Abstract
Many diseases are characterized by undesired or pathological neural activity. The local delivery of high frequency currents has been shown to be an effective method for blocking neural conduction in peripheral nerves and may provide a therapy for these conditions. To date, all studies of high frequency conduction block have utilized extraneural (cuff) electrodes to achieve conduction block. In this study we show that high frequency conduction block is feasible using intrafascicular electrodes.
Keywords: Nerve Block, High Frequency, Intrafascicular, Stimulation, Electrode
Introduction
Many diseases are characterized by undesired peripheral neural activity. For example, spasticity (in stroke, cerebral palsy, spinal cord injury and multiple sclerosis), chorea, tics and intractable hiccups are all associated with pathological motor activity. Numerous painful conditions including cancer pain, neuromas and neuralgias are associated with pathological afferent activity. These conditions are typically treated pharmacologically, but they may ultimately require surgical, chemical or radiation procedures which result in destruction of the nerve in treatment resistant cases [1, 2].
Numerous studies have shown that nerve conduction can be blocked through the delivery of high frequency alternating current (HFAC) to a peripheral nerve [3–5]. This type of nerve block has been shown to be a non-synaptic phenomenon localized to the site of the electrode [3]. The mechanism of action has been explored through modeling studies and is postulated to result from altered sodium channel dynamics due to membrane depolarization [6, 7] or diminished action potential amplitudes due to large potassium currents [8]. HFAC block has the potential to provide a non-destructive, nerve-sparing therapy for the conditions described above. To date, all studies of HFAC block have utilized extraneural (nerve cuff) electrodes. In this study we report that HFAC block is feasible using an intrafascicular electrode.
The technology for intrafascicular electrodes has advanced such that stable, biocompatible and chronically safe implementations are possible [9–11]. Electrodes employing microwires [12, 13], polymer substrates [14, 15] and silicon substrates [16–18] are in development. Intrafascicular electrodes provide both selective activation and recording from target fascicles or neurons in animal [12, 17, 19, 20] and acute human studies [21–23]. Because of their demonstrated potential for chronic implementation, fascicle-selectivity and minimally invasive placement [23], intrafascicular electrodes are an attractive option for HFAC block. This study demonstrates that HFAC block is possible using intrafascicular electrodes, which is of significant interest because of the potential to utilize this method for acute pain relief applications such as post-surgical pain.
Methods
Animal Experiments and Surgical Procedure
Experiments were performed in three adult Sprague-Dawley rats under institutional approval. The surgical procedure has been described previously [4]. The animals were anesthetized with intraperitoneal injections of Nembutal (phenobarbital sodium). An incision was made along the lateral aspect of the leg and thigh. The biceps femoris was reflected, and the sciatic nerve was exposed from 1 cm lateral to the spine to its distal branching into the tibial and common peroneal nerves. The sural and common peroneal nerves were severed. The gastrocnemius-soleus muscle complex was dissected, and the calcaneal tendon was severed from its distal attachment. The tibia was fixed to the table using a clamp, and the calcaneal tendon was attached to a force transducer using a toothed clamp.
A bipolar cuff electrode was placed on the proximal part of the sciatic nerve to provide supramaximal 20 μsec, 1 Hz stimulation pulses to the nerve. These pulses generated 1 Hz muscle twitches whose absence was used to verify neural conduction block [3, 4]. For two animals, two 45 gauge microwire electrodes (Nicolet #019-772900) were inserted into the tibial fascicle of the sciatic nerve just proximal to the tibial/common peroneal branch point. The microwires were used as a bipolar blocking electrode and were inserted using fine forceps or a 25 gauge needle. The wires had deinsulated tips (2mm long), and were placed with a 1.5 – 2mm edge-edge separation of the deinsulated contacts within the fascicle (inset of Figure 1). In the third animal, two 200 μm diameter Teflon-insulated tungsten microwires were inserted into the sciatic nerve. The microwires had 2 mm of Teflon insulation stripped from the end and were placed with a ~3mm edge-edge contact separation. For two of the animals, a 40 kHz charge-balanced sinusoidal current that was suprathreshold for nerve block was delivered to the nerve. In the third animal, randomized trials were performed to determine the threshold for block (the minimum current to produce complete motor block) at 20 kHz, 30 kHz and 40 kHz with a resolution of 100 μA using a procedure described previously [4].
Figure 1.
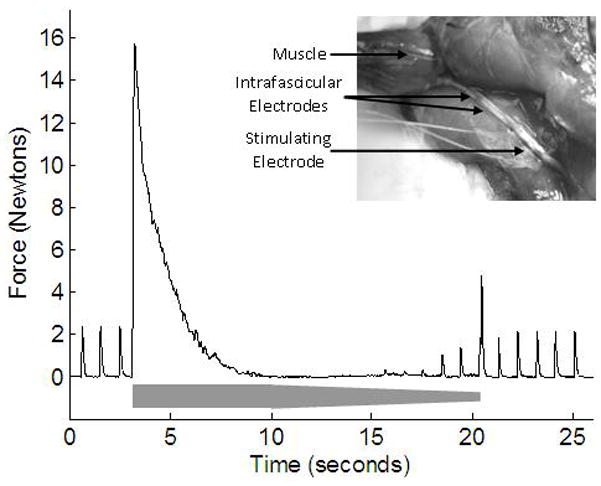
Recorded force of the gastrocnemius muscle during testing of HFAC nerve block threshold. The amplitude of the 20 kHz sinusoidal waveform is shown schematically in grey, starting at 2.2 mA. The absence of twitches between 10 to 14 s indicates complete conduction block. From 15 s, with further decrease in the HFAC amplitude, twitches reappear. The inset image shows the sciatic nerve preparation with a stimulating cuff electrode and intrafascicular blocking electrodes.
Results
A complete and reversible neural conduction block was achieved in all three animals with the delivery of a 40 kHz sinusoidal current via the bipolar intrafascicular electrodes. The current range used to achieve complete block across the three rats was 1.4 mA – 9.0 mA (peak values). Figure 1 shows an example of complete conduction block achieved using an intrafascicular electrode and is typical of complete HFAC motor block using this nerve preparation [4]. The trial demonstrates measurement of the block threshold by decreasing the blocking current amplitude until partial block resulted in small amplitude muscle twitches (from proximal stimulation). HFAC was delivered with an amplitude of 2.2 mA from 3 to 10 seconds in Figure 1 and was decremented in 0.1mA/sec steps until 21 seconds. The proximally generated nerve impulses are completely blocked by the intrafascicular electrode (as indicated by the absence of twitches) until the HFAC amplitude was decreased to below block threshold, where partial conduction block occurs. After the termination of the blocking current at ~21 seconds, the proximally generated twitches return as full nerve conduction is restored
Block thresholds were measured at three frequencies in one rat. Mean thresholds for the repeated randomized trials (four repeats) were 1.53 mA, 3.58 mA and 6.55 mA for 20 kHz, 30 kHz and 40 kHz, respectively. The standard deviation of the thresholds was small: 96μA, 50μA and 58μA, respectively.
Discussion
We have demonstrated that it is feasible to achieve complete neural conduction block of a mammalian peripheral nerve using intrafascicular electrodes. Observations such as the neural activation at the onset of the HFAC delivery, the increase in block threshold with frequency and rapid recovery of neural conduction are qualitatively consistent with a previous study in which conduction block was achieved using cuff electrodes [4]. Intrafascicular electrodes could potentially be used to provide selective blocking of individual fascicles in a nerve. This could be useful for applications such as selectively blocking spastic muscles or selectively blocking fascicles involved in acute or chronic pain. Additionally, intrafascicular electrodes are regularly used in human percutaneous electroneurography studies [23] and may be conducive to minimally invasive implantation techniques for an electrical nerve block therapy system.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Biomedical Imaging and Bioengineering Grant No. R01-EB-002091.
Abbreviations
- HFAC
High Frequency Alternating Current
Footnotes
Cleveland FES Center, Hamman 601, 2500 MetroHealth Drive, Cleveland, OH 44109
References
- 1.Lopez B, Hamlyn P, Zakrzewska J. Systematic Review of Ablative Neurosurgical Techniques for the Treatment of Trigeminal Neuralgia. Neurosurgery. 2004;54(4) doi: 10.1227/01.neu.0000114867.98896.f0. [DOI] [PubMed] [Google Scholar]
- 2.Steinbok P. Selective dorsal rhizotomy for spastic cerebral palsy: a review. Child’s Nervous System. 2007;23(9):981–990. doi: 10.1007/s00381-007-0379-5. [DOI] [PubMed] [Google Scholar]
- 3.Kilgore K, Bhadra N. Nerve conduction block utilizing high-frequency alternating current. Medical and Biological Engineering and Computing. 2004;42(3):394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 4.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle and Nerve. 2005;32(6):782. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 5.Bowman B, McNeal D. Response of Single Alpha Motorneurons to High-Frequency Pulse Trains. Appl Neurophysiol. 1986;49:121–138. doi: 10.1159/000100137. [DOI] [PubMed] [Google Scholar]
- 6.Williamson RP, Andrews BJ. Localized electrical nerve blocking. IEEE Transactions on Biomedical Engineering. 2005;52(3):362. doi: 10.1109/TBME.2004.842790. [DOI] [PubMed] [Google Scholar]
- 7.Bhadra N, et al. Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci. 2007;22:313–326. doi: 10.1007/s10827-006-0015-5. [DOI] [PubMed] [Google Scholar]
- 8.Tai Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005;13(3):415. doi: 10.1109/TNSRE.2005.847356. [DOI] [PubMed] [Google Scholar]
- 9.Lefurge T, et al. Chronically implanted intrafascicular recording electrodes. Annals of Biomedical Engineering. 1991;19(2):197. doi: 10.1007/BF02368469. [DOI] [PubMed] [Google Scholar]
- 10.Branner A, et al. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. Biomedical Engineering, IEEE Transactions on. 2004;51(1):146–157. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence S, et al. Long-Term Biocompatibility of Implanted Polymer-Based Intrafascicular Electrodes. Journal of Biomedical Materials Research. 2002;63(5):501. doi: 10.1002/jbm.10303. [DOI] [PubMed] [Google Scholar]
- 12.Smit JPA, Rutten WLC, Boom HBK. Endoneural selective stimulating using wire-microelectrode arrays. Rehabilitation Engineering, IEEE Transactions on. 1999;7(4):399–412. doi: 10.1109/86.808943. [DOI] [PubMed] [Google Scholar]
- 13.Bowman B, Erickson R. Acute and chronic implantation of coiled wire intraneural electrodes during cyclical electrical stimulation. Annals of Biomedical Engineering. 1985;13(1):75. doi: 10.1007/BF02371251. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence S, Dhillon G, Horch K. Fabrication and characteristics of an implantable, polymer-based, intrafascicular electrode. Journal of Neuroscience Methods. 2003;131(1–2):9. doi: 10.1016/s0165-0270(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 15.McNaughton TG, Horch K. Metallized polymer fibers as leadwires and intrafascicular microelectrodes. Journal of Neuroscience Methods. 1996;70(1):103. doi: 10.1016/S0165-0270(96)00111-2. [DOI] [PubMed] [Google Scholar]
- 16.Branner A. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Research Bulletin. 2000;51(4):293. doi: 10.1016/s0361-9230(99)00231-2. [DOI] [PubMed] [Google Scholar]
- 17.Branner A. Selective Stimulation of Cat Sciatic Nerve Using an Array of Varying-Length Microelectrodes. Journal of Neurophysiology. 2001;85(4):1585. doi: 10.1152/jn.2001.85.4.1585. [DOI] [PubMed] [Google Scholar]
- 18.Rutten WLC, et al. Neuro-electronic interfacing with multielectrode arrays. Engineering in Medicine and Biology Magazine, IEEE. 1999;18(3):47–55. doi: 10.1109/51.765188. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K, Horch K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. Biomedical Engineering, IEEE Transactions on. 1993;40(5):492–494. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Stein RB. Characterization of signals and noise rejection with bipolar longitudinal intrafascicular electrodes. Biomedical Engineering, IEEE Transactions on. 1999;46(2):226–234. doi: 10.1109/10.740885. [DOI] [PubMed] [Google Scholar]
- 21.Westling G, et al. Measurement of contractile and electrical properties of single human thenar motor units in response to intraneural motor-axon stimulation. J Neurophysiol. 1990;64(4):1331–1338. doi: 10.1152/jn.1990.64.4.1331. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa J. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. Journal of Physiology (Paris) 1983;342(1):633. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagbarth KE. Microelectrode recordings from human peripheral nerves (microneurography) Muscle and Nerve. 2002;(Suppl 11):S28. doi: 10.1002/mus.10144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


