/endocrine role of stem cells is supported by the evidence that MSC culturing media alone had a positive effect on regeneration after renal injury35 and by the finding that even adult stem cells from developmentally distant organs such as the brain hold the potential to contribute to renal regeneration.50 We recently found that the release of microvesicles and the transfer of genetic information may account for the beneficial effect of MSCs in the recovery of experimental AKI, further supporting a paracrine action.29
In conclusion, hAFSCs may provide an alternative source of stem cells for the treatment of AKI. However, although the mechanisms involved may be partially different, the efficacy of hAFSCs is not greater than that of MSCs, which represent a more accessible source of adult stem cells. Further studies in different models of AKI are needed to define the most suitable type of stem cell to be used in stem cell therapy of AKI.
Acknowledgements
We thank Federica Antico for technical assistance.
Footnotes
Supported by the Regione Piemonte, Biotechnology Platform, Targeted Project Pi-STEM and by Ministero Università e Ricerca Scientifica e Technologica. P.V.H. is a Marie Curie Fellow within the Kidstem Project, funded by the European Community.
References
- 1.Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390–2401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 2.Chhabra P, Brayman KL. The use of stem cells in kidney disease. Curr Opin Organ Transplant. 2009;14:72–78. doi: 10.1097/MOT.0b013e328320d2f5. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys BD, Duffield JS, Bonventre JV. Renal stem cells in recovery from acute kidney injury. Minerva Urol Nefrol. 2006;58:329–337. [PubMed] [Google Scholar]
- 5.Morigi M, Benigni A, Remuzzi G, Imberti B. The regenerative potential of stem cells in acute renal failure. Cell Transplant. 2006;15(Suppl 1):S111–S117. doi: 10.3727/000000006783982449. [DOI] [PubMed] [Google Scholar]
- 6.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041. [PubMed] [Google Scholar]
- 7.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 8.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 10.Humphreys BD, Bonventre JV. The contribution of adult stem cells to renal repair. Nephrol Ther. 2007;3:3–10. doi: 10.1016/j.nephro.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Condic ML. Alternative sources of pluripotent stem cells: altered nuclear transfer. Cell Prolif. 2008;41(Suppl 1):7–19. doi: 10.1111/j.1365-2184.2008.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastenberg ZJ, Odorico JS. Alternative sources of pluripotency: science, ethics, and stem cells. Transplant Rev (Orlando) 2008;22:215–222. doi: 10.1016/j.trre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Kuci S, Kuci Z, Latifi-Pupovci H, Niethammer D, Handgretinger R, Schumm M, Bruchelt G, Bader P, Klingebiel T. Adult stem cells as an alternative source of multipotential (pluripotential) cells in regenerative medicine. Curr Stem Cell Res Ther. 2009;4:107–117. doi: 10.2174/157488809788167427. [DOI] [PubMed] [Google Scholar]
- 14.Breymann C, Schmidt D, Hoerstrup SP. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev. 2006;2:87–92. doi: 10.1007/s12015-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 15.Graziano A, d'Aquino R, Laino G, Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21–26. doi: 10.1007/s12015-008-9013-5. [DOI] [PubMed] [Google Scholar]
- 16.Murohara T, Shintani S, Kondo K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr Pharm Des. 2009;15:2784–2790. doi: 10.2174/138161209788923796. [DOI] [PubMed] [Google Scholar]
- 17.Salgado AJ, Oliveira JT, Pedro AJ, Reis RL. Adult stem cells in bone and cartilage tissue engineering. Curr Stem Cell Res Ther. 2006;1:345–364. doi: 10.2174/157488806778226803. [DOI] [PubMed] [Google Scholar]
- 18.Antonucci I, Iezzi I, Morizio E, Mastrangelo F, Pantalone A, Mattioli-Belmonte M, Gigante A, Salini V, Calabrese G, Tete S, Palka G, Stuppia L. Isolation of osteogenic progenitors from human amniotic fluid using a single step culture protocol. BMC Biotechnol. 2009;9:9. doi: 10.1186/1472-6750-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- 20.Hoehn H, Salk D. Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods Cell Biol. 1982;26:11–34. doi: 10.1016/s0091-679x(08)61362-x. [DOI] [PubMed] [Google Scholar]
- 21.Orciani M, Emanuelli M, Martino C, Pugnaloni A, Tranquilli AL, Di Primio R. Potential role of culture mediums for successful isolation and neuronal differentiation of amniotic fluid stem cells. Int J Immunopathol Pharmacol. 2008;21:595–602. doi: 10.1177/039463200802100312. [DOI] [PubMed] [Google Scholar]
- 22.Prusa AR, Hengstschlager M. Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit. 2002;8:RA253–RA257. [PubMed] [Google Scholar]
- 23.Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, Warburton D, Atala A, De Filippo RE. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936–948. doi: 10.1111/j.1365-2184.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 25.Delo DM, De Coppi P, Bartsch G, Jr, Atala A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006;419:426–438. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- 26.Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- 27.Siegel N, Rosner M, Hanneder M, Freilinger A, Hengstschlager M. Human amniotic fluid stem cells: a new perspective. Amino Acids. 2008;35:291–293. doi: 10.1007/s00726-007-0593-1. [DOI] [PubMed] [Google Scholar]
- 28.Trovato L, De Fazio R, Annunziata M, Sdei S, Favaro E, Ponti R, Marozio L, Ghigo E, Benedetto C, Granata R. Pluripotent stem cells isolated from human amniotic fluid and differentiation into pancreatic β cells. J Endocrinol Invest. 2009;32:873–876. doi: 10.1007/BF03345764. [DOI] [PubMed] [Google Scholar]
- 29.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 31.Grange C, Bussolati B, Bruno S, Fonsato V, Sapino A, Camussi G. Isolation and characterization of human breast tumor-derived endothelial cells. Oncol Rep. 2006;15:381–386. [PubMed] [Google Scholar]
- 32.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 33.Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nephron Exp Nephrol. 2005;100:e40–e45. doi: 10.1159/000084111. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington, DC: 1996. [Google Scholar]
- 35.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 36.Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- 37.Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online. 2009;18(Suppl 1):17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- 38.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 39.Fang TC, Otto WR, Rao J, Jeffery R, Hunt T, Alison MR, Cook HT, Wright NA, Poulsom R. Haematopoietic lineage-committed bone marrow cells, but not cloned cultured mesenchymal stem cells, contribute to regeneration of renal tubular epithelium after HgCl2-induced acute tubular injury. Cell Prolif. 2008;41:575–591. doi: 10.1111/j.1365-2184.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 42.Yen TH, Alison MR, Cook HT, Jeffery R, Otto WR, Wright NA, Poulsom R. The cellular origin and proliferative status of regenerating renal parenchyma after mercuric chloride damage and erythropoietin treatment. Cell Prolif. 2007;40:143–156. doi: 10.1111/j.1365-2184.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci USA. 2005;102:3296–3300. doi: 10.1073/pnas.0406878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 45.Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, Lemley KV, Rosol M, Wu S, Atala A, Warburton D, De Filippo RE. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One. 2010;5:e9357. doi: 10.1371/journal.pone.0009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshino J, Monkawa T, Tsuji M, Hayashi M, Saruta T. Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol. 2003;14:3090–3101. doi: 10.1097/01.asn.0000101180.96787.02. [DOI] [PubMed] [Google Scholar]
- 47.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 50.Wang PH, Schwindt TT, Barnabe GF, Motta FL, Semedo P, Beraldo FC, Mazzali M, Dos Reis MA, Teixeira Vde P, Pacheco-Silva A, Mello LE, Camara NO. Administration of neural precursor cells ameliorates renal ischemia-reperfusion injury. Nephron Exp Nephrol. 2009;112:e20–e28. doi: 10.1159/000210575. [DOI] [PubMed] [Google Scholar]


