Abstract
Peptide-oligonucleotide conjugates (POCs) are molecular chimeras composed of a nucleic acid moiety covalently attached to a polypeptide moiety. POCs have been used in numerous applications from therapeutics to nanotechnology, and most recently as combinatorial agents in the assembly of bivalent protein affinity reagents. This unit describes the synthesis and purification of POC molecules using the heterobifunctional crosslinking reagent succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC), which enables amine-modified oligonucleotides to become covalently linked to cysteine-modified polypeptides. This solution-based protocol consists of a two-step synthesis followed by a single purification step.
Keywords: peptide-oligonucleotide conjugate, SMCC (succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate), fragment conjugation, antisense, DNA nanotechnology, synbodies, nanodisplay
INTRODUCTION
Peptide-oligonucleotide conjugates (POCs) possess unique properties that can be exploited in a wide range of applications from nanotechnology to drug delivery and antisense technology (Tung, 2000). Several synthetic protocols have been published that describe different approaches for making POC molecules (Singh, Spinelli et al., 2008). Most of these strategies involve in-line, solid-phase synthesis procedures in which the polypeptide and oligonucleotide are synthesized directly on a solid support (Grandas, Marchan et al., 2007). The in-line synthesis approach requires peptide and DNA synthesizers, along with protecting group strategies that are compatible with the solid-phase synthesis of both polymers.
Fragment conjugation represents a convenient alternative to in-line synthesis. The fragment conjugation strategy presented in this unit utilizes heterobifunctional crosslinking reagents, composed of an aliphatic chain containing a maleimide group on one side and an N-hydroxysuccinimide (NHS) on the other (Figure 1). This reagent allows cysteine-modified peptides to become covalently attached to amine-modified oligonucleotides. The fragment conjugation approach is highly versatile and can be used to create POCs that crosslink to any position in the peptide or nucleic acid sequence. Unlike the in-line synthesis approach, this technique is performed in solution using standard laboratory techniques without the need for specialized equipment and reagents.
Figure 1.
Example of heterobifunctional crosslinking reagents that contain maleimide and succinimidyl ester moieties, all of which are compatible with the protocol described in this unit.
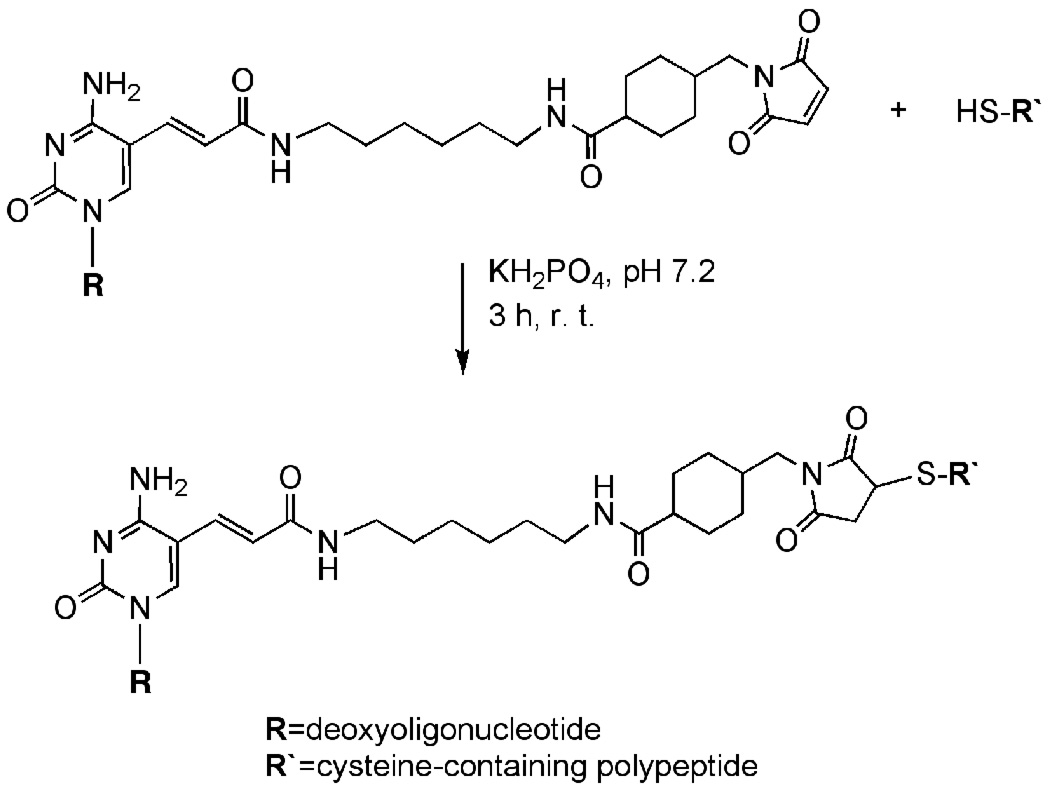
This unit describes the use of SMCC to covalently link amine-modified DNA to cysteine-containing peptides. The protocol was adapted from a previously published method (Harrison and Balasubramanian, 1998), and requires the use of high purity (>95%) amine-modified DNA. A purification method using denaturing polyacrylamide gel electrophoresis (urea-PAGE) is described in Basic Protocol 1. As shown in Figure 2, the primary amine modification on the DNA is reacted with the NHS ester moiety of the SMCC reagent (Basic Protocol 2) to attach the bifunctional molecule to the DNA. The SMCC-modified DNA is then conjugated to the polypeptide by a Michael-type addition of the sulfhydryl group of the cysteine residue to the maleimide moiety of the SMCC linker (Basic Protocol 3). Peptide-oligonucleotide conjugates can be purified using native-PAGE (Basic Protocol 4) or high performance liquid chromatography (HPLC) (Basic Protocol 5).
Figure 2.
Amine-modified oligonucleotide conjugation to a cysteine-containing peptide using the heterobifunctional crosslinking reagent SMCC.
PREPARATION OF AMINE-MODIFIED OLIGODEOXYNUCLEOTIDE
Amine-modified oligonucleotides are commercially available and can be purchased from several vendors including the W.M. Keck facility at Yale University and Integrated DNA Technologies (IDT). Most amine-modified DNA is synthesized using one of the four non-standard, amine-modified phosphoramidites (Figure 3). For modifications that occur at the 3’ terminus of the DNA strand, oligonucleotides can be synthesized from a universal linker or from a CPG column pre-charged with an amine moiety. The amine-modified DNA oligonucleotide must be purified prior to SMCC conjugation in order to remove any primary amines that would otherwise compete with the DNA for the crosslinking reagent. Many companies provide oligonucleotide purification, however this additional service can be cost prohibitive for many labs. Fortunately, amine-modified oligonucleotides are easy to purify by denaturing urea-PAGE, and a standard procedure is given here.
Figure 3.
Amine-modified deoxynucleotide phosphoramidite and CPG reagents available from Glen Research that can be used as building blocks to construct amine-modified deoxyoligonucleotides.
Materials
Amine-modified oligonucleotide
Bisacrylamide (Promega)
Acrylamide (Promega)
8 M urea (BDH)
50 mM ethylenediaminetetraacetic acid, pH 8.0 (EDTA, Pierce)
10X TBE buffer (1 M tris, 1 M boric acid, 10 mM EDTA, pH 8.3)
N’,N’,N’,N’, Tetraethylmethylenediamine (TEMED, Pierce)
10% (w/v) ammonium persulfate (APS, EMD Biosciences)
Running dye (0.05% (w/v) bromophenol blue and 0.05% (w/v) xylene cyanol in 1X TBE buffer)
200-proof ethanol (Sigma)
70% ethanol
3 M sodium acetate, pH 5.2 (Sigma)
Gel elution buffer (500 mM ammonium acetate, 10 mM magnesium acetate, 1 mM EDTA, pH 8.3)
Stir bar
Stir plate
Gel plates (19.7 × 16 cm and 19.7 × 18.5 cm)
Electrophoresis apparatus
Spacers (1.5 mm thick)
Comb (1 well with 2 maker lanes)
Power supply
Heat block
Disposable tubes (1.5 mL)
Plastic transfer pipette (pulled capillary)
Beaker (100 mL)
Plastic syringe (50 mL)
Plastic wrap
UV-active thin layer chromatography (TLC) plate
Handheld UV lamp- 254 nm
Microcentrifuge
Ultrafiltration spin filter tubes (0.45 µm, Millipore)
Black permanent marker
Razor blade or scalpel
Spatula
Vortex
Prepare urea-PAGE gel
-
1Prepare the gel plates, spacers, and comb using the manufacturer’s recommended protocol or as described in the following reference (Sharma and Yadav, 2009).Purification gels are 1.5 mm thick and often utilize a comb with a single well.
-
2In a 100 mL beaker, combine the acrylamide, bisacrylamide, urea, 10X TBE buffer and water using the amounts listed in Table 1.Oligonucleotides of ≤ 25 nucleotide bases are purified using a 20% polyacrylamide gel. Longer oligonucleotides may require a lower percentage of acrylamide.CAUTION: Acrylamide and bisacrylamide are hazardous. Use appropriate safety precautions and laboratory apparel.
-
3Stir the mixture using a stir bar and stir plate until the solution is homogeneous.To speed up the process, heat the mixture at 50 °C. Allow the solution to cool before moving to step 4.
-
4
Add 500 µL of 10% APS and 50 µL of TEMED to the 100 mL beaker and stir for 30 seconds.
-
5
Carefully pour the acylamide solution into the prepared gel plates to ensure that no leaks or air bubbles are present between the plates.
-
6
Insert the comb at the top of the gel and dislodge any remaining air bubbles by gently tapping on the glass.
-
7
Allow 30 minutes for the solution to polymerize between the gel plates.
Table 1.
Urea-PAGE Mixture
| Acrylamide % |
Acrylamide (g) |
Bisacrylamide (g) |
Urea (g) |
10X TBE buffer (mL) |
Deionized water (mL) |
10% APS (µL) |
TEMED (µL) |
|---|---|---|---|---|---|---|---|
| 6 | 5.7 | 0.3 | 42 | 10 | 40 | 500 | 50 |
| 8 | 7.6 | 0.4 | 42 | 10 | 40 | 500 | 50 |
| 10 | 9.5 | 0.5 | 42 | 10 | 40 | 500 | 50 |
| 12 | 11.4 | 0.6 | 42 | 10 | 40 | 500 | 50 |
| 14 | 13.3 | 0.7 | 42 | 10 | 40 | 500 | 50 |
| 16 | 15.2 | 0.8 | 42 | 10 | 40 | 500 | 50 |
| 18 | 17.1 | 0.9 | 42 | 10 | 40 | 500 | 50 |
| 20 | 19.0 | 1.0 | 42 | 10 | 40 | 500 | 50 |
Pre-run the urea-PAGE gel
-
8
Remove the bottom spacer and comb from the polymerized gel.
-
9
Rinse the gel plates with water to remove any polymerized acrylamide from the outside surface of the glass.
-
10
Place the gel plates in the gel electrophoresis apparatus and secure the plates as suggested by the manufacturer.
-
11
Fill the bottom reservoir of the gel apparatus with 1X TBE buffer. Remove any air bubbles trapped under the gel plates by tilting the apparatus to the side or using a syringe full of 1X TBE buffer to displace the bubbles.
-
12
Pour 1X TBE buffer into the top reservoir of the electrophoresis apparatus until the solution is ~3 cm above the gel.
-
13
Rinse the well of the gel with 1X TBE buffer to remove any residual polyacrylamide.
-
14Connect the electrophoresis apparatus to the power supply and set the power supply to 20 constant watts for 30 minutes.The ideal wattage for the gel will heat the glass plates so they are warm but not too hot to touch. Temperatures above ~ 70 °C can break the glass plates.
-
15
While the gel is pre-running, prepare the crude oligonucleotide.
Prepare the oligonucleotide
-
16Resuspend the oligonucleotide in deionized water for a final concentration of 1 µmole/mL.Add 1 mL of water to a 1 µmole scale DNA synthesis, and 200 µL of water to a 0.2 µmole scale DNA synthesis. If the pellet does not dissolve immediately, heat the mixture at 37 °C until homogeneous.
-
17
Transfer 1/4 the volume of the oligonucleotide solution to a 1.5 mL tube.
-
18
Add 1/5 the volume of 8 M urea and 1/10 the volume of 50 mM EDTA to the oligonucleotide solution in step 17.
-
19
Heat the mixture to 90 °C for 5 minutes to denature the DNA oligonucleotide.
Load and run the urea-PAGE gel
-
20
Once the gel has finished pre-running, disconnect the gel apparatus from the power supply and rinse the wells of the urea-PAGE gel with 1X TBE to remove any residual urea.
-
21
Load the denatured oligonucleotide into the large well of the urea-PAGE gel using a pulled capillary of a plastic transfer pipette.
-
22Load 5 µL of the running dye into the small wells located on each side of the large well.Running time will vary depending on the size of the oligonucleotide, but the two dyes can be used to approximate the location of the oligonucleotide in the gel according to the acrylamide percentage as shown in Table 2.
-
23
Reconnect the gel apparatus to the power supply and set the power at a constant 20 watts.
Table 2.
Migration of Running Dye in Relation to Oligonucleotide Length in Nucleotides (nt)
| Acrylamide % | Bromophenol Blue (nt) | Xylene Cyanol (nt) |
|---|---|---|
| 6 | 25 | 103 |
| 8 | 18 | 75 |
| 10 | 14 | 58 |
| 12 | 11 | 48 |
| 14 | 9 | 40 |
| 16 | 8 | 35 |
| 18 | 7 | 31 |
| 20 | 6 | 28 |
Gel extraction
-
24
Carefully remove the gel from the glass plates using a spatula to peal the gel away from the glass.
-
25
Wrap the gel front and back in a single layer of plastic wrap.
-
26
Place the gel on a UV-active TLC plate and image the gel by holding a handheld UV lamp turned on at 254 nm ~ 6 inches (16.24 cm) above the gel. The DNA will absorb the light and cast a shadow on the TLC plate.
-
27
Using a black permanent marker, trace the band corresponding to the oligonucleotide on the plastic wrap. Do this quickly, as the UV light will damage the DNA.
-
28
Turn the UV lamp off and cut the oligonucleotide band out of the gel using a clean razor or scalpel.
-
29
Cut the gel slice into 1–2 cm size pieces and transfer ~10 gel pieces to each 0.45 µm spin filter tube.
-
30
Place the tubes in the −20 °C freezer for 10 minutes.
-
31
Remove the tubes from the freezer and further crush the gel pieces with a spatula, being careful not to break the membrane in the spin filter.
-
32
Add 200 µL of gel elution buffer to each spin filter tube.
-
33Let the mixture sit at room temperature for 4 hours.Gentle agitation or rotation can increase the rate of diffusion.
-
34
Place the spin filter tubes in a microcentrifuge and spin for 5 minutes at 10,000 rpm.
-
35
Remove the filters from the tube and transfer the filtrate to a new 1.5 mL disposable tube.
-
36Repeat steps 32–35 two more times.As an alternative to the buffer diffusion method, electroelution can be used to recover oligonucleotide material from the gel pieces.
Ethanol precipitation
-
37
Dispense 200 µL of gel elution buffer into one or more 1.5 mL disposable tube.
-
38
Add 20 µL of 3 M sodium acetate and 800 µL of 200-proof ethanol to each tube.
-
39
Place each tube in the −80 °C freezer or on dry ice for 30 minutes.
-
40
Remove tubes from the freezer and centrifuge the mixture at 13,000 rpm for 30 minutes at 4 °C.
-
41
After centrifugation, a white pellet will be visible near the bottom of the tube.
-
42
Discard the supernatant and repeat steps 38–41 with 70% ethanol instead of 200-proof ethanol.
-
43
Carefully discard the supernatant and allow the pellet to air dry.
CONJUGATION OF SMCC TO THE AMINE-MODIFIED OLIGONUCLEOTIDE
The pure amine-modified oligonucleotide is conjugated to SMCC (Figure 4) through a coupling reaction between the primary amine on the DNA strand and the NHS ester moiety of SMCC. The rate of the reaction is dependent on the pH of the solution (Tournier, Wallach et al., 1998), and must be tightly controlled to avoid cross-reactivity of the amine with the maleimide moiety of the SMCC reagent (Brewer and Riehm, 1967). Optimal buffer conditions are pH 7.0 – 8.0 for both the initial coupling step between the amine-modified oligonucleotide and SMCC reagent, and the second coupling step between the peptide and SMCC-oligonucleotide.
Figure 4.
Conjugation of SMCC to an amine-modified deoxyoligonucleotide.
Materials
Pure amine-modified oligonucleotide (Basic Protocol 1)
100 mM KH2PO4 buffer, pH 7.2 (BDH)
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC, Pierce)
Acetonitrile (Sigma)
200-proof ethanol (Sigma)
70% ethanol
3 M sodium acetate, pH 5.2 (Sigma)
Disposable tubes (1.5 mL)
Microcentrifuge
Vortex
Conjugate SMCC to the DNA oligonucleotide
- Resuspend the purified oligonucleotide in 100 µL of ultrapure water.If you have multiple tubes, add a fraction of the volume of water to each tube, resuspend each pellet, and then combine the solution into one 1.5 mL tube.
- Quantify the oligonucleotide using UV absorbance and Beer’s Law. The extinction coefficient for the oligonucleotide sequence can be calculated based on the base composition.The extinction coefficient of the oligonucleotide can be calculated by adding together the extinction coefficients of each nucleobase in the sequence, see Table 3. The concentration of the oligonucleotide in solution can be calculated using Beer’s law (A254=clε), where c is the molar concentration, l is the pathlength of the UV cell (typically 1 cm), and ε is the molar extinction coefficient of the oligonucleotide sequence.
Transfer 20 nmoles of the oligonucleotide from step 1 to a new 1.5 mL tube.
Add 134 µL of 100 mM KH2PO4 buffer, pH 7.2 to the tube.
In a separate 1.5 mL tube, dissolve 1 mg of SMCC in 1 mL of acetonitrile for a final concentration of 3 mM.
Transfer 67.0 µL of SMCC (200 nmoles) solution to the oligonucleotide solution prepared in step 4.
Vortex the solution vigorously for 5 seconds. Centrifuge the tube.
Allow the reaction to take place at room temperature for 30 minutes.
- Conduct an Ethanol precipitation on the SMCC-oligonucleotide conjugate material as described in Basic Protocol 1.SMCC is soluble in ethanol while DNA is not. The majority of excess SMCC is separated from the SMCC-oligonucleotide conjugate when the ethanol fraction is removed. The SMCC-oligonucleotide conjugate is best stored as a dry solid at −20 °C.
Table 3.
Molar Extinction Coefficient for each Nucleobase
| Nucleobase | Extinction coefficient (ε) at 260 nm1 |
|---|---|
| T | 8700 M−1cm−1 |
| dC | 7400 M−1cm−1 |
| dG | 11500 M−1cm−1 |
| dA | 15400 M−1cm−1 |
Determined at 25 °C and neutral pH conditions (Fasman, 1975).
CONJUGATION OF SMCC-OLIGONUCLEOTIDE TO A CYSTEINE-CONTAINING PEPTIDE
The sulfhydryl group of a cysteine residue is used to conjugate the peptide to the maleimide functional group of the SMCC-oligonucleotide intermediate (Figure 5). This fragment conjugation strategy is limited to peptides that contain one cysteine residue per sequence, preferably at the N- or C-terminus. When incorporating a cysteine residue at the peptide terminus, the addition of a flexible amino acid linker, such as glycine-serine dipeptide, between the peptide and terminal cysteine residue provides a convenient spacer that can reduce interference between the functional region of the polypeptide and the DNA polymer. It is critical to ensure that all reagents used in the synthesis and deprotection of the polypeptide are removed prior to conjugation to the SMCC-oligonucleotide. Many commercial companies provide HPLC purification services to obtain peptides with high purity (often 70% to >98%). For this protocol, it is suggested to use peptides with > 98% purity.
Figure 5.
Conjugation of SMCC-oligonucleotide to a cysteine-containing polypeptide.
Materials
SMCC-oligonucleotide (Basic Protocol 2)
100 mM KH2PO4 buffer, pH 7.2 (BDH)
Polypeptide (>98% purity)
Siliconized disposable tubes (1.5 mL, Fisher)
Microcentrifuge
Vortex
Conjugate SMCC-oligonucleotide to Peptide
- Resuspend the peptide in water for a final concentration of 1 mg/mL solution.Depending on the amino acid composition of the polypeptide, different solvents may be necessary to dissolve the peptide. Solvents such as acetonitrile, methanol, acetic acid and dimethyl sulfoxide (DMSO) are compatible dissolving agents. Do not use any solvents that contain sulfhydryl groups.
- Resuspend the SMCC-oligonucleotide conjugate in 200 µL of 100 mM KH2PO4 buffer, pH 7.2 and transfer to a 1.5 mL siliconized tube.Polypeptides can nonspecifically bind to standard polypropylene tubes, therefore siliconized tubes are used to reduce the peptide interaction with the tube.
Add 100 nmoles of peptide to the tube in step 2.
Vortex the solution for 5 seconds.
Centrifuge the tube briefly.
Let the reaction take place at room temperature for at least 3 hours.
PURIFICATION OF PEPTIDE-OLIGONUCLEOTIDE CONJUGATES USING NATIVE-PAGE
POCs can be purified using standard urea-PAGE (Basic Protocol 1), native-PAGE (Basic Protocol 4), or HPLC (Basic Protocol 5) purification. Urea-PAGE differs from native-PAGE purification by running the gel under non-denaturing conditions and stabilizing the gel temperature at 4 °C. Urea-PAGE purification can be used to purify the POC product, however we, along with others, have observed the degradation of some POC sequences at high temperatures caused by the urea-PAGE running conditions (Portela, Albericio et al., 2007). For these POC sequences, native-PAGE or HPLC purification is used. HPLC is a relatively fast method for POC purification, however retention times of the starting reagents and POC product may vary based on the amino acid composition of the peptide. Native-PAGE provides a convenient alternative to HPLC purification and optimal resolution between the oligonucleotide and POC product is easy to obtain.
Materials
Crude POC material (Basic Protocol 3)
40% acrylamide/bisacrylamide 19:1 solution (BioRad)
10X TAE-Mg2+ (400 mM tris, 200 mM acetic acid, 20 mM EDTA, 125 mM magnesium acetate, pH 7.5)
N’,N’,N’,N’, Tetraethylmethylenediamine (TEMED, Pierce)
10% ammonium persulfate (APS, EMD Biosciences)
Glycerol (BDH)
Native running dye (0.05% (w/v) bromophenol blue, 0.05% (w/v) xylene cyanol, 80% (v/v) glycerol)
NAP-10 (GE Lifesciences)
Beaker (100 mL)
Siliconized disposable tubes (1.5 mL, Fisher)
Stir bar
Stir plate
Syringe (50 mL)
Microcentrifuge
Gel plates (19.7 × 16 cm and 19.7 × 18.5 cm)
Spacers (1.5 mm thick)
Comb (10 wells)
Electrophoresis apparatus
Power supply
SyberGold (Invitrogen, optional)
Prepare native-PAGE gel
-
1
Prepare the gel plates, spacers, and comb using the manufacturer’s recommended protocol or as described in the following reference (Sharma and Yadav, 2009).
-
2Combine the 40% acrylamide/bisacrylamide solution, 10X TAE-Mg2+ buffer and water in a 100 mL beaker using the amounts listed in Table 4 for a given acrylamide percentage.For oligonucleotides with <25 bases, use a 20% acrylamide gel concentration.
-
3
Stir the mixture using a stir bar and stir plate until the solution is homogeneous.
-
4
Add 500 µL of 10% APS and 50 µL of TEMED and mix for 30 seconds.
-
5
Carefully pour the solution into the prepared gel plates to ensure that no leaks or air bubbles are present between the plates.
-
6
Insert the 10-well comb at the top of the gel and dislodge any remaining air bubbles by gently tapping on the glass.
-
7
Allow 60 minutes for the solution to polymerize between the plates.
Table 4.
Native-PAGE Mixture
| Gel % | 40% Acrylamide/bisacrylamide 19:1 (mL) |
10X TAE-Mg2+ buffer (mL) |
Deionized water (mL) |
10% APS (µL) |
TEMED (µL) |
|---|---|---|---|---|---|
| 6 | 6 | 4 | 30 | 500 | 50 |
| 8 | 8 | 4 | 28 | 500 | 50 |
| 10 | 10 | 4 | 26 | 500 | 50 |
| 12 | 12 | 4 | 24 | 500 | 50 |
| 14 | 14 | 4 | 22 | 500 | 50 |
| 16 | 16 | 4 | 20 | 500 | 50 |
| 18 | 18 | 4 | 18 | 500 | 50 |
| 20 | 20 | 4 | 16 | 500 | 50 |
Pre-run the native-PAGE gel
-
8
Remove the bottom spacer and comb from the polymerized gel.
-
9
Rinse the gel plates with water to remove any polymerized material on the surface of the glass.
-
10
Place the gel plates in the gel electrophoresis apparatus and secure the plates as suggested by the manufacturer.
-
11
Fill the bottom reservoir of the gel apparatus with 1X TAE-Mg2+ buffer. Remove any air bubbles trapped under the gel plates by tilting the apparatus to the side or using a syringe full of 1X TAE-Mg2+ buffer to displace the bubbles.
-
12
Pour 1X TAE-Mg2+ buffer in the top reservoir of the electrophoresis apparatus to ~3 cm above the top of the gel.
-
13
Rinse the wells of the gel with 1X TAE-Mg2+ buffer to remove any residual polyacrylamide.
-
14Connect the electrophoresis apparatus to the power supply and set the power to a constant 200 volts for 30 minutes.Native gel electrophoresis should be run using constant voltage to prevent the gel plates from heating. It is best to place the gel apparatus in a 4 °C room or connect the apparatus to a water bath with water cycling at 4 °C to keep the gel plates cool.
-
15
While the gel is pre-running, prepare the POC material by adding 1/10 the volume of glycerol and 1/10 the volume of 10X TAE-Mg2+ buffer to the crude POC solution in step 6 of Basic Protocol 3.
Run the native-PAGE gel for POC purification
-
16
After the pre-run, disconnect the gel apparatus from the power supply and rinse the 10 wells with 1X TAE-Mg2+ buffer.
-
17
Load 50 µL of prepared POC material into each well and 5 µL of native running dye in the two outermost wells flanking the POC material.
-
18
Reconnect the gel apparatus to the power supply and start the gel running at 200 volts at 4 °C. Running time will vary with gel percentage and length of the oligonucleotide.
Isolation of POC material
-
19Recover the POC material from the gel as described in Gel extraction of Basic Protocol 1.If the POC material is prepared on a small scale or is not detectable by UV, use SyberGold staining methods as described by manufacturer. If the POC material is stained with SyberGold and eluted as described in Gel extraction, conduct an Ethanol precipitation after step 29 of Isolation of POC material to remove any excess SyberGold.
-
20
To remove excess salts from the POC solution, prepare a NAP-10 column by washing the column with 10 column volumes of water.
-
21
Combine the purified POC solution in a 1.5 mL siliconized tube.
-
22
Add water to the tube until the final volume of the POC solution is 1 mL.
-
23
Add the 1 mL of POC to the top of the NAP-10 column.
-
24
Label four 1.5 mL siliconized tubes as elutions #1–4.
-
25
Place tube #1 below the NAP-10 column for collection purposes.
-
26
Add 0.5 mL of water to the top of the NAP-10 column and collect the output 0.5 mL in the 1.5 mL tube from step 25.
-
27
Repeat steps 25–26 three more times with tubes #2–4.
-
28Lyophilize the four tubes to dryness.If you notice tube #4 contains a gel like material, repeat steps 22–28.
-
29Resuspend the POC material in 100 µL of water and quantify using UV absorbance as described in Basic Protocol 1.The POC material is quantified based on the oligonucleotide sequence since the contribution of absorbance at 254 nm caused by the amino acids is considered negligible compared to the absorbance of the DNA nucleobases.
-
30Calculate the overall synthesis yield by taking the ratio of product obtained in step 29 (in moles) to the starting amount of pure DNA from Basic Protocol 2 (20 nanomoles).The average yield of POC material after synthesis and purification by native-PAGE is 10% to 30%.
PURIFICATION OF PEPTIDE-OLIGONUCLEOTIDE CONJUGATES USING REVERSE PHASE-HPLC
As an alternative to urea- and native-PAGE purification, reverse phase-HPLC (RP-HPLC) can be used to purify POCs. Described below is a RP-HPLC protocol for the purification of POC material that has been slightly modified from a previously published method (Harrison and Balasubramanian, 1998). Initial analysis of the POC material is performed by injecting a small amount of the crude POC material onto the HPLC column and running the complete gradient method to obtain an analytical chromatogram trace. Migration of the POC material is monitored using absorbance at 254 nm, and retention times will vary depending on the sequence composition of both the peptide and DNA polymer. Additional injections of the oligonucleotide and SMCC-oligonucleotide intermediate may be needed to determine which peak corresponds to the POC. Once the peaks in the analytical chromatogram trace have been assigned, either an analytical or semi-preparative HPLC column (depending on the amount of POC material) is used to purify the remaining POC material.
Materials
Crude POC material (Basic Protocol 3)
HPLC mobile phase A: 0.1 M ammonium acetate, pH 7.0
HPLC mobile phase B: acetonitrile (HPLC grade, Sigma)
Nanopure water
- High-performance liquid chromatography (HPLC) system with:
- Injector (autosampler preferred), sample loop, and syringe (for manual injections)
- Binary pumping system
- UV/Vis detector with wavelength detection between 200 and 300 nm
- Analytical column: reverse phase column (i.e., Source 5RPC ST 4.6/150, Amersham)
- Semi-preparative column: reverse phase column (i.e., Source 15RPC ST 4.6/100, Amersham)
- Automatic fraction collector (optional)
Lyophilizer
Disposable siliconized tubes (1.5 mL, Fisher)
Prepare HPLC instrument and RP-HPLC analytical injection
-
1
Connect the analytical RP-HPLC column to the HPLC instrument as directed by the manufacturer’s handbook.
-
2
Turn the HPLC instrument on and set the UV detector to 254 nm.
-
3
Turn on the binary pumps and begin pumping 100% mobile phase B through the column at a flow rate of 0.8 mL/min for 5 minutes.
-
4
Change the solvent from 100% mobile phase B to 95% mobile phase A over 1 minute with a continuous flow rate of 0.8 mL/min. Flow 95% mobile phase A through the column until a flat baseline is achieved.
-
5
Program the HPLC gradient system to start at 95% mobile phase A and increase the percentage of mobile phase B over time as shown in Table 5.
-
6
Start the gradient program while tracking the absorbance at 254 nm. Continue to do full gradient cycles until the baseline is flat for the entire gradient program.
-
7
Dilute 5 µL of POC material from Basic Protocol 3 in 20 µL of 0.1 M ammonium acetate, pH 7.0.
-
8
Inject 25 µL of POC sample from step 7 into the sample column of the HPLC instrument and elute the POC material by starting the gradient program described in Table 5.
Table 5.
Gradient of Mobile Phase B for RP-HPLC Purification of Crude POC Materiala
| Elapse time (min) | Percentage mobile phase Bb |
|---|---|
| 0 | 5 |
| 5 | 5 |
| 30 | 30 |
| 31 | 100 |
| 35 | 100 |
| 36 | 5 |
| 40 | 5 |
Gradient conditions are based on a 0.8 mL/min flow rate using an analytical Amersham Source 5RPC ST 4.6/150 column at room temperature with a 40 minute injection cycle. Mobile phase A: 0.1 M ammonium acetate, pH 7.0. Mobile phase B: acetonitrile.
Percentage is at elapsed time.
Purify crude POC material using analytical or semi-preparative RP-HPLC
-
9
Depending on the amount of POC material, use an analytical or semi-preparative column accordingly.
-
10
Prepare the remaining 95 µL POC material from Basic Protocol 3 in 405 µL of 0.1 M ammonium acetate, pH 7.0.
-
11Repeat step 8 five times by injecting 100 µL of POC material from step 10 and running the same gradient listed in Table 5.Elute the material at a flow rate of 0.8 mL/min when using an analytical column or 3 mL/min for semi-preparative column. Collect the assigned POC fraction as determined in the analytical chromatogram using an automatic fraction collector or manually by observing the chromatogram in real time.
-
12
Exchange the purified POC material into water using a Nap-10 column as described in steps 21–27 of Isolation of POC material.
-
13
Lyophilize the fractions to dryness.
-
14Resuspend the POC material in 100 µL of water and quantify using UV absorbance as described in Basic Protocol 1.The POC material is quantified based on the oligonucleotide sequence since the contribution of absorbance at 254 nm by the amino acids is considered negligible compared to the absorbance of the nucleobases.
-
15Calculate the overall synthesis yield by taking the ratio of product obtained in step 14 (in moles) to the starting amount of pure DNA from Basic Protocol 2 (20 nanomoles).The average yield of POC material after synthesis and purification by RP-HPLC is 30% to 50%.
COMMENTARY
Background Information
Several methods exist to conjugate polypeptides to synthetic DNA and RNA (Roberts and Szostak, 1997; Venkatesan and Kim, 2006; Moroder, Steger et al., 2009). The most commonly used method of peptide-oligonucleotide construction is in-line solid phase synthesis. Using this technique, the peptide and oligonucleotide strands are synthesized sequentially using automated synthesizers. Typically, the peptide is synthesized first on a solid support, and a modified N-terminal or the natural C-terminal hydroxyl group is used to create a phosphodiester linkage between the peptide and the first nucleotide (Haralambidis, Duncan et al., 1990; Tung, 2000). The remaining oligonucleotide sequence is then elongated using standard phosphoramidite chemistry on a DNA synthesizer. Although the in-line solid phase synthesis method is straightforward, the strategy is limited to amino acid sequences with chemical protecting groups that are compatible with oligonucleotide synthesis and deprotection chemistries (Venkatesan and Kim, 2006).
Fragment conjugation is an alternative strategy to the solid phase method of creating POC molecules. Since this approach uses independently synthesized and deprotected peptides and oligonucleotides, fragment conjugation overcomes the need for compatible protecting groups and allows for the conjugation of longer polymers containing any combination of amino acids and nucleotides. Also, because the peptide and oligonucleotide polymers are commercially available, the fragment conjugation method is applicable to most laboratories.
The fragment conjugation method presented here utilizes the heterobifunctional crosslinking reagent SMCC. This method was originally reported by Harrison and Balasubramanian in 1997 to characterize the hybridization properties of POCs (Harrison and Balasubramanian, 1997; Harrison and Balasubramanian, 1998). The functionality of the SMCC molecule allows for a wide range of oligonucleotide and peptide sequences to be conjugated. However, one constraint of this fragment conjugation method is that the peptide sequence must contain only one cysteine residue. One added benefit of this strategy is that it is compatible with other chemical modifications including biotin, 2,4-dinitrophenyl (DNP), psoralen, puromycin, and fluorescein labels.
Applications
The protein recognition and cellular uptake capabilities of polypeptides, along with the base pair recognition of oligonucleotides, allows for a wide range of application for POC molecules (Leonetti, Degols et al., 1990; Soukchareun, Tregear et al., 1995). POCs were originally used for antisense technology where viral or cellular gene expression is controlled by hybridizing a small DNA sequence to the mRNA region that encodes a gene of interest. POCs are well suited for this type of application because the peptide portion of the molecule is able to recognize a particular cell type and transport the oligonucleotide sequence across the cell membrane where the oligonucleotide can selectively hybridize with mRNA and inhibit translation.
Since the original use of POC molecules as antisense reagents, many new applications have emerged that extend the use of POCs into the field of bionanotechnology. One effort focuses on the advancement of structural DNA nanotechnology in which the nucleic acid moiety of the POC molecules are used to direct individual peptides to specific locations on the surface of a DNA nanostructure. In the first example, the c-myc peptide was conjugated to a short 20-nt single-stranded DNA molecule that was complimentary in sequence to a DNA probe presented on the surface of a two-dimensional DNA nanoarray (Williams, Lund et al., 2007). When hybridized, the tiled array produced a nanoscale pattern of evenly spaced parallel lines separated by 64 nanometers. When probed with the cognate anti-c-myc antibody, the antibody specifically recognized the c-myc peptide displayed on the surface of the nanoarray. This technology of displaying peptides on the surfaces of DNA nanostructures was termed nanodisplay. A second example of nanodisplay recently appeared when POCs displayed on DNA nanotubes were used to template-direct the nucleation of gold nanoparticles from soluble chemical precursors. Transmission electron microscopy images demonstrated the formation of gold nanoparticles of a discrete size evenly spaced on the surface of the DNA nanotube (Stearns, Chhabra et al., 2009). Together, these two examples demonstrate the utility of POC molecules as chemical reagents that can be used to precisely organize biological and inorganic materials on the nanoscale.
More recently, POC molecules were used to create bivalent protein affinity reagents, termed synbodies (Williams, Diehnelt et al., 2009). This chemical design relies on the principle of multivalency in which peptides with affinity to non-overlapping epitopes on a protein surface can be used to create bivalent affinity reagents that bind their target proteins with significantly higher affinity than the individual peptides alone. Synbodies are constructed by synthesizing a small library of POCs using two peptides that are independently conjugated to complimentary strands of synthetic DNA. When the POCs are hybridized together, the two peptides are displayed on the dsDNA scaffold with varying spatial separation and orientation depending on the location of the amine modification within the DNA sequence. By positioning the peptides at different positions along the DNA backbone it is possible to quickly determine the optimal distance required to achieve high affinity binding. The synbody with the optimal peptide separation and orientation has ~1,000-fold improvement in binding affinity to the target protein when compared to the individual peptides that make up the bivalent reagent. The creation of synbodies demonstrates a unique application of POC molecules to produce nanoscale, bivalent protein affinity reagents.
Critical Parameters and Troubleshooting
When constructing POCs using the fragment conjugation protocol there are several critical parameters that need to be addressed. Of principal importance is the need for high purity (>95%) oligonucleotides and peptides that are free of unwanted amine and thiol impurities commonly found in solid-phase synthesis reagents. It is also important to be aware of denaturants and buffers that contain molecules that can react with SMCC.
Second, alternative purification strategies must be employed when scaling the reaction to concentrations greater than 20 nmoles per tube. In the described method, ethanol precipitation is used to remove the excess SMCC, however, this separation method is not sufficient for large-scale conjugation reactions. Alternatively, it is advised to use HPLC or urea-PAGE to purify the SMCC-oligonucleotide product from the excess SMCC for large-scale conjugations. Example chromatograms of SMCC-oligonucleotide and crude POC material using RP-HPLC is shown in Figure 6.
Figure 6.

Reverse phase-HPLC analysis of A) the c-myc peptide (EQKLISEEDLC), B) crude POC material (EQKLISEEDLC-ACCAGCTGTGCAGGCCTCGC), and C) 1 nanomole of SMCC-oligonucleotide spiked into the POC mixture (B. Williams Ph.D. thesis).
Validation of peptide-oligonucleotide conjugation can be performed using two different methods: gel mobility shift assay or mass spectrometry (MS). Depending on the amino acid and nucleotide composition of the POC, it can be difficult to crystallize the POC material using a matrix when performing matrix-assisted laser desorption ionization (MALDI) MS due to the different matrixes needed for the peptide and oligonucleotide polymers. For optimal matrix crystallization, it is best to try all of the following matrixes when performing MALDI-MS analysis of POC material; 4-hydroxy-α-cyanocinnamic acid, sinapinic acid, 3-hydroxypicolinic acid, and 2,4,6-trihydroxyacetophenone/diammonium salt (Tung, 2000). An example of MALDI-MS analysis of crude and pure POC material using sinapinic acid is shown in Figure 7. Electrospray ionization (ESI) MS (Tengvall, Auirola et al., 2003) and analytical native-PAGE are alternative methods of analyzing POC material that do not require matrix optimization. An example of native-PAGE mobility shift analysis of POC material is shown in Figure 8.
Figure 7.

MALDI mass spectroscopy analysis of A) SMCC-oligonucleotide and B) purified POC material (B. Williams Ph.D. thesis).
Figure 8.
Native-PAGE mobility shift analysis of POC material. Retardation of mobility is observed between the ssDNA (ACCAGCTGTGCAGGCCTCGC) (lane1), SMCC-oligonucleotide (SMCC-ACCAGCTGTGCAGGCCTCGC) hybridized to its complimentary strand (lane 2) and POC (EQKLISEEDLC-ACCAGCTGTGCAGGCCTCGC) hybridized to its complimentary strand (lane 3), thereby confirming the presence of the polypeptide (Williams, Lund et al., 2007). Image reproduced with permission from Wiley-VCH Verlag GmbH & Co.
Anticipated Results
As with all synthetic steps, the yield of the final product depends on the purity of the starting materials, with higher purity peptides and DNA giving higher overall yields. Using the methods described above, a yield of 2–6 nmoles of pure POC material can be obtained from native-PAGE purification and 6–10 nmoles from RP-HPLC purification when conducting a 20 nanomole conjugation reaction. The yield may vary depending on the polypeptide sequence and the location of the amine-modified nucleobase in the DNA sequence. To optimize the yield of the reaction, vary the ratio of oligonucleotide to SMCC during the first conjugation step and the peptide to SMCC-oligonucleotide during the second conjugation step.
Time Consideration
The synthesis and purification of peptide-oligonucleotide conjugates using either the native-PAGE or HPLC purification methods can be performed in 2–3 days. Additional material can be generated in the same time frame by conducting multiple 20 nanomole reactions in parallel.
Acknowledgements
We would like to thank members of the Chaput lab for helpful suggestions on the development of the POC protocol. B.W. was the recipient of an NSF-IGERT Fellowship and most of this work was supported with a grant from the National Institutes of Health (CA126622-01) to J. C.
Literature Cited
- Brewer CF, Riehm JP. Evidence for possible nonspecific reactions between N-ethylmaleimide and proteins. Anal. Biochem. 1967;18:248–255. [Google Scholar]
- Grandas A, Marchan V, Debethune L, Pedroso E. Stepwise solid-phase synthesis of nucleopeptides. Curr. Protoc. Nucleic Acid Chem. 2007:4.22.1–4.22.54. doi: 10.1002/0471142700.nc0422s31. [DOI] [PubMed] [Google Scholar]
- Haralambidis J, Duncan L, Angus K, Tregear GW. The synthesis of polyamide-oligonucleotide conjugate molecules. Nucleic Acids Res. 1990;18:493–499. doi: 10.1093/nar/18.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JG, Balasubramanian S. A convenient synthetic route to oligonucleotide conjugates. Bioorg. Med. Chem. Lett. 1997;7:1041–1046. [Google Scholar]
- Harrison JG, Balasubramanian S. Synthesis and hybridization analysis of a small library of peptide-oligonucleotide conjugates. Nucleic Acids Res. 1998;26:3136–3145. doi: 10.1093/nar/26.13.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti JP, Degols G, Lebleu B. Biological activity of oligonucleotide-poly(L-lysine) conjugates: mechanism of cell uptake. Bioconj. Chem. 1990;1:149–153. doi: 10.1021/bc00002a010. [DOI] [PubMed] [Google Scholar]
- Moroder H, Steger J, Graber D, Fauster K, Trappl K, Marquez V, Polacek N, Wilson DN, Micura R. Non-hydrolyzable RNA-peptide conjugates: A powerful advance in the synthesis of mimics for 3 prime-peptidyl tRNA termini. Angew. Chem. 2009;121:4116–4120. doi: 10.1002/anie.200900939. [DOI] [PubMed] [Google Scholar]
- Portela C, Albericio F, Eritja R, Castedo L, Mascarenas JL. Ds-oligonucleotide-peptide conjugates featuring peptides from the leucine-zipper region of Fos as switchable receptors for the oncoprotein Jun. ChemBioChem. 2007;8:1110–1114. doi: 10.1002/cbic.200700115. [DOI] [PubMed] [Google Scholar]
- Roberts RW, Szostak JW. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl. Acad. Sci. USA. 1997;94 doi: 10.1073/pnas.94.23.12297. 122297-112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Yadav BR. Denaturing urea-polyacrylamide gel electrophoresis (PAGE) based microsatellite analysis. 2009 http://www.protocol-online.org.
- Singh Y, Spinelli N, Defrancq E. Chemical strategies for oligonucleotide-conjugates synthesis. Curr. Org. Chem. 2008;12:263–290. [Google Scholar]
- Soukchareun S, Tregear GW, Haralambidis J. Preparation and characterization of antisense oligonucleotide-peptide hybrids containing viral fusion peptides. Bioconj. Chem. 1995;6:43–53. doi: 10.1021/bc00031a004. [DOI] [PubMed] [Google Scholar]
- Stearns LA, Chhabra R, Sharma J, Liu Y, Petuskey WT, Yan H, Chaput JC. Template-directed nucleation and growth of inorganic nanoparticles on DNA scaffolds. Angew. Chem. Int. Ed. 2009;48:8494–8496. doi: 10.1002/anie.200903319. [DOI] [PubMed] [Google Scholar]
- Tengvall U, Auirola S, Antopolsky M, Azhaev A, Beigelman L. Characterization of antisense oligonucleotide-peptide conjugates with negative ionization electrospray mass spectrometry and liquid chromatography-mass spectrometry. J. Pharma. Biomed. Anal. 2003;32:581–590. doi: 10.1016/s0731-7085(03)00165-1. [DOI] [PubMed] [Google Scholar]
- Tournier EJM, Wallach J, Blond P. Sulfosuccinimidyl 4-(N-maleimidomethyl)-1-cyclohexane carboxylate as a bifunctional immobilization agent. Optimization of the coupling conditions. Anal. Chimica Acta. 1998;361:33–44. [Google Scholar]
- Tung C-H. Preparation and application of peptide-oligonucleotide conjugates. Bioconj. Chem. 2000;11:605–618. doi: 10.1021/bc0000334. [DOI] [PubMed] [Google Scholar]
- Venkatesan N, Kim BH. Peptide conjugates of oligonucleotides: Synthesis and applications. Chem. Rev. 2006;106:3712–3761. doi: 10.1021/cr0502448. [DOI] [PubMed] [Google Scholar]
- Williams BAR, Diehnelt CW, Belcher P, Greving M, Woodbury NW, Johnston SA, Chaput JC. Creating protein affinity reagents by combining peptide ligands on synthetic DNA scaffolds. J. Am. Chem. Soc. 2009;131:17233–17241. doi: 10.1021/ja9051735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BAR, Lund K, Liu Y, Yan H, Chaput JC. Self-assembled peptide nanoarrays: an approach to studying protein-protein interactions. Angew. Chem. Int. Ed. 2007;46:3051–3054. doi: 10.1002/anie.200603919. [DOI] [PubMed] [Google Scholar]