Abstract
The anthropoid primate placenta appears to be unique in producing corticotropin-releasing hormone (CRH). Placental CRH is involved in an endocrine circuit key to the production of estrogens during pregnancy. CRH induces cortisol production by the maternal and fetal adrenal glands, leading to further placental CRH production. CRH also stimulates the fetal adrenal glands to produce dehydroepiandrostendione sulfate (DHEAS) which the placenta converts into estrogens. There are at least two patterns of maternal circulating CRH across gestation among anthropoids. Monkeys examined to date (Papio and Callithrix) have an early-to-mid gestational peak of circulating CRH, followed by a steady decline to a plateau level, with a possible rise near parturition. In contrast, humans and great apes have an exponential rise in circulating CRH peaking at parturition. To further document and compare patterns of maternal circulating CRH in anthropoid primates, we collected monthly blood samples on 14 squirrel monkeys (Saimiri boliviensis) and 10 owl monkeys (Aotus nancymaae) during pregnancy. CRH immunoreactivity was measured from extracted plasma by solid-phase RIA. Both squirrel and owl monkeys displayed a mid-gestational peak in circulating CRH: days 45–65 of the 152-day gestation for squirrel monkeys (mean±SEM CRH = 2694±276 pg/ml) and days 60–80 of the 133-day gestation for owl monkeys (9871±974 pg/ml). In squirrel monkeys, circulating CRH declined to 36% of mean peak value by two weeks before parturition and then appeared to increase; the best model for circulating CRH over gestation in squirrel monkeys was a cubic function, similar to previous results for baboons and marmosets. In owl monkeys, circulating CRH appeared to plateau with no subsequent significant decline approaching parturition, although a cubic function was the best fit. This study provides additional evidence for a mid-gestational peak of maternal circulating CRH in ancestral anthropoids that has been lost in the hominoid lineage.
Keywords: placenta, anthropoid, corticotropin-releasing hormone, gestation
Introduction
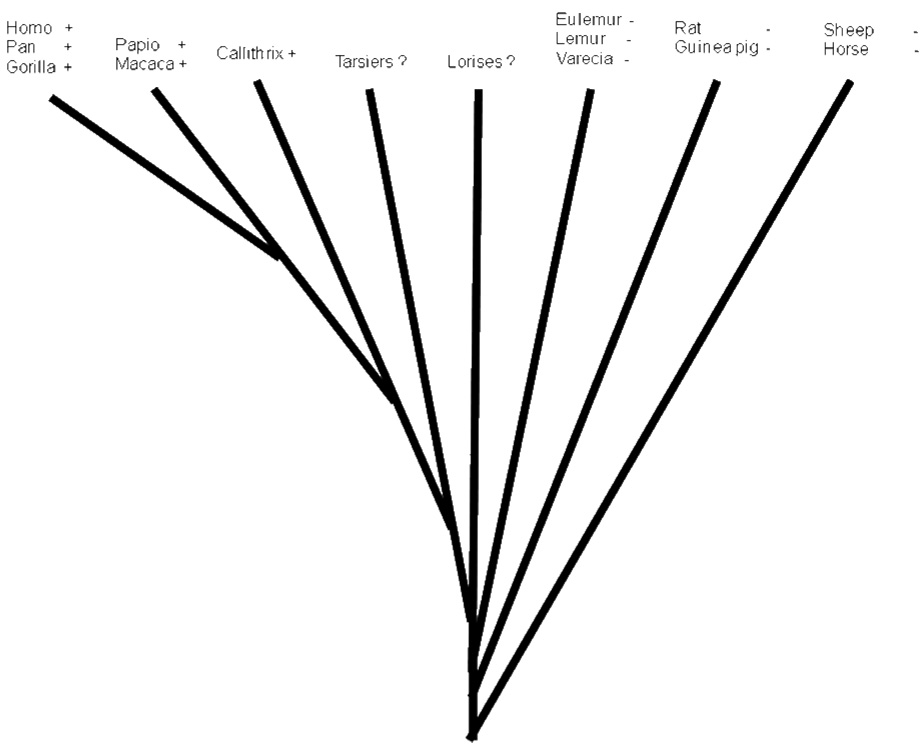
Placental production of corticotropin-releasing hormone (CRH) appears to be an anthropoid primate adaptation [Robinson et al., 1989; Bowman et al., 2001; Power and Schulkin, 2006]. Shortly after CRH was first isolated from sheep hypothalamus [Vale et al., 1981] the peptide was detected in serum obtained from pregnant women [Sasaki et al., 1984]. The source of the CRH in maternal circulation was determined to be the placenta [Grino et al., 1987; Frim et al., 1988]. Subsequently, circulating CRH was detected in pregnant chimpanzees [Smith et al., 1999], gorillas [Robinson et al., 1989; Smith et al., 1999], baboons [Goland et al., 1992; Smith et al., 1993], macaques [Robinson et al., 1989; Giussani et al., 1998; Bowman et al., 2001], and common marmosets [Bowman et al., 2001; Power et al., 2006]; but not in rats or guinea pigs [Robinson et al., 1989] nor in several species of Malagasy prosimian primates [Robinson et al., 1989; Bowman et al., 2001]. African and Asian prosimians (lorises and tarsiers) have yet to be examined. Thus the placentas of humans and apes, Old World monkeys, and New World monkeys all appear to synthesize CRH and release it into maternal circulation; but no other primates nor any non-primate mammals are known to do so (Fig 1).
Figure 1.
Simple phylogenetic representation of species tested for presence/absence of maternal circulating CRH during pregnancy. + indicates CRH detected; − indicates CRH not detected; ? means no species in these taxa have been tested. Data from Power and Schulkin, 2006.
One function of placental CRH is to stimulate the maternal and fetal adrenal glands to synthesize and release steroids. This maternal-placental-fetal endocrine circuit serves to produce the increasing concentration of maternal circulating estrogens as pregnancy progresses. In most mammalian species, the increase in circulating estrogens across pregnancy is accomplished by placental conversion of progestins to estrogens [Smith et al., 2005]. The anthropoid primate placenta does not express to any appreciable extent a key enzyme (17α-hydoxylase-17,20-lyase) required for this process [Kallen, 2004; Rainey et al., 2004]. Thus, in anthropoids circulating progesterone does not decrease as estrogen increases over pregnancy [Smith et al., 2005]; rather both continually rise in concentration. In anthropoids, placental production of estrogens is dependent instead upon the substrate steroid dehydroepiandrostendione sulfate (DHEAS), produced by the fetal adrenal in response to stimulation by placental CRH [Rainey et al., 2004].
The temporal profile of maternal circulating CRH in humans is very consistent. Circulating CRH is undetectable until approximately the end of the first trimester. From then on, circulating concentrations of CRH increase exponentially over pregnancy, peaking at parturition [Goland et al., 1986; Campbell et al., 1987; Sasaki et al., 1987; McLean et al., 1995]. After the placenta is expelled from the uterus, circulating CRH rapidly returns to undetectable levels. This pattern is consistent with the proposed endocrine feedback system between maternal and fetal adrenals and the placenta. Similar to its feed-forward effect on CRH levels in certain brain regions such as the amygdala [Makino et al., 1994; Watts and Sanchez-Watts, 1995], cortisol induces CRH production in placenta [Robinson et al., 1988; Jones et al., 1989]. Placental CRH acts on the maternal and fetal adrenal glands to induce production of cortisol (predominantly from the maternal adrenals) and DHEAS (predominantly from the fetal adrenals). Cortisol released by the maternal adrenal glands and to a lesser extent by fetal adrenal glands acts on the placenta to induce greater CRH production. This positive feedback system results in increasing production by fetal adrenal glands of DHEAS, which is converted by the placenta into estrogens [Rainey et al., 2004]. The system is putatively essential for the appropriate timing of human parturition [Lockwood, 2004].
The temporal profile of maternal circulating CRH over gestation in the great apes is qualitatively the same as that of humans [Smith et al., 1999; Power and Schulkin, 2006]. However, the temporal profile seen in pregnant monkeys (baboons and common marmosets) appears to be fundamentally different [Power and Schulkin, 2006]. Instead of an exponential increase in circulating CRH across gestation, these monkey species exhibit a pattern best described by a cubic function, with an early-to-mid gestational rise to a peak value, followed by a decline in circulating CRH concentration until shortly before parturition when it appears to rise again [Power and Schulkin, 2006]. The fact that an Old World monkey and a New World monkey share a qualitatively similar pattern of maternal circulating CRH across gestation that differs from the common pattern seen in humans and apes suggests that the monkey pattern may be the ancestral pattern whereas the human and ape pattern is derived.
However, results from only two species do not constitute overwhelming evidence for a consistent pattern of CRH expression during gestation in monkeys. Until more taxa can be examined the full picture of the anthropoid patterns of maternal circulating CRH remains uncertain. Accordingly, the present research was designed to investigate the pattern of maternal circulating CRH in two additional New World monkey species: the squirrel monkey (Saimiri boliviensis) and the owl monkey (Aotus nancymaae).
Methods
Subjects
This research was carried out under the authorization of existing protocols approved by the Institutional Animal Care and Use Committee of the University of South Alabama and was in compliance with all applicable U.S. laws and the ASP Principles for the Ethical Treatment of Non Human Primates. Squirrel monkeys (Saimiri boliviensis boliviensis) in this study were part of a national resource for Neotropical Primate Research and Resources (CNPRR) maintained at the University of South Alabama Center. The squirrel monkeys were housed in social groups of between 15 and 35 animals, containing one adult male and between 10 – 15 adult females with their offspring. Housing consisted of indoor pens measuring approximately 4.5 m × 2.5 m × 1.5 m, connected by two round, 14” diameter doors. Social groups had access to two to three pens depending on its size. All animals were fed a commercial New World Primate diet that had a guaranteed analysis of crude fat ≥ 9%. The diet was supplemented three times weekly with chopped vegetables, including celery, bell peppers, squash, and beans. Grapes, peanuts, and meal worms were fed sparingly as positive reinforcers when animals presented for clinical observations. Water was available ad libitum. The light dark schedule was maintained to track the local sunrise and sunset, so animals were exposed to long and short days annually.
The owl monkeys were housed indoors at the University of South Alabama vivarium, an Association for the Assessment and Accreditation of Laboratory Animal Care, International-accredited facility. The female owl monkeys in this report were pair-housed with a mate and offspring < 6 months of age, if present, in stainless steel vertical cages 0.81 m × 0.7 m × 1.8 m equipped with a 25 cm × 20 cm × 27.5 cm nestbox. The monkeys were fed a mixed diet consisting of ZuPreem® Primate Diet canned (Mission, KS), ¼ orange, and Laboratory Fiber-Plus® Monkey Diet (PMI® Nutrition International, St. Louis, MO) daily. The diet was supplemented with PRIMA-Treats® (Bio-Serve, Frenchtown, NJ), fruits and vegetables, and water was available ad libitum. Light/dark cycle was offset with lights coming on at 3 am and going off at 3 pm. Red lights came on during the dark cycle to enable observation of these nocturnal primates during their more active phase. Temperature was maintained at 26–27 °C. All of the owl monkey dams had had a previous live birth.
Blood samples were obtained from 14 pregnant squirrel monkeys (median age = 6.5 years, range = 4 to 11 years; median parity = 1, range = 0 to 6) and 10 pregnant owl monkeys (median age = 6 years, range = 4 to 14 years; median parity = 4, range = 3 to 11) across gestation (2 – 5 samples per female). Blood was collected from manually restrained animals via the femoral vein. The restraint was for less than three minutes, and has been shown not to affect measured values of other reproductive hormones (Yoeman et al., 1988). Samples were collected in EDTA, centrifuged (3,000 RPM for 10 minutes) and then plasma pipette off. All blood samples were obtained between 8am and 10am. All samples from squirrel monkeys were taken during gestation; for nine of the ten owl monkeys, there were samples taken either before or after pregnancy in addition to the pregnancy samples. All females produced a term birth. All owl monkey births were live births; one female produced twins. In the squirrel monkeys, four of the 14 births were term stillbirths.
Gestational age was estimated for each sample from the date of birth, assuming a 152-day gestation for squirrel monkeys [LEW, SVG, unpublished data] and a 133-day gestation for owl monkeys [Hunter et al., 1979]. In the cases of stillbirths in squirrel monkeys a determination was made that the births were term based on an examination of the dead infant. There were 46 blood samples collected from the 14 squirrel monkey dams (2 – 4 samples from each dam) ranging from an estimated 11 days of gestation to 145 days of gestation. There were 39 blood samples collected from the 10 owl monkey dams (2 – 4 samples from each dam) ranging from an estimated 4 days of gestation to 128 days of gestation. An additional sample from each of 9 of the owl monkey samples collected outside of gestation was assayed.
CRH extraction and radioimmunoassay
CRH peptide was extracted using the established method described in Power and colleagues [2006]. Plasma samples were thawed at room temperature, centrifuged (900× g, 5 min, 4° C), and returned to ice. Supernatant (0.2 mL) was transferred to low-binding, screw-cap polypropylene tubes (Axygen, Union City, CA), to which ice-cold 100% methanol (0.8 ml) was added. After vortexing, samples were rotated (15 min, 4° C) and centrifuged (2000× g, 20 min, RT). Then, the supernatant was transferred into a polypropylene tube containing 2 µl of 1% Triton X-100, dried in a Savant Speed Vac Concentrator overnight, and then stored at −80°C until immunoassay.
For assay, samples were reconstituted in 0.2 mL of gelatin assay buffer (0.15 M K2HPO4, 0.2 mM ascorbic acid, 0.1% gelatin, pH 7.5). Plasma CRH immunoreactivity content was quantified with a sensitive and specific solid-phase radioimmunoassay (RIA) adapted from Zorrilla and colleagues [2001]. Immulon-4 HBX Removawell 96-well plates (Thermo Fisher Scientific, Rochester, NY) were coated with protein A/G (1 mg/100 µl 1 M NaHC03/well, pH 9.0; Pierce Biotechnology, Rockford, IL) for 48 hours. Plates were rinsed with wash buffer (0.15 M KH2PO4 supplemented with 0.2 mM ascorbic acid and 0.1% Tween-20, pH 7.5) to dislodge loose protein A/G. Wells were incubated for 7 days at 4° C with 40 µl anti-CRF serum (rC70, generously provided by W. Vale, The Salk Institute, La Jolla, CA) at a titer of 1:400,000 in gelatin assay buffer. After three rinses to dislodge loose antibody, 40 µl of sample or standard (6 to 20,000 pg/ml) in duplicate, was incubated for 4 days at 4°C. Following incubation, 40 µl of [125I-Tyr0]-r/h corticotropin-releasing factor (~10,000 cpm/40 µl; Perkin Elmer, Boston, MA) was added to each well and incubated for an additional 24 hours at 4° C. Wells were rinsed with wash buffer, blotted dry, and separated. Residual radioactivity was counted by an Apex Automatic Gamma Counter. A four-parameter logistic curve fit model was used for interpolation of the standard curves (SigmaPlot 10.0, Systat Software, Point Richmond, CA).
The rC70 antiserum, raised in rabbit against full-length synthetic rat/human CRF by the Vale laboratory, has been well-characterized for radioimmunoassay [Vale et al., 1983], and used extensively in previous studies to measure CRF immunoreactivity in non-human primate species [Hsu and Price, 2009] including squirrel monkeys [Basset & Foote, 1992; Cha & Foote, 1988; Foote & Cha, 1988]. Here, ln dose-ln cpm response dilution analysis demonstrated parallelism of Aotus monkey samples with human CRF standards across 600 – 20,000 pg/ml concentrations, which spanned the range of samples. Even dilution analysis of Saimiri sciureus samples around the assay’s limit of sensitivity (6 – 600 pg/ml) still demonstrated parallelism with the rat/human CRF standards (albeit both yielding regressions of shallow slope). The assay’s mean limit of sensitivity, defined as 2 standard deviations of cpm from the zero concentration, was 499 pg/ml (~25 pg/well). The mean intra-assay coefficient of variation across all samples was 19–21% and 16% for samples with more typical concentrations of between 500 and 2000 pg/ml. Typical inter-assay coefficients of variation for this assay are 20% or less [Cottone et al., 2009]. To reduce the influence of plate-to-plate variability on results, most samples of a given species were run on a single plate (78% and 67% for squirrel and owl monkey, respectively), all samples from a given subject were analyzed within the same plate, and gestational age was equally represented across the two plates required for each species.
Statistical analysis
Mean ± SEM are given for CRH values at key time points. Differences were tested by ANOVA. The patterns of maternal CRH across gestation were modeled using the SPSS® curve estimation function (SPSS® 16.0, SPSS Inc, Chicago Il.) The data were tested for linear, quadratic, cubic, and exponential relationships with gestational age. The mean residual values for each female of maternal circulating CRH by gestational age between squirrel monkey dams that produced a live infant versus a stillborn infant were tested by ANOVA. The pattern of the residuals (i.e. always positive, always negative, mixed) between mothers of live and stillborn infants was tested by chi-square.
Results
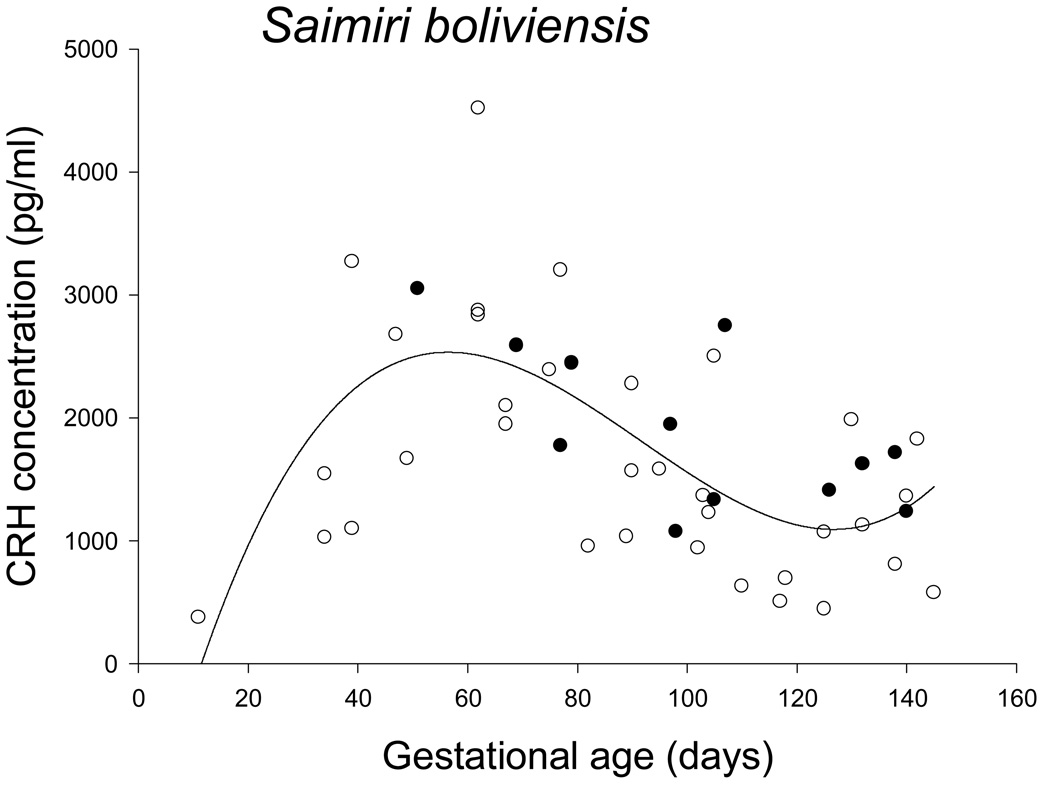
The pattern of maternal circulating CRH across pregnancy in squirrel monkeys was qualitatively similar to that found in the common marmoset by Power and colleagues [2006]. Circulating CRH concentration rapidly increased to a peak between days 45 and 70 of gestation (mean CRH = 2694 ± SEM 276 pg/ml), and then declined to a mean of 961 ± SEM 213 pg/ml between days 110 and 130 of gestation, after which it appeared to begin to increase again. The pattern was best described by a cubic function (R2 = .481) with a peak at about day 55 of gestational age, an inflection point at day 95 of gestational age, followed by a local minimum about two weeks prior to parturition, or around day 136 of gestational age (Figure 2). Although the curve estimations for linear (R2 = .135), quadratic (R2 = .234) and exponential (R2 = .085) functions were all significant, they were significantly less good fits to the data than was the cubic function.
Figure 2.
Maternal circulating CRH in pregnant squirrel monkeys; open circles identify samples from live births and filled circles identify samples from term stillbirths. The best fit curve was a cubic function.
Infant birth weight was not associated with residual deviations of circulating CRH from the fit cubic function in squirrel monkeys. There also appeared to be no qualitative difference in the gestational pattern of circulating CRH between Saimiri females producing live or stillbirths (Figure 2), with no significant mean difference between the residuals from the cubic function estimation between live (−84.4 ± 151.1 pg/ml) and still (171.2 ± 201.9 pg/ml) births (ANOVA: F = 0.881, df = 1,12, p = .367). However, three females had residual values for circulating CRH always below zero (1 stillbirth and 2 live births), 9 females had residual values that were both negative and positive (1 stillbirth and 8 live births), and two 2 females had residual values that were always positive (2 stillbirths). This pattern was significantly different from chance (chi-square: p = .041), with stillbirths overrepresented in those females that showed consistently higher values than the mean cubic function (2 of 2 births, 100%) as compared to those with both negative and positive residuals or consistently lower values (2 of 12 births, 17%) (chi-square: p < .02).
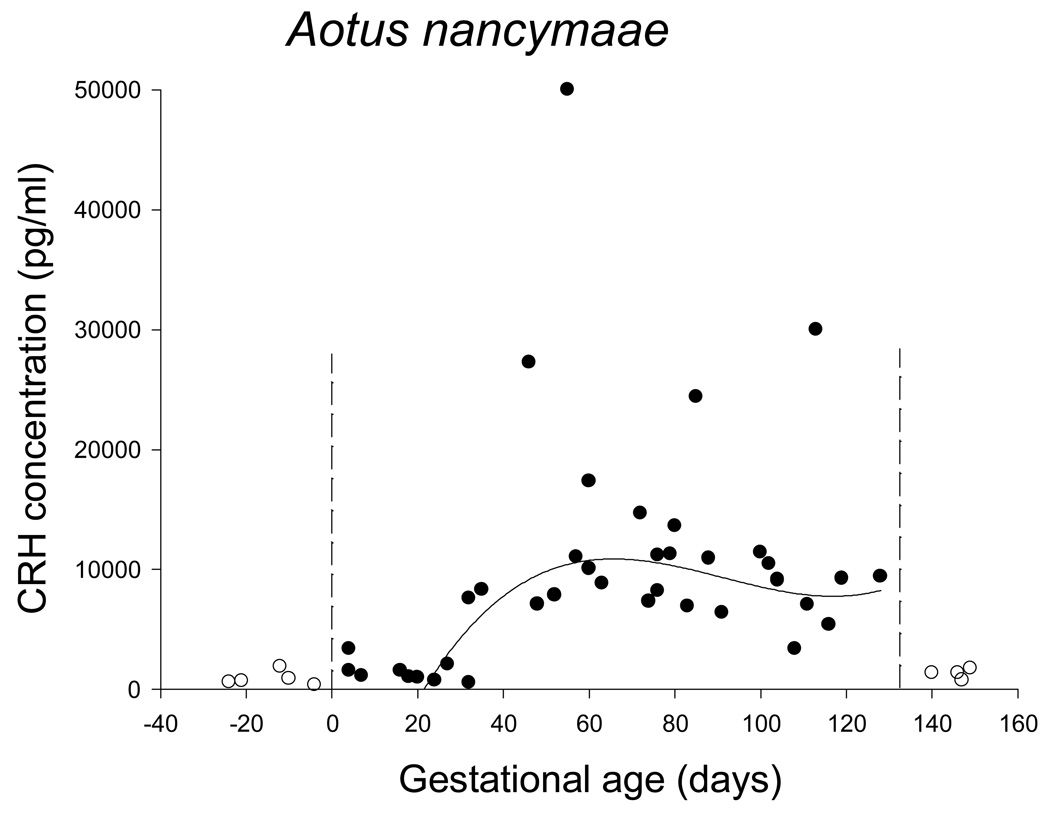
The pattern of circulating CRH concentration was not as clear in the owl monkeys (Figure 3). Firstly, nonpregnant values for CRH immunoreactivity were not zero, although the values were consistent and much lower than pregnant values (1078 ± SE 177 pg/ml versus 9949 ± SE 1514 pg/ml, p = .008). Before day 30 of gestation maternal circulating CRH concentration was not significantly different from baseline, nonpregnant values (1559 ± SE 299 pg/ml, p=.176). After day 30 of gestation mean CRH concentration was significantly higher than nonpregnant or early (before day 30) values (12114 ± SE 1699 pg/ml, p=.001). There was no clear peak, however, and later gestation samples did not differ from mid gestation samples. A linear regression of CRH after day 30 of gestation was not significant. Although the quadratic, cubic and exponential curve estimations all returned significant results, none of the R2 values were greater than .332. There were 4 samples with very high values for CRH (> 20,000 pg/ml); eliminating these outliers and running the curve estimation from day 20 of gestation to eliminate part of the long tail of baseline values in early gestation dramatically improved the fit for the cubic functions (R2 = .551), as illustrated in Figure 3. The female that gave birth to twins did not have exceptional values for circulating CRH.
Figure 3.
Maternal circulating CRH in pregnant owl monkeys. Open circles identify samples from before or after gestation; closed circles identify samples from during gestation. Vertical dashed lines indicate the beginning and end of gestation. Excluding the four highest values during gestation and all values before day 20 of gestation resulted in the best fit curve for maternal circulating CRH across gestation being a cubic function (shown).
Discussion
Placental expression of CRH appears to be a unique reproductive adaptation for anthropoid primates. A key function of placental CRH expression is to stimulate the fetal adrenal zone to synthesize and release DHEAS, which the placenta then converts to estrogens. Another function is to stimulate the maternal adrenal gland and the transitional zone of the fetal adrenal gland (both directly and through stimulation of the fetal pituitary) to synthesize and release cortisol. Cortisol serves to drive the maternal-placental-fetal adrenal axis through a positive feed-back system [Robinson et al., 1988] which results in increasing estrogen production over gestation [Lockwood, 2004; Rainey et al., 2004]. Maternal cortisol also is likely involved in many of the metabolic adjustments necessary for successful pregnancy, such as the increase in maternal metabolic rate and insulin resistance [Damjanovic et al., 2009]. Circulating cortisol in the fetus serves to mature fetal organs. Thus the progression of gestation is appropriately linked to the timing of fetal development, and infants are born with organs (especially lungs) appropriately matured for extra-uterine life [McLean et al., 1995; Challis et al., 2000].
The results from this study provide further evidence that there are two patterns of placental CRH expression and release among anthropoid primates, with monkeys differing from the ape-human pattern. Maternal circulating CRH in squirrel (Figure 2) and owl monkeys (Figure 3) is qualitatively similar to that of baboons and common marmosets (Figure 4), with a peak in mid gestation.
Figure 4.
The patterns of maternal circulating CRH across pregnancy in (A) baboons (Papio hamydryas), data from Goland et al., 1992; and (B) common marmosets (Callithrix jacchus), data from Power et al., 2006. The arrow indicates a value from a triplet pregnancy that subsequently aborted.
The function of the early peak in maternal circulating CRH common to all monkeys so far studied is unknown. In common marmosets (Callithrix jacchus) peak maternal circulating CRH coincides with a period of significant fetal growth and development [Power et al., 2006], implying that placental CRH performs additional important functions beyond the endocrine circuit mentioned above that coordinates the progression and ending of pregnancy. These other possible functions may be lineage-specific (i.e. monkeys differ from apes), but also might reflect different functions for CRH at different times during gestation, or for different target tissues. Placental CRH produced during gestation is likely to have multiple effects on many maternal and fetal organs besides the pituitary and adrenals, and these effects will likely be tissue-specific.
Maternal excretion of estrogens and cortisol has been shown to increase in early-to-mid gestation in monkey species that have been studied. For example, maternal urinary excretion of cortisol significantly increased in successful common marmoset pregnancies starting around the tenth week of gestation, reached a peak value by the 14th week and then was relatively constant until parturition [Tardif et al., 2005]. The timing of the increase in urinary cortisol corresponds to peak maternal circulating CRH in this species (Figure 4). In several New World monkeys, maternal urinary estrogen excretion is known to increase early in gestation and then to decline after mid-gestation, though, of course, remaining elevated relative to the nonpregnant state: Callithrix jacchus [Eastman et al., 1984; Tardif et al., 2005; Saltzman et al., 2008], Callimico goeldii [Ziegler et al., 1990], Saguinus fuscicollis [Heistermann and Hodges, 1995], and S. oedipus [Ziegler et al., 1987]. In C. jacchus, the increase in excreted estrogen to a peak value again roughly corresponds to the time of peak maternal CRH. CRH has been shown to stimulate estrogen production in cultured human placental cells [You et al., 2006]. Urinary chorionic gonadotropin (CG) excretion peaks in C. jacchus at about 60 days of gestation, and then rapidly declines [Saltzman et al., 2008]; thus urinary CG excretion declines as maternal CRH increases. Finally, maternal urinary androgen excretion in another marmoset (C. geoffroyi) steadily increases to a peak between days 70 and 100 of gestation and then rapidly declines [French et al., 2009], again mirroring to a large extent the pattern of maternal circulating CRH in the closely related C. jacchus. Thus, the early-to-mid gestational peak in maternal circulating CRH may indeed be associated with maternal-placental-fetal steroid production circuits in monkeys.
Interpretation of the two known different patterns of anthropoid placental CRH expression is complicated by the fact that most, though possibly not all, anthropoid primates exhibit detectable levels of circulating CRH binding protein (CRH-BP) of either hepatic or placental origin [Bowman et al., 2001]. Circulating CRH-BP has been detected in pregnant and nonpregnant humans and chimpanzees, pregnant gorillas, and in nonpregnant orangutans, gibbons, lion-tailed macaques, common marmosets and squirrel monkeys, but not in ruffed lemur, horse or sheep. Surprisingly, CRH-BP was not detected in nonpregnant mandrill, baboon and spider monkey [Bowman et al., 2001]. Thus hepatic expression of CRH-BP is not consistent among anthropoids, being expressed in apes, but variably among monkeys. Human placenta expresses CRH-BP [Petraglia et al., 2005]; studies of pregnant monkeys are required to determine whether circulating CRH-BP is a common feature of anthropoid pregnancy. If so, the time course of circulating maternal CRH-BP will be important to determine in non-human primates, given its putative regulation of “free” CRH levels in human pregnancy [Linton et al., 1993; McLean et al., 1995].
The pattern of maternal circulating CRH-BP during gestation is known for humans [Linton et al., 1993] and gorillas [Smith et al., 1999] (stable until the third trimester, then a decrease in maternal serum CRH-BP concentration), and chimpanzees [Smith et al., 1999] (stable throughout gestation), but remains to be determined in other anthropoids. In humans, the concentration of maternal serum CRH-BP in early pregnancy is probably sufficient that most placental CRH secreted into the maternal compartment is bound. Placental CRH is thought to have its major effects on maternal physiology in the third trimester, as the concentration of CRH begins to equal and then exceed that of CRH-BP. It is unknown whether the early-to-mid gestational peak in placental CRH expression in baboons, common marmosets, squirrel monkeys, and owl monkeys results in substantial free CRH in maternal circulation.
Maternal physiology and behavior can affect placental CRH expression [Erickson et al., 2001; Herrmann et al., 2001], and it is reasonable to hypothesize that placental CRH released into the maternal compartment can and will have significant effects on maternal physiology. The same is true for fetal physiology. In human pregnancy, maternal circulating CRH has been suggested to serve as a signal of adversity [Schulkin, 1999]. Pregnancies destined to be delivered preterm have early and higher increases of maternal circulating CRH [McLean et al., 1995; Hobel et al., 1999; Majzoub et al., 1999; Smith et al., 2009]. Maternal circulating CRH is higher in preeclampsia [Goland et al., 1995] and several other pregnancy complications including multiple gestations [Warren et al., 1990]. In this small sample, the timing of maternal CRH was not different between live births and stillbirths in squirrel monkeys. However, the two females that consistently had circulating CRH values above the mean values of the cubic function both delivered a still born infant, a frequency significantly greater than those of females with more mixed or consistently low CRH values across pregnancy. The owl monkey who delivered twins did not exhibit any difference in maternal circulating CRH. In contrast, common marmoset peak maternal circulating CRH depended on litter size, being highest in triplet pregnancies (Figure 4). Aborted pregnancies in common marmosets are associated with a lack of an increase of maternal urinary cortisol excretion [Tardif et al., 2005]. A single case of an aborted triplet pregnancy was associated with a low value for maternal circulating CRH during the expected peak time period [Power et al., 2006]. However, the low values for cortisol and CRH likely represented placental failure, rather than a specific regulatory disruption. Overall, the data are not yet sufficient to specify whether or how disruptions in the pattern of maternal circulating CRH associate with pregnancy complications or poor birth outcomes in monkeys, but the present report advances the hypothesis that consistently high maternal CRH during pregnancy may be associated with increased risk of stillbirth.
The non-zero values for CRH immunoreactivity outside of pregnancy in Aotus is puzzling. It is possible that CRH from some organ other than placenta is reaching peripheral circulation in owl monkeys; a low level of circulating CRH immunoreactivity has been found in the horse, with no effect of pregnancy [Ellis et al., 1994]. Alternatively, there may be a circulating factor in Aotus blood that cross-reacts with CRH antibodies. The CRH protein family consists of at least four ligands (CRH and urocortins I, II and III), two receptors with multiple splice variants, and the binding protein. CRH immunoreactivity values were not different from those outside of pregnancy in early owl monkey gestation; but after day 30 values were significantly elevated until parturition. Moreover, dilution analysis demonstrated parallelism of estimated CRH signal in Aotus samples across the range of pregnancy-associated elevations with signal from authentic human CRH standard. Thus we have confidence in the existence of a pregnancy-associated rise in circulating CRH in Aotus, which we hypothesize derives from placental CRH production.
The consistency in the qualitative pattern of maternal circulating CRH found in an Old World monkey (Figure 4) and now in three species of New World monkey (Figures 2, 3, and 4) suggests that it represents the ancestral pattern that arose before the last common ancestor of Old and New World monkeys. The pattern seen in great apes and humans thus is likely derived. Data from the smaller ape species (hylobatids) and from tarsiers and lorises would be helpful to further elucidate the evolutionary path of placental CRH expression in primates, as would data from more Old World monkey species, especially colobines.
Acknowledgements
This research was partially supported by PHS grants P40 RR01254, 5R24 RR020052 and P01 DK26741. The views and conclusions expressed by the authors are their own and do not necessarily represent the official views of the National Institutes of Health (NIH) or the National Center for Research Resources (NCRR). The research was conducted in full compliance with the laws of the United States of America; the research protocols were approved by the Institutional Animal Care and Use Committee of the University of South Alabama.
References
- Bassett JL, Foote SL. Distribution of corticotropin-releasing factor-like immunoreactivity in squirrel monkey (Saimiri sciureus) amygdala. J Comp Neurol. 1992;323:91–102. doi: 10.1002/cne.903230108. [DOI] [PubMed] [Google Scholar]
- Bowman ME, Lopata A, Jaffe RB, Golos TG, Wickings J, Smith R. Corticotropin-releasing hormone-binding protein in primates. Am J Primatol. 2001;53:123–130. doi: 10.1002/1098-2345(200103)53:3<123::AID-AJP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, Lowry PJ. Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab. 1987;64:1054–1059. doi: 10.1210/jcem-64-5-1054. [DOI] [PubMed] [Google Scholar]
- Cha CI, Foote SL. Corticotropin-releasing factor in olivocerebellar climbing-fiber system of monkey (Saimiri sciureus and Macaca fascicularis): parasagittal and regional organization visualized by immunohistochemistry. J Neurosci. 1988;8:4121–4137. doi: 10.1523/JNEUROSCI.08-11-04121.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic SS, Stojic RV, Lalic NM, Jotic AZ, Macut DP, Ognjanovic SI, Petakov MS, Popovic BM. Relationship between basal metabolic rate and cortisol secretion throughout pregnancy. Endocr. 2009;35:262–268. doi: 10.1007/s12020-008-9137-z. [DOI] [PubMed] [Google Scholar]
- Eastman SAK, Makawati DW, Collins WP, Hodges JK. Pattern of excretion of urinary steroid metabolites during the ovarian cycle and pregnancy in the marmoset monkey. J Endocrinl. 1984;102:19–26. doi: 10.1677/joe.0.1020019. [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Livesey JH, Donald RA. Horse plasma corticotrophin-releasing hormone (CRH): characterisation and lack of a late gestational rise or a plasma CRH-binding protein. J Endocrinol. 1994;143:455–460. doi: 10.1677/joe.0.1430455. [DOI] [PubMed] [Google Scholar]
- Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, Schulkin J. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86:2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- Foote SL, Cha CI. Distribution of corticotropin-releasing-factor-like immunoreactivity in brainstem of two monkey species (Saimiri sciureus and Macaca fascicularis): an immunohistochemical study. J Comp Neurol. 1988;276:239–264. doi: 10.1002/cne.902760208. [DOI] [PubMed] [Google Scholar]
- French JA, Smith AS, Birnie AK. Maternal androgen levels in female marmosets (Callithrix geoffroyi) vary across trimesters but do not vary with the sex ratio of litters. Gen Comp Endocrinol. 2009 doi: 10.1016/j.ygcen.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. 1988;82:287–292. doi: 10.1172/JCI113585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Winter JA, Jenkins SL, Tame JD, Abrams LM, Ding X-Y, Nathanielsz PW. Changes in fetal plasma corticotropin-releasing hormone during androtenedione-induced labor in the rhesus monkey: lack of an effect on the fetal hypothalamo-pituitary-adrenal axis. Endocrinol. 1998;139:2803–2810. doi: 10.1210/endo.139.6.6044. [DOI] [PubMed] [Google Scholar]
- Goland RS, Wardlaw SL, et al. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab. 1986;63:1199–1203. doi: 10.1210/jcem-63-5-1199. [DOI] [PubMed] [Google Scholar]
- Goland RS, Wardlaw SL, Fortman JD. Plasma corticotropin-releasing factor concentrations in the baboon during pregnancy. Endocrinology. 1992;131:1782–1786. doi: 10.1210/endo.131.4.1396323. [DOI] [PubMed] [Google Scholar]
- Goland RS, Tropper PJ, Warren WB, Stark RI, Jozak SM, Conwell IM. Concentrations of corticotropin-releasing hormone in the umbilical cord blood of pregnancies complicated by preeclampsia. Reprod Fertil Develop. 1995;7:1227–1230. doi: 10.1071/rd9951227. [DOI] [PubMed] [Google Scholar]
- Grino M, Chrousos GP, Margioris AN. The corticotropin-releasing hormone gene is expressed in human placenta. Biochem Biophys Res Commun. 1987;148:1208–1214. doi: 10.1016/s0006-291x(87)80261-9. [DOI] [PubMed] [Google Scholar]
- Heisterman M, Hodges JK. Endocrine monitoring of the ovarian cycle and pregnancy in the saddle-back tamarin (Saguinus fuscicollis) by measurement of steroid conjugates in urine. Am J Primatol. 1995;35:117–128. doi: 10.1002/ajp.1350350204. [DOI] [PubMed] [Google Scholar]
- Herrmann TS, Siega-Riz AM, Hobel CJ, Aurora C, Dunkel-Schetter C. Prolonged periods without food intake during pregnancy increase risk for elevated maternal corticotrophin-releasing hormone concentrations. Am J Obstet Gynecol. 2001;185:403–412. doi: 10.1067/mob.2001.115863. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol. 2009;512:825–848. doi: 10.1002/cne.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Martin RD, Dixson AF, Rudder BCC. Gestation and inter-birth intervals in the owl monkey (Aotus trivirgatus griseimembra) Folia Primatol. 1979;31:165–175. doi: 10.1159/000155881. [DOI] [PubMed] [Google Scholar]
- Jones SA, Brooks AN, Challis JR. Steroids modulate corticotropin-releasing hormone production in human fetal membranes and placenta. J Clin Endocrinol Metab. 1989;68:825–830. doi: 10.1210/jcem-68-4-825. [DOI] [PubMed] [Google Scholar]
- Kallen CB. Steroid hormone synthesis in pregnancy. Obstet Gynecol Clin N Am. 2004;31:795–816. doi: 10.1016/j.ogc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Woods RJ, Eben F, Wolfe CD, Behan DP, Potter E, Vale WW, Lowry PJ. Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76:260–262. doi: 10.1210/jcem.76.1.8421097. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ. The initiation of parturition at term. Obstet Gynecol Clinics N Amer. 2004;31:935–947. doi: 10.1016/j.ogc.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Majzoub JA, McGregor JA, Lockwood CJ, Smith R, Taggart MS, Schulkin J. A central theory of preterm and term labor: putative role for corticotropin-releasing hormone. Am J Obstet Gynecol. 1999;180:S232–S241. doi: 10.1016/s0002-9378(99)70707-6. [DOI] [PubMed] [Google Scholar]
- McLean M, Bistis A, Davies JJ, Woods R, Lowry PJ, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Florio P, Vale WW. Placental expression of neurohormones and other neuroactive molecules in human pregnancy. In: Power ML, Schulkin J, editors. Birth, distress and disease: Placenta-brain interactions. Cambridge, UK: Cambridge University Press; 2005. pp. 16–73. [Google Scholar]
- Power ML, Schulkin J. Functions of corticotropin-releasing hormone in anthropoid primates: from brain to placenta. Am J Hum Biol. 2006;18:431–447. doi: 10.1002/ajhb.20521. [DOI] [PubMed] [Google Scholar]
- Power ML, Bowman ME, Smith R, Zieglar TE, Layne DG, Schulkin J, Tardif SD. The pattern of maternal serum corticotropin-releasing hormone concentration during pregnancy in the common marmoset (Callithrix jacchus) Am J Primatol. 2006;68:181–188. doi: 10.1002/ajp.20215. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Rehman KS, Carr BR. Fetal and maternal adrenals in human pregnancy. Obstet Gynecol Clin N Am. 2004;31:817–835. doi: 10.1016/j.ogc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci USA. 1988;85:5244–5248. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Arbiser JL, Emanuel RL, Majzoub JA. Species-specific placental corticotropin releasing hormone messenger RNA and peptide expression. Moll Cell Endocrinol. 1989;62:337–341. doi: 10.1016/0303-7207(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Liedl KJ, Salper OJ, Pick RR, Abbott DH. Post-conception reproductive competition in cooperatively breeding common marmosets. Horm Behav. 2008;53:274–286. doi: 10.1016/j.yhbeh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT. Immunoreactive corticotropin-releasing factor is present in human maternal serum during the third trimester of pregnancy. J Clin Endocrinol Metab. 1984;59:812–814. doi: 10.1210/jcem-59-4-812. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Shinkawa O, Margioris AN, Liotta AS, Sato S, Murakami O, Go M, Shimizu Y, Hanew K, Yoshingaga K. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor and delivery. J Clin Endocrinol Metab. 1987;64:224–229. doi: 10.1210/jcem-64-2-224. [DOI] [PubMed] [Google Scholar]
- Schulkin J. CRH signals adversity in both the placenta and the brain: regulation by glucocorticoids and allostatic overload. J Endocrinol. 1999;161:340–356. doi: 10.1677/joe.0.1610349. [DOI] [PubMed] [Google Scholar]
- Smith R, Chan E-C, Bowman ME, Harewood WJ, Phippard AF. Corticotropin-releasing hormone in baboon pregnancy. J Clin Endocrinol Metab. 1993;76:1063–1068. doi: 10.1210/jcem.76.4.8473382. [DOI] [PubMed] [Google Scholar]
- Smith R, Wickings J, Bowman MB, Belleoud A, Bubreuil G, Davies JJ, Madsen G. Corticotropin-releasing hormone in Chimpanzee and Gorilla pregnancy. J Clin Endocrinol Metab. 1999;84:2820–2825. doi: 10.1210/jcem.84.8.5906. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, Nicholson R, Clifton V, Zakar T. The regulation of human parturition. In: Power ML, Schulkin J, editors. Birth, distress and disease: Placenta-brain interactions. Cambridge, UK: Cambridge University Press; 2005. pp. 74–87. [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman MA, McGrath SA, Bisits AM, McElduff P, Giles WB, Smith DW. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Ziegler TE, Power M, Layne DG. Endocrine changes in full term pregnancies and pregnancy loss due to energy restriction in the common marmoset (Callithrix jacchus) J Clin Endocrinol Metab. 2005;90:335–339. doi: 10.1210/jc.2004-1064. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;78:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vale W, Vaughan J, Yamamoto G, Bruhn T, Douglas C, Dalton D, Rivier C, Rivier J. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–577. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]
- Warren WB, Goland RS, Wardlaw SL, Stark RI, Fox HE, Conwell IM. Elevated maternal plasma corticotropin releasing hormone levels in twin gestation. J Perinat Med. 1990;18:39–44. doi: 10.1515/jpme.1990.18.1.39. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. J Physiol. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoeman RR, Williams LE, Hazelton JM, Ricker RB, Abee RC. The effects of brief manual restraint, prior conditioning and ketamine sedation on lutenizing hormone and estradiol levels in the female Bolivian squirrel monkey. Am J Primatol. 1988;14:454–455. [Google Scholar]
- You X, Yang R, Tang X, Gao L, Ni X. Corticotropin-releasing hormone stimulates estrogen biosynthesis in cultured human trophoblasts. Biol Reprod. 2006;74:1067–1072. doi: 10.1095/biolreprod.105.049361. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Eman S, Bridson WE, Snowdon CT. Urinary gonadotropin and estrogen excretion during postpartum estrus, conception and pregnancy in the cotton-top tamarin (Saguinus Oedipus Oedipus) Am J Primatol. 1987;12:127–140. doi: 10.1002/ajp.1350120202. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Snowdon CT, Warneke M, Bridson WE. Urinary excretion of oestrone conjugates and gonadotropins during pregnany in the Goeldi’s monkey. J Reprod Fertil. 1990;89:163–168. doi: 10.1530/jrf.0.0890163. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]