Abstract
Akt-mediated signaling plays an important role in blood vascular development. In this study, we investigated the role of Akt in lymphatic growth using Akt-deficient mice. First, we found that lymphangiogenesis occurred in Akt1−/−, Akt2−/−, and Akt3−/− mice. However, both the diameter and endothelial cell number of lymphatic capillaries were significantly less in Akt1−/− mice than in wild-type control mice, whereas there was only a slight change in Akt2−/− and Akt3−/− mice. Second, valves present in the small collecting lymphatics in the superficial dermal layer of the ear skin were rarely observed in Akt1−/− mice, although these valves could be detected in the large collecting lymphatics in the deep layer of the skin tissues. A fluorescence microlymphangiography assay showed that the skin lymphatic network in Akt1−/− mice was functional but abnormal as shown by fluorescein isothiocyanate-dextran draining. There was an uncharacteristic enlargement of collecting lymphatic vessels, and further analysis showed that smooth muscle cell coverage of collecting lymphatic vessels became much more sparse in Akt1-deficient mice than in wild-type control animals. Finally, we showed that lymphatic vessels were detected in compound Akt-null mice and that lymphangiogenesis could be induced by vascular endothelial growth factor-C delivered via adenoviral vectors in adult mice lacking Akt1. These results indicate that despite the compensatory roles of other Akt isoforms, Akt1 is more critically required during lymphatic development.
The lymphatic system provides an important conduit for the maintenance of tissue fluid homeostasis and transport of macromolecules as well as immune cells into the blood circulation and is also implicated in inflammation and tumor metastasis.1–6 During the last decade, extensive studies have been carried out in the field of lymphatic research, which have resulted in great insights into the molecular network underlying lymphatic development. Prox1, a homeobox transcription factor, has been shown to be a fate-determining factor for lymphatic endothelial cell differentiation,7,8 and its expression is controlled by Sox18.9 Activation of vascular endothelial growth factor receptor-3 signaling by its ligand vascular endothelial growth factor (VEGF)-C/-D plays crucial roles for lymphatic endothelial cell proliferation, migration, and survival during the formation of the lymphatic network.10,11 Various other genes have also been identified as participating in lymphatic development.2,5
The serine/threonine protein kinase Akt (also named PKB) acts as a major signal transducer downstream of phosphoinositide-3 kinase.12,13 Three isoforms of Akt exist in mammalian cells, including Akt1, Akt2, and Akt3, and are encoded by three separate genes, which have approximately 85% sequence identity.14 Akt can be activated by many angiogenic growth factors such as VEGF and angiopoietin-1, which are mediated by their corresponding receptor tyrosine kinases.15–18 It has been shown that VEGF-mediated activation of Akt is critical for vessel patterning in zebrafish19 and vascular development of the chorioallantois in chick embryos.20 Although Akt plays an important role in blood vascular development including cell survival and cell growth and differentiation by orchestrating a number of signaling pathways in endothelial cells,13,21 the contribution of each Akt isoform in this process has not been fully elucidated. A recent study has demonstrated that Akt1 is the predominant isoform in mouse blood vascular endothelial cells, and endothelial cell proliferation and migration in response to VEGF were impaired when Akt1 was deleted.22
So far, the role of Akt-mediated signaling in lymphatic development has been poorly understood. Mice homozygous for Pik3r1, which encodes p85α as well as two smaller variants (p55α and p50α) acting as regulatory subunits of class IA phosphoinositide 3-kinases, were shown to have chylous ascites accumulation into the abdomen after birth.23 Further investigation demonstrated that there was defective lymphatic development and valve formation in Pik3r1−/− mice.24 In this study, we further show that Akt can be activated on VEGF-C induction in primary lymphatic endothelial cells. Although lymphatic vessel growth occurred in the single Akt knockout mice (Akt1−/−, Akt2−/−, or Akt3−/−), loss of Akt1 led to abnormal lymphatic development including the reduced lymphatic endothelial cell numbers and vessel size, defective valve development, and smooth muscle cell coverage of collecting lymphatics. This result indicates that Akt1 is the major isoform in mediating signals for lymphatic development.
Materials and Methods
Knockout Models
Akt1, Akt2, and Akt3 knockout mice were generated as described previously25–27 and backcrossed into the C57BL/6 background. All animal experiments were performed in accordance with the institutional guidelines of the Model Animal Research Institute of Nanjing University.
Cell Culture
NCI-H460 cells were obtained from American Type Culture Collection (Manassas, VA) and were maintained in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 2 mmol/L l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal calf serum (PromoCell, Heidelberg, Germany). Primary human lymphatic endothelial cells (hLECs) were isolated from human foreskin as described previously.28 hLECs were cultured in 10 μg/ml fibronectin-coated plates with endothelial growth medium containing growth supplements from Promocell.
Production of Recombinant Adenoviruses and in Vivo Delivery of VEGF-C Via Adenoviral Vector
The cDNA encoding human VEGF-C was cloned into the transfer vector (pShuttle-IRES-EGFP) of the AdEasy vector system (Qbiogene, Carlsbad, CA). Recombinant adenoviruses (AdVEGF-C/GFP and AdGFP) were produced following the manufacturer's instruction. NCI-H460 cells used for expression analysis were transduced with AdVEGF-C/GFP or AdGFP (multiplicity of infection 50). Expression of VEGF-C was confirmed by Western blot analysis of the conditioned medium as described previously,29 and green fluorescent protein (GFP) expression by the examination under a fluorescence microscope.
Approximately 2 × 108 plaque-forming units of AdVEGF-C/GFP or AdGFP control viruses were injected subcutaneously into mouse ears. Tissues were collected for histological analysis within 2 weeks after adenoviral transduction.
Immunohistochemistry
Antibodies used in this study included rabbit anti-mouse LYVE-1 (Abcam Inc., Cambridge, MA) or rat anti-mouse LYVE-1 (a kind gift from Dr. Gou Young Koh, KAIST, Daejeon, Republic of Korea), rabbit anti-human Prox1 (Abcam, and a kind gift from Dr. Tatiana V. Petrova, University of Lausanne, Lausanne, Switzerland), goat anti-mouse Integrin-α9 (R&D Systems, Minneapolis, MN), rabbit anti-human phosphorylated endothelial nitric oxide synthase (eNOS) (Upstate Biotechnology, Charlottesville, VA), hamster anti-mouse platelet/endothelial cell adhesion molecule-1 (PECAM-1) (Millipore Corporation, Billerica, MA), or monoclonal rat anti-mouse PECAM-1 (PharMingen, San Diego, CA), and Cy3-conjugated mouse anti-mouse α-smooth muscle actin (Sigma-Aldrich). For whole-mount staining of blood vascular and lymphatic vessels, tissues were fixed in 4% paraformaldehyde, blocked with 3% milk and 0.3% Triton X-100 in PBS, and incubated with antibodies against LYVE-1 and PECAM-1 overnight at 4°C. Alexa 594-, Alexa 488-, or Cy5-conjugated secondary antibodies (Molecular Probes, Eugene, OR) were used for staining, and samples were then mounted with Vectashield (Vector Laboratories, Burlingame, CA) and analyzed with a Leica confocal microscope.
For staining of tissue sections, tissues were fixed in 4% paraformaldehyde overnight at 4°C, and paraffin sections (6 μm) were immunostained with antibodies as described above. The signals were amplified using the tyramide signal amplification system (PerkinElmer Life and Analytical Sciences, Waltham, MA). Peroxidase activity was developed using 3-amino-9-ethyl carbazole (Sigma-Aldrich), and sections were counterstained with hematoxylin before analysis. For staining of cryosections, tissues were fixed in 4% paraformaldehyde for 2 hours on ice, incubated in 20% sucrose/PBS overnight and embedded in O.C.T. compound (Tissue-Tek, Sakura Finetek USA, Inc., Torrance, CA). Sections (10 μm) were used for immunostaining as described above.
Cell Proliferation Analysis
Mice were injected with BrdU (0.1 mg/g b.wt., Sigma-Aldrich) 2 hours before sample collection. Skin tissues were collected and processed for histological analysis. 5-Bromo-2′-deoxyuridine (BrdU) incorporation was detected using mouse anti-BrdU (Roche Diagnostics, Indianapolis, IN) antibody, followed by staining with Alexa 488-conjugated secondary antibody (Roche Diagnostics). Lymphatic endothelial cells were identified by immunostaining for Prox1 as described above. BrdU+/Prox1+ cells were examined under a fluorescence microscope and quantified.
Western Blot Analysis
hLECs were starved overnight in serum-free basal medium and then were stimulated for 30 minutes with 100 ng/ml recombinant human VEGF-C (R&D Systems). Cells were lysed in the lysis buffer (1% Nonidet P-40, 20 mmol/L Tris-HCl [pH 7.5], 150 mmol/L NaCl, 5 mmol/L EDTA, 2 mmol/L Na3VO4, 100 μmol/L phenylmethylsulfonyl fluoride with proteinase inhibitor cocktail [Roche Diagnostics]) and cleared of particulate material by centrifugation. Total lysates were analyzed by SDS-polyacrylamide gel electrophoresis and blotted with antibodies against Akt or phospho-Akt (Cell Signaling Technology, Danvers, MA).
Quantification of Lymphatic Diameter
For the quantification of lymphatic capillary size in ear skin, eight images (with ×100 magnification) representing different regions of whole ear skin (bottom side) were taken under a fluorescence microscope and kept constant for all of the samples. Five horizontal lines were evenly laid on the images, and the diameters of lymphatic vessels crossed with these lines were measured and analyzed using Image Pro Plus (MediaCybernetics, Inc., Bethesda, MD).
Fluorescence Microlymphangiography
The functionality of the lymphatic network in Akt-null mice was assayed by fluorescence microlymphangiography using fluorescein isothiocyanate (FITC)-conjugated dextran (FITC-dextran 2000, Sigma-Aldrich). FITC-dextran (2 μl) was injected intradermally into mouse ears or tails. The lymphatic vessels were examined using a fluorescence dissection microscope (MZFLIII, Leica, Wetzlar, Germany).
Statistics
Statistical analyses were performed with the unpaired Student's t-test. All P values are two-tailed.
Results
Alteration of Lymphatic Capillary Size in Akt1−/− Mice
To investigate the role of Akt in lymphatic development, we examined the skin sections from adult Akt1−/−, Akt2−/−, and Akt3−/− and wild-type (WT) control mice by immunostaining for the lymphatic marker LYVE-1.30 Compared with the WT control (Figure 1A), in the single Akt gene knockout mice lymphatic vessels could be detected (Figure 1, B–D), and there was no obvious difference in lymphatic vessel number. However, whole-mount immunostaining with ear skin revealed that the diameter of capillary lymphatics in Akt1−/− mice was significantly less than that of the WT control mice (Figure 1, E–H). The size of the lymphatic capillaries was quantified using Image-Pro Plus (MediaCybernetics, Inc.). As shown in Figure 1I, the diameter of skin lymphatic capillaries in Akt1−/− mice was approximately half of that in WT mice. Lymphatic size was also slightly less in Akt2−/− and Akt3−/− mice than that of the wild-type control. Consistently, the change in lymphatic capillary size was also seen in internal organs such as the intestine of Akt1-null mice (Supplemental Figure 1, see http://ajp.amjpathol.org). We also examined blood vessels of skin in Akt1−/− mice by whole-mount immunostaining for pan-endothelial cell marker PECAM-1. As shown in Supplemental Figure 2 (see http://ajp.amjpathol.org), there was no obvious difference in blood vessel diameter between the Akt1−/− and WT mice.
Figure 1.
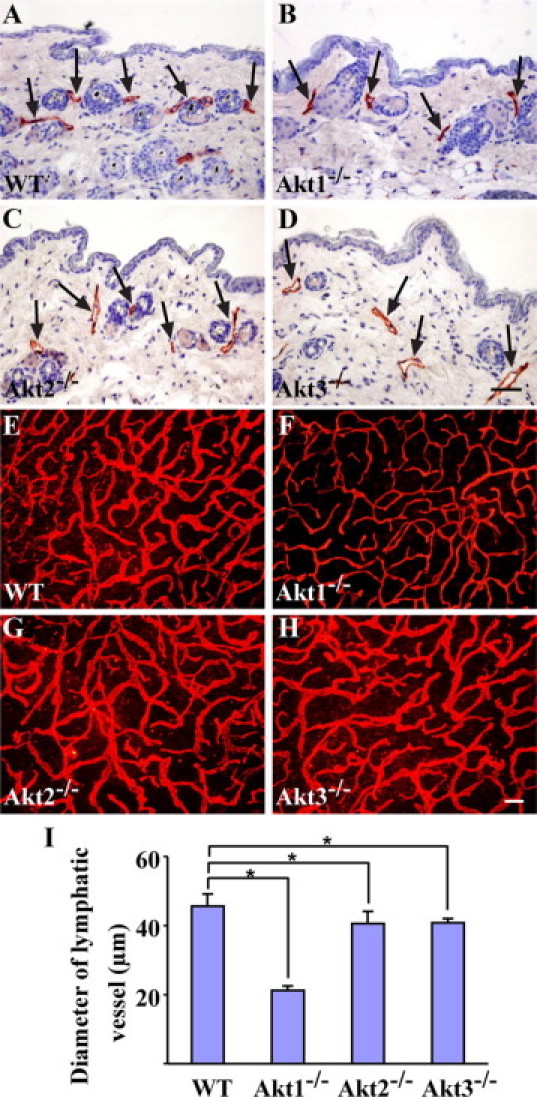
Histological analysis of lymphatic vessels in single Akt knockout mice. A–D: Immunohistochemical staining for LYVE-1 with skin sections of WT (A), Akt1−/− (B), Akt2−/− (C), and Akt3−/− mice (D). Arrows point to lymphatic vessels. E–H: Whole-mount staining for LYVE-1 with ear skins of WT (E), Akt1−/− (F), Akt2−/− (G), and Akt3−/− mice (H). I: Quantification of lymphatic vessel diameters from WT, Akt1−/−, Akt2−/−, and Akt3−/− mice (WT: 45.7 ± 3.5 μm; Akt1−/−: 21.2 ± 1.4 μm, P < 0.0001; Akt2−/−: 40.5 ± 3.6 μm, P = 0.0295; Akt3−/−: 40.8 ± 1.2 μm, P = 0.0088; mean ± SD; n = 6 for each group). *P < 0.05. Scale bars: 50 μm (D); 200 μm (H).
To find out the reason for the alteration of lymphatic vessel diameter in Akt1-null mice, we analyzed the lymphatic endothelial cell (LEC) number in skin lymphatics by immunostaining for LYVE-1 and Prox1 (Figure 2, A–F). Quantification of LEC number revealed that total LECs per grid was about half in lymphatic capillaries of Akt1-null mice compared with that of control mice (Figure 2G). The difference remained the same after the normalization of LEC number by lymphatic length (LECs/per 100-μm lymphatic vessel) (Figure 2H). We further examined lymphatic endothelial cell proliferation by BrdU labeling and immunostaining for Prox1 with skin tissues at postnatal day 1. There was only a small trend toward the decrease of the percentage of BrdU+ lymphatic endothelial cells (BrdU+/Prox1+) in Akt1-null mice (Supplemental Figure 3, see http://ajp.amjpathol.org). Apoptotic lymphatic endothelial cells were also hardly observed by terminal deoxynucleotidyl transferase dUTP nick-end labeling staining in both WT and Akt1-null mice (data not shown). In addition, we analyzed the eNOS expression and its phosphorylation status in lymphatic vessels by immunostaining, and there was no obvious difference in the total eNOS (data not shown) and phosphorylated eNOS levels (Supplemental Figure 4, see http://ajp.amjpathol.org) between Akt1 deficient and control mice.
Figure 2.
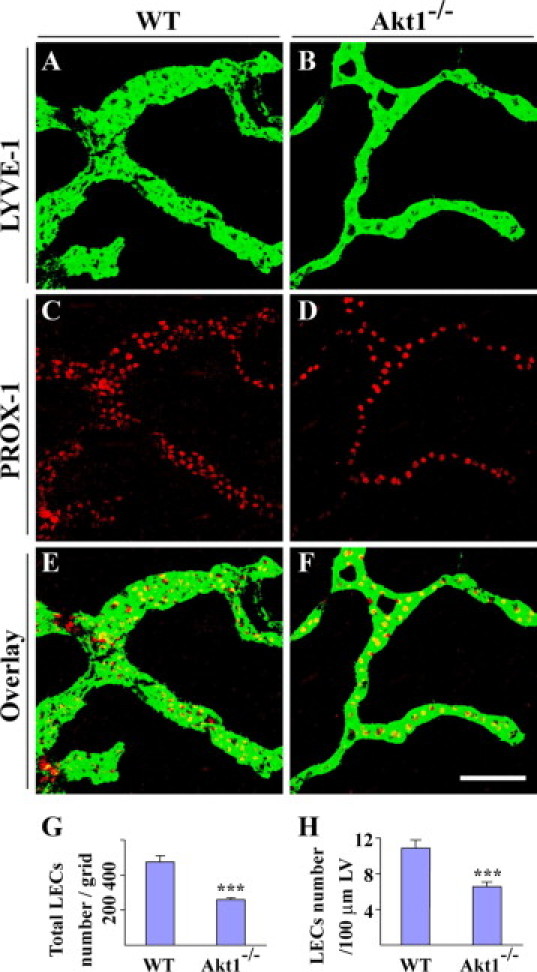
Fewer endothelial cell numbers in lymphatic capillaries of Akt1−/− mice. A–F: Whole-mount staining for LYVE-1 (green) and PROX1 (red) with ear skins of WT (A, C, and E) and Akt1−/− mice (B, D, and F). G: Quantification of total LEC number in WT and Akt1−/− mice (WT: 473.7 ± 34.7 cells/grid; Akt1−/−: 258.6 ± 11.7 cells/grid; mean ± SD; n = 6 for each group; P < 0.0001). H: Quantification of LEC numbers per 100-μm lymphatic vessel (LV) in WT and Akt1−/− mice (WT: 11.0 ± 0.9 cells, Akt1−/−: 6.6 ± 0.5 cells; mean ± SD; n = 6 for each group; P < 0.0001). ***P < 0.0001. Scale bar: 100 μm.
Lack of Valve Formation in Small Collecting Lymphatics of Akt1-Null Mice
We further examined lymphatic valve formation in Akt1-null mice. The bottom side of ear skin adjacent to cartilage contains mainly lymphatic capillaries and small collecting lymphatics, and large collecting lymphatics are present in the deep layer of tissues at the upper side of ear skin. Analysis of ear skin from wild-type mice by whole-mount immunostaining with PECAM-1 showed that lymphatic valves were strongly stained although lymphatic vessels were faintly positive for PECAM-1 (Figure 3, A, B, F, and G, arrows). There was also sparse smooth muscle cell coverage with valve-containing small collecting lymphatics (Figure 3C). Note that regions with smooth muscle cell (SMC) coverage in lymphatic vessels were negative for LYVE-1 staining (Figure 3, C–E, arrowheads). Interestingly the valves and smooth muscle cell coverage were rarely observed in lymphatic vessels in the corresponding area of Akt1−/− mice (Figure 3, F–J). The absence of lymphatic valves was validated by immunostaining for LYVE-1 (Figure 3, M, N, S, and T) and integrin-α9 (Figure 3, K, L, Q, and R), a marker for lymphatic valves.31 Consistently, there were integrin-α9 positive structures observed in the lymphatic vessels at the bottom side of ear skin in wild-type mice (Figure 3, K and O, arrows), but these were rarely observed in Akt1−/− mice (Figure 3, L and P). However, valves could be detected in large collecting lymphatics in the deep layer of tissues at the upper side of ear skin in both wild-type (Figure 3, Q and U, arrows) and Akt1-null mice (Figure 3, R and V, arrows).
Figure 3.
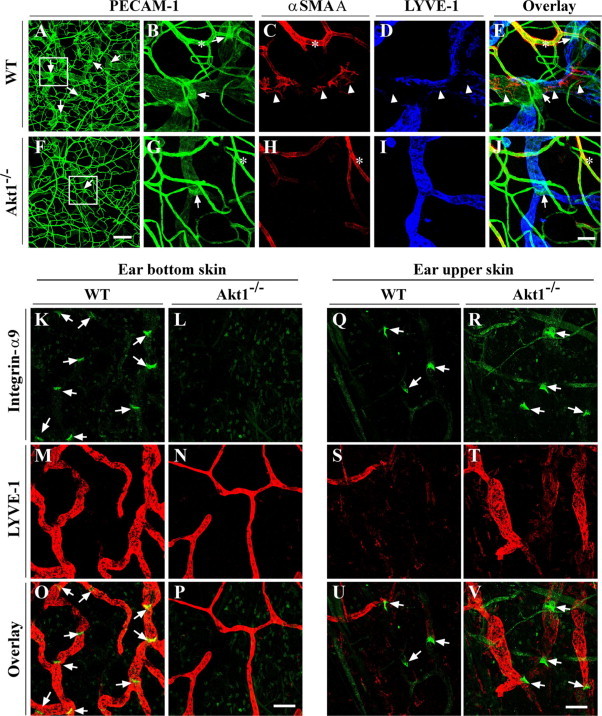
Defective lymphatic valve formation in Akt1-deficient mice. A–J: Whole-mount staining for PECAM-1, α-smooth muscle actin (SMA), and LYVE-1 with ear skin of WT and Akt1−/− mice. B–E and G–J: High-magnification view of boxed regions in A and F, respectively. Arrows indicate lymphatic valves with strong PECAM-1 staining; arrowheads indicate regions in lymphatic vessels with SMC coverage, which are negative for LYVE-1 staining; asterisk indicates blood vessels with smooth muscle cell coverage. K–V: Whole-mount staining for LYVE-1 and integrin-α9 with ear skin of WT and Akt1−/− mice. K–P: At the bottom side of ear skin adjacent to cartilage, integrin-α9-positive lymphatic valves can be seen in WT (K and O) but not Akt1−/− mice (L and P). Q–V: In both WT (Q and U) and Akt1−/− mice (R and V), integrin-α9-positive valves can be seen in the large collecting lymphatic vessels in the deep layer of skin tissues at the upper side of the ear. Arrows point to the valves. Scale bars: 200 μm (F); 50 μm (J); 100 μm (P and V).
Defective Remodeling of Collecting Lymphatic Vessels in Akt1-Deficient Mice
To examine the functionality of the lymphatic vessels in Akt1-null mice, FITC-dextran was injected intradermally for the visualization of lymphatic draining. The lymphatic network in Akt1-null mice was shown to have the draining function (Figure 4B) compared with that of wild-type mice (Figure 4A). However, there was an obvious delay with FITC-dextran draining as demonstrated in the tails of Akt1-null mice (Supplemental Figure 5, see http://ajp.amjpathol.org). There were no functional defects observed with collecting lymphatic vessels in Akt2- or Akt3-null mice (data not shown). We also found that some regions of collecting lymphatic vessels appeared abnormally enlarged in Akt1-null mice (Figure 4B, arrowhead). The enlargement of collecting lymphatic vessels in Akt1−/− mice was further confirmed by whole-mount immunostaining of ear skin for PECAM-1 and LYVE-1 (Figure 4, D, F, and H, arrows), and collecting lymphatic vessels of wild-type mice are shown as controls in Figure 4, C, E, and G (arrows). The enlargement of collecting lymphatics was accompanied by a significant increase in lymphatic endothelial cell numbers (WT: 16.8 ± 1.7 cells/100 μm lymphatic vessel; Akt1−/−: 25.3 ± 5.9 cells/100 μm lymphatic vessel, mean ± SD; n = 4 for each group; P < 0.05). This may result from the lack of contact inhibition of endothelial cell proliferation by vascular smooth muscle cells.
Figure 4.
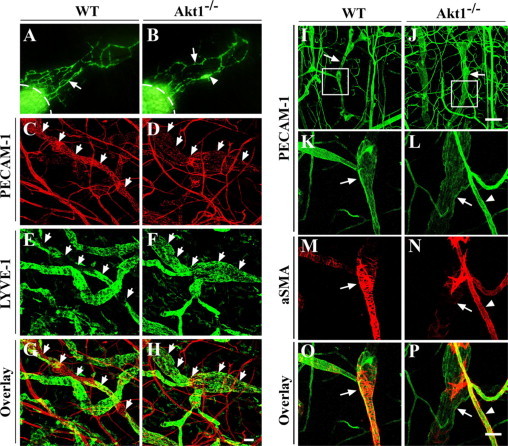
Defective smooth muscle cell coverage with collecting lymphatic vessels in Akt1-null mice. A and B: FITC-dextran microlymphangiography to examine the functionality of the collecting lymphatic vessels in ear skin of WT (A) and Akt1−/− mice (B). Arrows in A and B point to functional collecting lymphatic vessels. Arrowhead in B points to an enlarged region of the collecting lymphatic vessels in Akt1-null mice. Dashed lines indicate the injection sites. C–H: Whole-mount immunostaining for PECAM-1 (red) and LYVE-1 (green) with ear skin of WT (C, E, and G) and Akt1−/− (D, F, and H) mice. Arrows point to collecting lymphatic vessels. I–P: Whole-mount immunostaining for PECAM-1 and α-smooth muscle actin (aSMA) with ear skin from WT and Akt1−/− mice. K–P: High-magnification view of boxed regions in I and J, respectively. Arrows indicate collecting lymphatic vessels with SMC coverage. Arrowheads indicate blood vessels with normal SMC coverage. Note that there is much sparser SMC coverage with collecting lymphatic vessels in Akt−/− mice in comparison with that of the WT control. Scale bars: 50 μm (H); 200 μm (J); 50 μm (P).
Further analysis by immunostaining for α-smooth muscle actin showed that there was much sparser smooth muscle cell coverage with collecting lymphatics in Akt1-deficient mice (Figure 4, J, L, N and P, arrows) than that in wild-type mice. Note that there was dense coating of smooth muscle cells in large collecting lymphatics except in the valve region in wild-type mice (Figure 4, I, K, M and O, arrows). There were no obvious defects with SMC investment with blood vessels in Akt1-null mice (Figure 4, L, N, and P, arrowhead).
Induction of Lymphangiogenesis by VEGF-C in Adult Mice Lacking Akt1
We further analyzed the expression of Akt isoforms in lymphatic endothelial cells and activation of Akt by VEGF-C stimulation. In cultured human lymphatic endothelial cells, all three Akt isoforms can be detected at the mRNA level by RT-PCR analysis (data not shown). Treatment with VEGF-C induced an increase of Akt phosphorylation in lymphatic endothelial cells (Figure 5A).
Figure 5.
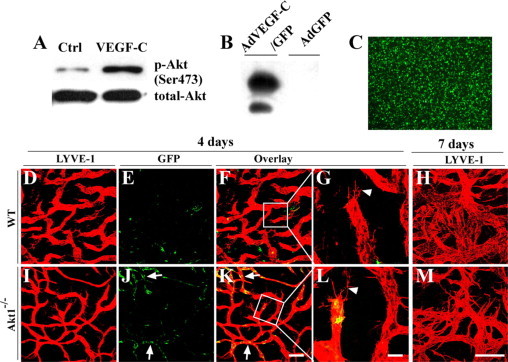
Induction of lymphangiogenesis with VEGF-C delivered by adenoviral vectors in adult mice lacking Akt1. A: Cultured human LECs were starved overnight and then stimulated with recombinant human VEGF-C. Cells were then harvested for Western blot analysis of total Akt and phosphorylated Akt. B and C: Western blot analysis of VEGF-C (B) in the conditioned medium of NCI-H460 cells transduced with AdVEGF-C/GFP, and expression of GFP was examined under a fluorescence microscope (C). D–M: Whole-mount immunostaining for LYVE-1 (red) with the mouse ear skin at day four (D–G and I–L) or day seven (H and M) after transduction with AdVEGF-C/GFP. Arrows in J and K indicate the GFP+ lymphatic endothelial cells, and arrowheads in G and L point to the lymphatic sprouts. Scale bars: 200 μm (K); 50 μm (L); 100 μm (M).
To investigate whether the loss of Akt would have any effect on lymphangiogenesis in adults, mice were treated with VEGF-C delivered via adenoviral vector. Recombinant adenoviruses overexpressing human VEGF-C and GFP (AdVEGF-C/GFP) were examined for VEGF-C expression by Western blot analysis (Figure 5B), and GFP expression was confirmed under a fluorescence microscope (Figure 5C). Induction of lymphangiogenesis by AdVEGF-C/GFP in vivo was analyzed by whole-mount immunostaining of mouse ear skin for LYVE-1 after the administration of recombinant adenoviruses via subcutaneous injection. VEGF-C induced numerous lymphatic sprouts by day 4 (Figure 5, D–G and I–L, arrowhead), and massive lymphatic vessel growth by day 7 (Figure 5, H and M), after adenoviral transduction in both Akt1-null (Figure 5, I–M) and wild-type mice (Figure 5, D–H). It is noteworthy that there are more GFP+ lymphatic endothelial cells in the Akt1-null mice (Figure 5, J–L, arrows) after AdVEGF-C/GFP treatment. This result suggests that there may be more adenoviruses retained in the skin lymphatic vessels of Akt1-null mice, resulting in increased adenoviral transduction of LECs. This finding supports the above observation (Supplemental Figure 5, see http://ajp.amjpathol.org) that the lymphatic draining function in Akt1−/− mice is not as efficient as that of wild-type controls.
Detection of Lymphatic Vessels in Akt Compound Knockout Mice
To further investigate the compensatory role of Akt isoforms in lymphatic development, we analyzed lymphatic vessels in Akt compound knockout mice. Mice with deletion of both Akt2 and Akt3 (Akt2−/−/Akt3−/−) survived well, and there were no obvious defects in the formation of the lymphatic network (Figure 6, A and C). Because mice with deletion of both Akt1 and Akt3 (Akt1−/−/Akt3−/−) are embryonic lethal before the emergence of lymphatic vessels,32 we analyzed mice homozygous for Akt1 deletion and heterozygous for Akt3 deletion (Akt1−/−/Akt3+/−). Consistent with the phenotype of Akt1-null mice, the lymphatic capillary size was less than that of wild-type control (Figure 6, B and D), and there was no obvious difference between Akt1−/−/Akt3+/− and Akt1−/− mice. Lymphatic vessels could be detected in the skin of Akt1−/−/Akt2−/− mice at postnatal day 1 (Figure 6E), although they died shortly after birth,33 and skin tissues from Akt1+/−/Akt2+/− mice were used as control (Figure 6F). The results suggest that Akt1 is the major player in lymphatic development, whereas the Akt2 or Akt3 isoform can partially compensate for its loss during lymphatic development, as summarized in the schematic illustration (Figure 6G).
Figure 6.
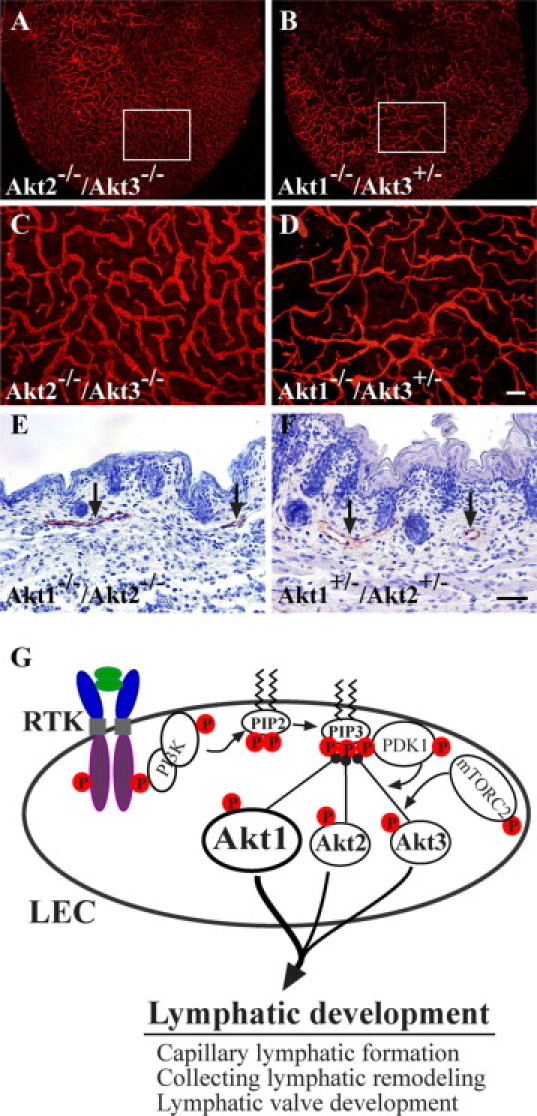
Compensatory roles of Akt isoforms in lymphatic development. A–D: Whole-mount immunostaining for LYVE-1 with ear skin of compound knockout mice (Akt2−/−/Akt3−/− [A] and Akt1−/−/Akt3+/− [B]). C and D: High-magnification view of the boxed regions in A and B, respectively. E and F: Immunohistochemical staining for LYVE-1 with skin sections from Akt1−/−/Akt2−/− (E) and Akt1+/−/Akt2+/− (F) at postnatal day one. Arrows point to lymphatic vessels. G: Schematic illustration to summarize the roles of Akt in lymphatic development. In LECs, three Akt isoforms are expressed, and Akt can be activated downstream of various receptor tyrosine kinases (RTK). Akt1 is the most required isoform in lymphatic development although Akt2 or Akt3 can partially compensate for its absence. Scale bars: 200 μm (D); 50 μm (F).
Discussion
In this study we have investigated the role of Akt in lymphatic development. We show that lymphangiogenesis occurred in mice null for Akt1, Akt2, or Akt3 during embryonic development and also in adult mice when induced by VEGF-C. However, loss of Akt1 led to reduced size in lymphatic capillary vessels and defects in smooth muscle cell coverage with collecting lymphatic vessels and valve development, whereas there was no obvious abnormality observed in mice with deletion of either Akt2 or Akt3 or both Akt2 and Akt3. This finding indicates that despite the partial compensatory role of Akt isoforms in lymphatic development, Akt1 is the most required isoform in lymphangiogenesis.
In mammals, three Akt genes exist, and they compensate for each other in a variety of biological functions because of their high sequence identity and ubiquitous expression.32,34–38 Consistently, we have found that lymphatic vessels could be detected in mice with genetic deletion of a single Akt isoform or with combined deficiency of Akt isoforms. There were no obvious defects with the lymphatic network observed in Akt2−/−/Akt3−/− mice. However, abnormalities in lymphatic formation were observed when Akt1 is absent. The diameter of capillary lymphatic vessels is significantly less in Akt1−/− mice than that in wild-type controls. The findings indicate that Akt1 alone is sufficient for maintaining lymphatic development, whereas Akt2 or Akt3 can only partially compensate for its loss. Akt1 has been shown to be the main isoform in blood vascular endothelial cells.22 The dominant role of Akt1 in lymphatic vessel growth suggests that Akt1 may also be the major isoform in lymphatic endothelial cells although transcripts for three Akt genes can be detected in LECs by RT-PCR (data not shown). It is therefore likely that the survival signal for LECs may be attenuated in Akt1-null mice, which leads to the reduced LEC number and capillary size. However, apoptotic lymphatic endothelial cells could hardly be detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling staining in both wild-type and Akt1-null mice (data not shown). There was only a slight decrease in lymphatic endothelial proliferation by BrdU labeling in Akt1-null mice, and the difference was not statistically significant compared with that in wild-type controls (Supplemental Figure 2, see http://ajp.amjpathol.org). Although eNOS has been shown to be the immediate signaling molecule downstream of Akt,39,40 loss of Akt1, Akt2, or Akt3 alone does not seem to have a strong effect on eNOS phosphorylation. We show that phospho-eNOS could be detected in lymphatic endothelial cells of Akt1−/− mice and other Akt isoform-deficient mice, and there was no obvious difference in the level of phosphorylated eNOS by immunostaining between Akt1-null and wild-type mice (Supplemental Figure 3, see http://ajp.amjpathol.org). This may be due to the compensatory mechanisms by the remaining Akt isoforms and/or other protein kinases such as protein kinase A.22,41 Compensation of Akt isoforms in lymphatic growth was also validated by the occurrence of lymphangiogenesis on VEGF-C induction in adult Akt1-null mice. Furthermore, Akt has the potential to phosphorylate multiple substrates.42 It is possible that several molecular pathways are disrupted in lymphatic endothelial cells of Akt1-null mice, which altogether contributes to the defects observed with lymphatic development. The details about altered downstream signals in lymphatic endothelial cells in the absence of Akt1 or other Akt isoforms are yet to be investigated.
In addition to the decrease in lymphatic capillary size, there were also remodeling defects with collecting lymphatic vessels. We observed that the skin collecting lymphatic vessels of Akt1-null mice were abnormally enlarged. Immunohistochemical analysis showed that there was insufficient coverage of collecting lymphatic vessels by smooth muscle cells in Akt1-deficient mice. Impaired SMC recruitment to the collecting lymphatic vessels has been shown in Ang2-null mice.43 On the other hand, ephrinB2 has been shown to be expressed by collecting lymphatic endothelium, and the abnormal coverage of SMC with capillary lymphatics has been shown in ephrinB2-mutant mice with loss of the C-terminal PDZ interaction site.44 An increase in SMC coverage with lymphatic vessels has also been shown in Foxc2-deficient mice, because of the increased platelet-derived growth factor-B expression by lymphatic endothelial cells.45 It is not clear how these distinct signaling pathways coordinate to regulate lymphatic vessel remodeling and maturation. However, Akt has been shown to be an important mediator downstream of various receptor protein kinases such as Tie2, platelet-derived growth factor receptor-β, and EphB4, which are critically required for vascular remodeling and maturation involving vascular differentiation and mural cell recruitment and its interaction with endothelial cells for stabilizing vasculature.15,18,46–50 This finding suggests that Akt plays an important role in mediating the signals for the remodeling of primary lymphatic vessels into a mature network and that defects with lymphatic remodeling may be due to the insufficient recruitment of SMCs or poor interactions between SMCs and LECs.
Interestingly, in this study, we also observed that lymphatic valves are rarely seen in the small collecting lymphatic vessels but are present in large ones in Akt1−/− mice. Lymphatic valves are tiny semilunar structures, which are important for preventing lymph backflow during their contraction. Formation of vascular valves involves several important cellular events including endothelial to mesenchymal transformation followed by cell proliferative expansion, migration, and remodeling into valve leaflets.51 So far there is little understanding about the molecular regulation of lymphatic valve development. Defective development of lymphatic valves has also been reported in mice with genetic deletion of the Foxc2 gene,45 in ephrinB2 mutant mice,44 in mice with endothelial cell-specific deletion of Itga9,31 and in Pik3r1-deficient mice.24 Based on this study and findings of other researchers, it seems that the phosphoinositide-3 kinase-Akt pathway plays an important role in lymphatic valve development. The detailed cellular and molecular mechanisms underlying this process require further investigation.
Finally, there was no obvious change in blood vessel size by whole-mount immunostaining for PECAM1 with ear skin (Supplemental Figure 2, see http://ajp.amjpathol.org) and internal organs such as retina (data not shown) of Akt1-null mice, although the blood vessel basement membrane was reported to be thinner with reduced laminin content.22 This finding is consistent with studies by other researchers showing that Akt1 is not essential for blood vascular growth during embryonic development because Akt1-null mice are viable, but rather it has an important role during pathological angiogenesis.22,52–54 Our findings are also in agreement with the observation that there is no abnormal blood vessel growth observed in Pik3r1-null mice despite defective lymphatic development.24 In addition to the difference in molecular profiles between blood vascular and lymphatic endothelial cells,55,56 the distinct phenotype in response to the loss of Akt1 could also be related to the structural difference between blood vascular and lymphatic capillaries. In blood vessels, there is continuous basement membrane and also supporting pericytes, whereas lymphatic capillaries do not have supporting cells and the basement membrane is discontinuous. The LECs may be more sensitive to the Akt-mediated signaling for survival than blood vascular endothelial cells.
To summarize, loss of Akt1 led to the reduced size of lymphatic capillaries and defects in the maturation of collecting lymphatic vessels and valve development, and Akt2 or Akt3 could partially compensate for its loss during lymphatic development. The distinct requirement of Akt isoforms in vascular development may reflect their differential expression levels in endothelial cells. However, it is also possible that there may exist Akt isoform-specific substrates in different types of cells. Further investigation in this direction would help us understand the differential requirement of Akt in blood vascular and lymphatic development.
Acknowledgements
We thank Yanlan Cao, Dong Liang (Nanjing University), and the staff of the MARC Animal Facility (Nanjing University) for excellent technical assistance and help with animal work. The Friedrich Miescher Institute for biomedical research is part of the Novartis Research Foundation.
Footnotes
Supported by the National Natural Science Foundation of China (grants 30771069, 30671038, and 30930028), the National Key Basic Research Program of China (grant 2006CB943502), and the Ministry of Education of China (NCET: Program for New Century Excellent Talents in University).
F.Z., Z.C., and L.Z. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Contributor Information
Zhongzhou Yang, Email: yangzz@nicemice.cn.
Yulong He, Email: yhe20005@yahoo.com.
Web Extra Material
References
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 4.He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 6.Baluk P, McDonald DM. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann NY Acad Sci. 2008;1131:1–12. doi: 10.1196/annals.1413.001. [DOI] [PubMed] [Google Scholar]
- 7.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 8.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Françcois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 10.Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 11.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 12.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 13.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 14.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: aKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 16.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 17.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 19.Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 20.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85α. Nat Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- 24.Mouta-Bellum C, Kirov A, Miceli-Libby L, Mancini ML, Petrova TV, Liaw L, Prudovsky I, Thorpe PE, Miura N, Cantley LC, Alitalo K, Fruman DA, Vary CP. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85α, p55α, and p50α. Dev Dyn. 2009;238:2670–2679. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase Bα/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 26.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 27.Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–1859. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tammela T, He Y, Lyytikka J, Jeltsch M, Markkanen J, Pajusola K, Yla-Herttuala S, Alitalo K. Distinct architecture of lymphatic vessels induced by chimeric vascular endothelial growth factor-C/vascular endothelial growth factor heparin-binding domain fusion proteins. Circ Res. 2007;100:1468–1475. doi: 10.1161/01.RES.0000269043.51272.6d. [DOI] [PubMed] [Google Scholar]
- 30.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of Akt1/protein kinase Bα (PKBα) and Akt3/PKBγ on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones PF, Jakubowicz T, Hemmings BA. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991;2:1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altomare DA, Guo K, Cheng JQ, Sonoda G, Walsh K, Testa JR. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11:1055–1060. [PubMed] [Google Scholar]
- 36.Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bγ with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999;274:9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- 37.Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA. Identification of a human Akt3 (protein kinase Bγ) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun. 1999;257:906–910. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- 38.Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, Gardner J, Cheng JQ, Testa JR, Tsichlis PN. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993;8:745–754. [PubMed] [Google Scholar]
- 39.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 40.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, Hassid A, Busse R, Fleming I. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res. 2005;97:1236–1244. doi: 10.1161/01.RES.0000195611.59811.ab. [DOI] [PubMed] [Google Scholar]
- 42.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 43.Gale N, Thurston G, Hackett S, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte M, Jackson D, Suri C, Campochiaro P, Wiegand S, Yancopoulos G. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 44.Mäkinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 46.Hellström M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 47.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 48.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 51.Shworak NW. Angiogenic modulators in valve development and disease: does valvular disease recapitulate developmental signaling pathways? Curr Opin Cardiol. 2004;19:140–146. doi: 10.1097/00001573-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Bβ is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 54.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


