Abstract
Changes in anterior knee laxity (AKL), genu recurvatum (GR) and general joint laxity (GJL) were quantified across days of the early follicular and early luteal phases of the menstrual cycle in 66 females, and the similarity in their pattern of cyclic variations examined. Laxity was measured on each of the first 6 days of menses (M1–M6) and the first 8 days following ovulation (L1–L8) over two cycles. The largest mean differences were observed between L5 and L8 for AKL (0.32mm), and between L5 and M1 for GR (0.56°) and GJL (0.26)(P<.013). At the individual level, mean absolute cyclic changes in AKL (1.8±0.7mm, 1.6±0.7mm), GR (2.8±1.0°, 2.4±1.0°) and GJL (1.1±1.1, 0.7±1.0) were more apparent, with minimum, maximum and delta values being quite consistent from month to month (ICC2,3=.51–.98). Although the average daily pattern of change in laxity was quite similar between variables (Spearman correlation range 0.61 and 0.90), correlations between laxity measures at the individual level were much lower (range −0.07 to 0.43). Substantial, similar, and reproducible cyclic changes in AKL, GR and GJL were observed across the menstrual cycle, with the magnitude and pattern of cyclic changes varying considerably among females.
Keywords: joint laxity, menstrual cycle, anterior cruciate ligament, knee
INTRODUCTION
Joint laxity continues to be a variable of interest as we seek to uncover the underlying risk factors for ACL injury in females.1 Measures of anterior knee laxity (AKL)2–6, genu recurvatum (GR) 5,7 and general joint laxity (GJL)6,8–10 are greater in females compared to males, and both retrospective9,11–14 and prospective6,15 studies implicate an association between greater AKL, GR and/or GJL and risk of ACL injury. These findings are largely based on laxity measures taken at a single time point, yet there is evidence that joint laxity may change appreciably in response to changes in sex hormone concentrations across the menstrual cycle.16–19 Hence, the risk associated with greater magnitudes of joint laxity may also change cyclically. This periodicity is supported by epidemiological studies noting a higher proportion of ACL injuries in the follicular (both early and late) compared to luteal phase.20–24
To date, studies examining cyclic variations in joint laxity have been limited to AKL. In studies that used actual hormone concentrations to define cycle phase, females were reported to have greater AKL in the periovulatory and luteal phases of the menstrual cycle compared to the early follicular phase (menses).16–19 However, other studies examining a particular phase based on specified day(s) of the cycle have reported no cyclic changes in AKL, suggesting there is no uniform time in the cycle where all females experience these cyclic changes.2,25–28 This is consistent with findings that the magnitude and timing of cyclic changes in AKL can vary considerably among individual females18,29, in part due to the individual variability in the timing and amplitude of their sex hormone concentration changes.29,30 Cyclic variations in GJL and GR have yet to be examined. Characterizing the extent to which these variables change across the cycle is important for future prospective study designs, as the risk associated with these factors may also vary over time (perhaps in some females more than others). Also unknown is the extent to which the pattern of cyclic variations is consistent across laxity variables, or within an individual from month to month. Both have implications for determining hormone responsiveness within an individual, i.e. whether changes in a single laxity variable is sufficiently representative of changes in other laxity variables, and whether data obtained in a given month is representative for that individual over time.
We examined the change in magnitude of AKL, GR and GJL across the early follicular and early luteal phases of the menstrual cycle over two cycles, and examined the similarity in these cyclic variations between the two cycles and between the three laxity variables. Based on previous studies of AKL, our expectation was that consistent cyclic increases in GR and GJL would be observed between days in the early follicular to days in the early luteal phase of the menstrual cycle, but that the magnitude of these cyclic variations would vary considerably among females. We also expected that the pattern of cyclic variations between AKL, GR and GJL would be similar.
METHODS
Seventy females (21.5±2.6yrs, 163.9±6.6cm, 60.9±8.9kg) participated as part of a larger study on hormone mediated changes in knee joint laxity and knee joint neuromechanics. Participants were recreationally active between 2.5 and 10 hours per week for the past 3 months; had normal menstrual cycles lasting 26–32 days that varied no more than ± 1 day between months and did not use oral contraceptives or other hormone stimulating medications for the past 6 months; had no history of pregnancy or plans to become pregnant; had a body mass index < 30 (BMI = wt/ht2); were non-smokers; had no history of injury involving the osteochondral surface, ligament, tendon, capsule, or menisci; had no connective tissue disorders; and consumed no alcohol 24 hours prior to any test session. Participants signed a University approved consent form prior to enrollment.
Because previous work has identified considerable variability in the timing and magnitude of AKL changes from the early follicular to luteal phases29,30, we tracked females for two months, measuring AKL, GR and GJL each morning during the first 6 days following the onset of menses (per self report) and the first 8 days following evidence of ovulation. These test days were selected to best capture individual cyclic variations as previous research has identified minimum and maximum AKL values during these time points.16,17,19 To estimate day of ovulation, participants were provided an ovulation kit (CVS One Step Ovulation Predictor [sensitivity 20 mIU/ml LH, accuracy 99%]; CVS Corporation, Woonsocket, RI) to begin using on day 8 of their menstrual cycle. All data were obtained in the morning hours (7:00 – 9:00a.m.) prior to any physical activity, with each tested as close as possible to the same time each day within that 2 hour window (generally ± 30 minutes). While it was not possible to blind investigators to the general time of a female’s cycle, one investigator obtained the laxity measures on each day while blinded to the subject’s previous measures, and a separate investigator handled subject scheduling and data entry. All participants were tested on the dominant stance leg (preferred stance limb when kicking a ball). Prior to testing, participants attended a session to familiarize them with all study requirements. Participants were instructed to avoid any unusual or strenuous activity 2 days prior to any test day (defined as activity beyond what they normally and consistently perform on a daily basis), and to defer their daily exercise routine until after each test session. These instructions were intended to control for exercise related changes in laxity27, and to prevent muscle soreness and other changes in muscle tension from confounding daily laxity recordings. A brief questionnaire administered each morning ensured participants were adhering to study requirements.
Prior to initiation of the study, two investigators were trained on the laxity measures and established excellent intratester (day-to-day) reliability [Intraclass correlation coefficient and standard error of measurement ICC2,k(SEM) for Tester 1 = 0.96(0.3mm) for AKL, 0.97(0.5°) for GR, and 0.99(0.3pts) for GJL; Tester 2 = 0.97(0.4mm) for AKL, 0.97(0.3°) for GR, and 0.98(0.3pts) for GJL) and inter-tester reliability [ICC2,k(SEM) = .96(0.5mm) for AKL, .98(0.4°) for GR, and .98(.2pts) for GJL] on a group of 16 subjects measured on two occasions, 24–48 hours apart. To optimize measurement consistency within an individual, one examiner performed all measurements. AKL was measured as the anterior displacement of the tibia relative to the femur when a 133N posterior-to-anterior directed load was applied to the tibia using the KT-2000™ Knee Arthrometer (MEDmetric® Corp; San Diego, CA). With the knee flexed to 25±5°, three posterior directed forces were applied to the tibia to establish a zero reference point, followed by an anterior directed force of 133N to measure AKL. Surface electromyographic electrodes monitored any measurable muscle activity or guarding. To reduce measurement error, all positions were marked to ensure reproducible positioning day to day, the thighs were stabilized with a Velcro strap to minimized lower extremity rotation, and a bubble level on the device ensured a direct A-P line of pull. The device was calibrated annually to maintain accurate readings. GJL was measured with the Beighton and Horan Joint Mobility Index.31 Mobility was measured at the trunk, and bilaterally at the 5th finger, thumb, elbow, and knee, with each joint receiving a score of 1 for each criteria met: 5th finger extension ≥ 90°, ability to passively flex the wrist and abduct the thumb so that the thumb touched the volar aspect of the forearm, active elbow hyperextension ≥ 10 degrees, standing postural knee hyperextension ≥10 degrees, and ability to flex the trunk and easily place the palms of the hands flat on the floor while keeping the knees extended. The cumulative score (range 0–9) from all joints was recorded as GJL. GR was measured in supine with the distal shank on a 4 inch bolster. A 12″ goniometer equipped with extendable rods was aligned with the axis over the lateral femoral epicondyle, the stationary arm with the greater trochanter and the movable arm with the lateral malleolus. The measurement was taken while the participant contracted their quadriceps and maximally extended their knee. Genu recurvatum was recorded in degrees as a positive value.
Daily measurements for AKL, GR, and GJL obtained from the first 6 days of menses (M1–M6) and the first 8 days of the early luteal phase (L1 – L8) over two consecutive menstrual cycles were recorded for each female. At each time point, three measures were taken of AKL and GR and averaged for analysis. Separate linear repeated measures mixed models for AKL, GR, and GJL determined whether laxity values differed by cycle (controlling for day) or day (controlling for cycle), or whether there was significant cycle by day interaction. Given the known individual variability in the pattern of laxity changes across the menstrual cycle18,29, we also examined laxity changes on an individual level by extracting the minimum, maximum and delta (maximum–minimum) values for each laxity variable within each female and month. Repeated measures ANOVA and intraclass correlation coefficients (ICC2,3) and standard error of measurements (SEM) were then used to examine the consistency in these values across cycles. Finally, to better compare the patterns of variability across the 14 days for the three laxity variables on the same scale, we also plotted the z-scores for each set of daily means—from each daily mean we subtracted the overall mean for that variable, and divided by the standard deviation of the daily means for that variable. We then computed Spearman correlation coefficients for each set of comparisons of daily means of AKL, GJL, and GR, and also for each set of comparisons of absolute maximums, minimums, and deltas (maximum–minimum) of AKL, GJL, and GR. Alpha level was set for all analyses at P<.05.
RESULTS
Three of the 70 enrolled subjects were excluded because of multiple missed data sessions, and 1 was excluded due to a very short luteal phase resulting in incomplete data. Study compliance was very good for the remaining 66 subjects; occasional missed sessions (illness, travel, etc) resulted in 3 or fewer data points missing from any given test day from M1 – L6. For days L7 and L8, 5 and 8 data points were missing, respectively, due to shortened luteal phases in some subjects (i.e. they began their next cycle before 8 days post ovulation could be collected), and schedule conflicts in others (e.g. end of data collection extending into holidays).
Table 1 presents the daily group means for each laxity variable, stratified by cycle and also averaged across the two cycles. Linear mixed models revealed significant differences in AKL (P=.013), GR (P<.001) and GJL (P<.001) by day. The largest mean difference was observed between days L5 and L8 for AKL (0.32mm), and between M1 and L5 for GR (0.56°) and GJL (0.26). There were no significant main effects of cycle (P range .100 – .992), and no significant cycle*day interactions (P range .080 – .712) for any of the laxity variables, suggesting that, for each variable, the pattern of daily changes were generally consistent across the two cycles.
Table 1.
Daily Means ± Standard Deviations of Each Laxity Variable for Cycle 1, Cycle 2 and their Combined Average
| DAY | Anterior Knee Laxity (mm) | Genu Recurvatum (°) | General Joint Laxity (score) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | C1 C2 Avg | Cycle 1 | Cycle 2 | C1 C2 Avg | Cycle 1 | Cycle 2 | C1 C2 Avg | |
| Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | |
| M1 | 6.6 ± 2.2 | 6.5 ± 2.3 | 6.6 ± 2.2 | 3.7 ± 3.3 | 3.4 ± 3.2 | 3.6 ± 3.3 | 1.7 ± 1.8 | 1.9 ± 1.6 | 1.8 ± 1.7 |
| M2 | 6.6 ± 2.0 | 6.6 ± 2.2 | 6.6 ± 2.1 | 3.7 ± 3.2 | 3.8 ± 3.4 | 3.7 ± 3.3 | 1.8 ± 1.7 | 1.9 ± 1.6 | 1.8 ± 1.7 |
| M3 | 6.8 ± 2.1 | 6.6 ± 2.1 | 6.7 ± 2.1 | 3.8 ± 3.4 | 3.7 ± 3.4 | 3.8 ± 3.4 | 1.8 ± 1.6 | 2.1 ± 1.6 | 1.9 ± 1.6 |
| M4 | 6.7 ± 2.2 | 6.6 ± 2.3 | 6.7 ± 2.2 | 3.8 ± 3.5 | 3.8 ± 3.4 | 3.8 ± 3.4 | 1.9 ± 1.7 | 2.0 ± 1.7 | 2.0 ± 1.7 |
| M5 | 6.6 ± 2.1 | 6.6 ± 2.3 | 6.6 ± 2.2 | 3.7 ± 3.5 | 3.9 ± 3.4 | 3.8 ± 3.5 | 1.9 ± 1.7 | 2.0 ± 1.6 | 2.0 ± 1.6 |
| M6 | 6.6 ± 2.1 | 6.5 ± 2.3 | 6.6 ± 2.2 | 3.8 ± 3.3 | 3.8 ± 3.2 | 3.8 ± 3.3 | 1.9 ± 1.7 | 2.0 ± 1.6 | 1.9 ± 1.6 |
| L1 | 6.5 ± 2.3 | 6.6 ± 2.3 | 6.6 ± 2.3 | 3.7 ± 3.5 | 3.9 ± 3.5 | 3.8 ± 3.5 | 2.0 ± 1.6 | 2.0 ± 1.7 | 2.0 ± 1.7 |
| L2 | 6.5 ± 2.3 | 6.8 ± 2.3 | 6.7 ± 2.3 | 3.9 ± 3.6 | 4.1 ± 3.6 | 4.0 ± 3.6 | 2.0 ± 1.7 | 2.1 ± 1.7 | 2.0 ± 1.7 |
| L3 | 6.6 ± 2.2 | 6.9 ± 2.2 | 6.8 ± 2.2 | 3.9 ± 3.5 | 4.1 ± 3.5 | 4.0 ± 3.5 | 1.9 ± 1.7 | 2.1 ± 1.7 | 2.0 ± 1.7 |
| L4 | 6.6 ± 2.1 | 6.8 ± 2.2 | 6.7 ± 2.2 | 3.9 ± 3.3 | 4.2 ± 3.5 | 4.0 ± 3.4 | 2.0 ± 1.7 | 2.1 ± 1.7 | 2.1 ± 1.7 |
| L5 | 6.7 ± 2.2 | 6.9 ± 2.3 | 6.8 ± 2.3* | 4.1 ± 3.3 | 4.2 ± 3.4 | 4.2 ± 3.3* | 2.1 ± 1.6 | 2.0 ± 1.7 | 2.1 ± 1.7* |
| L6 | 6.6 ± 2.3 | 6.8 ± 2.2 | 6.7 ± 2.2 | 3.9 ± 3.3 | 4.2 ± 3.3 | 4.0 ± 3.3 | 2.0 ± 1.5 | 2.1 ± 1.7 | 2.0 ± 1.6 |
| L7 | 6.5 ± 2.2 | 6.6 ± 2.1 | 6.6 ± 2.2 | 3.9 ± 3.2 | 3.7 ± 3.4 | 3.8 ± 3.3 | 1.9 ± 1.5 | 2.0 ± 1.7 | 2.0 ± 1.6 |
| L8 | 6.4 ± 2.4 | 6.5 ± 2.2 | 6.5 ± 2.3 | 3.6 ± 3.4 | 3.9 ± 3.4 | 3.7 ± 3.4 | 1.8 ± 1.5 | 2.0 ± 1.6 | 1.9 ± 1.6 |
Largest mean difference at L5 compared to L8 (AKL), and M1 (GR and GJL) (all P>.05)
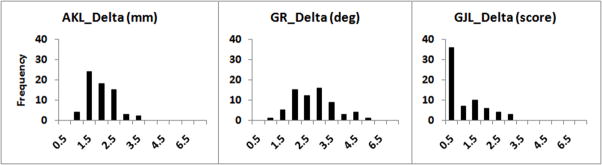
Table 2 presents the means, standard deviations, and ranges for absolute minimum, maximum and delta values for each laxity variable obtained at the individual level, stratified by cycle. Although general joint laxity (GJL) scores ranged from 0 to 7 in these data, 45% of all the GJL scores across all subjects and days were either 0 or 1, and 31% of subjects had a GJL score of either 0 or 1 for their maximum value. Across both cycles, the minimum and maximum values of each of the three variables were significantly different from one another (P<.01). Further, for each variable, the difference between minimum and maximum values was somewhat smaller for cycle 2 compared to cycle 1 (P range 0.004 – 0.012). Reliability estimates indicate a high level of month to month consistency for minimum and maximum laxity values within an individual (ICC2,3 range = .95–.98), and lower consistency (ICC2,3 range = .51–.73) for delta values (primarily due to the smaller between subject variance in this measure – see Figure 1 for the distribution of the delta values). These results suggest that there are no consistent differences in minimum, maximum, or delta laxity values across the two cycles, which further confirms the results of repeated measures mixed models. Thus, for the remaining analyses described below, we combined data across the two cycles.
Table 2.
Means, standard deviations (sd), ranges and reliability coefficients for absolute minimum, maximum and delta laxity values obtained at the individual level across two menstrual cycles
| CYCLE 1 | CYCLE 2 | Reliability | ||||
|---|---|---|---|---|---|---|
| Mean ±Sd | (Range) | Mean ±Sd | (Range) | ICC2,3 | SEM | |
| AKL (mm) | ||||||
| Minimum | 5.7 ± 2.0 | (2.0–12.4) | 5.8 ± 2.1 | (1.7–12.6) | 0.98 | 0.26 |
| Maximum* | 7.6 ± 2.3 | (3.4–14.4) | 7.4 ± 2.3 | (2.7–14.5) | 0.98 | 0.32 |
| Delta† | 1.8 ± 0.7 | (0.7–3.7) | 1.6 ± 0.6 | (0.7–3.0) | 0.71 | 0.36 |
| GR (deg) | ||||||
| Minimum | 2.5 ± 3.4 | (−2.7–14.0) | 2.7 ± 3.3 | (−2.0–14.0) | 0.97 | 0.63 |
| Maximum* | 5.3 ± 3.4 | (0.0–15.0) | 5.2 ± 3.4 | (0.0–15.0) | 0.97 | 0.55 |
| Delta† | 2.8 ± 1.0 | (1.0–6.3) | 2.4 ± 1.0 | (1.0–5.0) | 0.51 | 0.7 |
| GJL (score) | ||||||
| Minimum | 1.4 ± 1.6 | (0.0–7.0) | 1.6 ± 1.6 | (0.0–7.0) | 0.95 | 0.35 |
| Maximum* | 2.4 ± 1.8 | (0.0–7.0) | 2.3 ± 1.7 | (0.0–7.0) | 0.96 | 0.33 |
| Delta† | 1.1 ± 1.1 | (0.0–4.0) | 0.7 ± 1.0 | (0.0–4.0) | 0.73 | 0.56 |
AKL = anterior knee laxity, GR = genu recurvatum, GJL = general joint laxity;
Maximum > minimum;
Cycle 2 < Cycle 1
Figure 1.
Distribution of Individual Delta (maximum – minimum) Laxity Values Averaged over Two Cycles
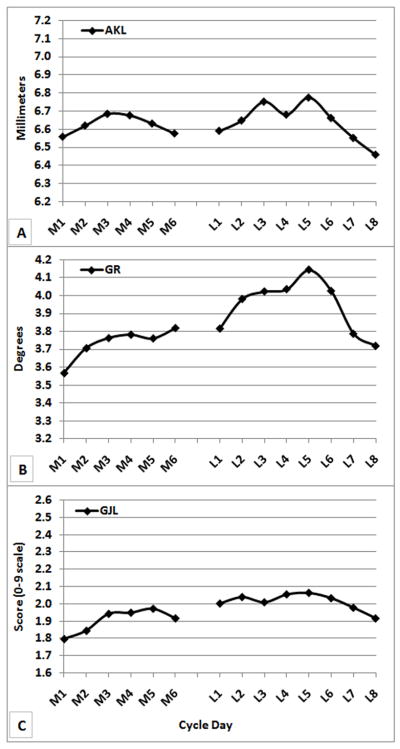
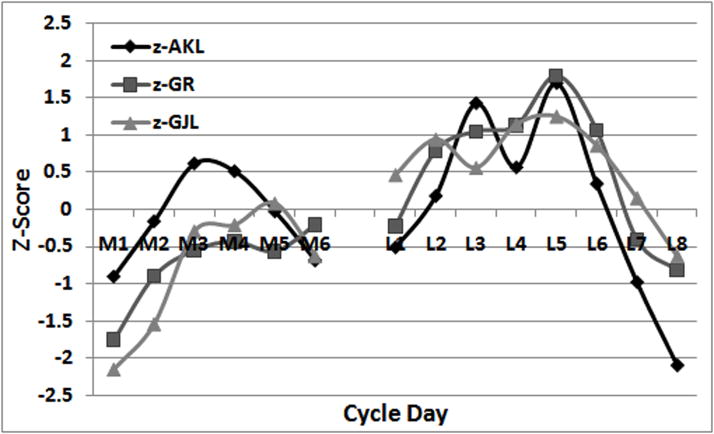
Plots of average means by day of AKL (Figure 2A), GR (Figure 2B), and GJL (Figure 2C) suggest a similar pattern of change across the menstrual cycle. Spearman correlations among these average daily means were 0.62 for AKL and GR, 0.61 for AKL and GJL, and 0.91 for GR and GJL. This similarity is more apparent in Figure 3, where the standardized values of the daily means for each variable are plotted on the same axes. The significant main effect for day (p<0.013) as reported in each of the repeated measures models for AKL, GR and GJL is readily apparent in these figures. All variables reach their average maximum on day L5. GR and GJL reach their average minimum daily value on M1, while AKL reaches its average minimum on L8. However, this similarity in the pattern of change in the average daily means for AKL, GR and GJL hides much individual-level variability in these values across the cycle. As can be seen from the standard deviations reported in Table 1, they are relatively large in magnitude compared to the means, particularly for GR and GJL.
Figure 2.
Group Means averaged across cycles for a) AKL (mm), b) GR (deg), c) GJL (score)
Figure 3.
Standardized values (z-scores) of daily means for AKL, GR, and GJL
To further characterize individual-level variability, we computed the proportion of females who reached their own maximum (minimum) laxity value on the same day that the group average maximum (minimum) was reached. Using data from cycle 1, 21.4% reached their maximum AKL on day L5 (the day of the average maximum), while an additional 30% reached maximum AKL within 2 days of L5. For GR, 24.3% reached their maximum on day L5, while an additional 57.1% reached maximum within 2 days of L5. Similar proportions (21.4% and 25.7%) reached their minimum AKL and GR, respectively, on the same day the average minimum value was reached (day L8 for AKL, day M1 for GR.), and an additional 31.4% and 27.1% reached their minimum AKL and GR, respectively, within 2 days of the average minimum. We did not carry out these computations for GJL, given the small range of scores on this variable for most subjects.
The Spearman correlation coefficients for the relationships between laxity variables at the individual level were 0.43 for AKL and GR, −0.07 for AKL and GJL, and 0.16 for GR and GJL. These Spearman correlations are considerably lower than those computed for the comparisons of average daily means at the group level, suggesting a relatively large amount of inter-individual variability in pattern of daily values of the three variables.
DISCUSSION
Our primary findings suggest that, on average, at the group level, similar and substantial cyclic changes occur in AKL, GR and GJL across the menstrual cycle. To our knowledge, this is the first study to demonstrate such similarity. Not unexpectedly, however, given what is known about menstrual cycle variability among women, the patterns and magnitudes of cyclic changes in these laxity variables were considerably different across females. This variability is apparent when comparing the significant but relatively small changes in daily mean values (Table 1) to the standard deviations (also in Table 1) and to the substantially larger average delta values (Table 2). This variability is further reinforced by the apparent dispersion in the time when individual females obtained their minimum or maximum laxity values compared to the day that the group mean minimum and maximums values occurred. Finally, the crude correlations between AKL, GR, and GJL at the individual level were comparatively low (−0.07 – 0.43). While one might interpret these variations to simply represent random error in the data, we do not believe this is the case for two reasons. First, the intra-individual changes observed in the majority of women were substantially larger than what could reasonably be explained by random error alone, as on average, intra-individual deltas typically exceeded 4–6 times the expected measurement error (0.3 to 0.4mm for AKL, 0.3–0.5° for GR, 0.2–0.3pts for GJL). Second, although the timing and magnitude of cyclic variations were quite variable between women, the pattern of change within an individual was quite consistent from one month to the next.
When comparing our findings in this larger cohort with previous research examining cyclic changes in AKL, some important observations are noted. First, the largest mean difference we observed between days is in line with previous studies, where the largest mean difference reported across measured time points range from 0.26 – 0.60mm in most studies2,16,18,25,26,28, but was higher in two others (0.8–1.4mm)17,19. Second, our results support earlier work18,29,30 that the magnitude of cyclic changes in AKL (as well as that now observed for GR and GJL) is quite variable across females; not all females experience substantial cyclic changes in their laxity values. When examining the maximum change in AKL across days within individuals, these changes average 1.8mm, but ranged from 0.7–3.7mm. This average change was higher than one study which reported an average change of 1.42mm (range not reported) in recreationally active females when compared across three time points32, but smaller than what was reported for a group of more sedentary females when measured daily across one complete menstrual cycle (3.2mm, range 1.5–5.3mm).29 Our findings also agree with previous work that the timing of the cyclic changes are quite variable among women, and may not always be confined to the peri-ovulatory and early luteal phases.29 This was recently demonstrated by Park et al.18 who compared laxity values in 26 females across the follicular, peri-ovulatory and mid luteal days of the cycle, and reported that 10 of 26 females recorded their highest laxity value in the follicular phase. In the present study, close to 50% of the subjects had their minimum or peak laxity value more than 2 days away from the day of mean maximum or minimum, and it was not uncommon to observe rising or declining laxity values during days of menses. Although these cyclic variations during menses have been suggested to be a delayed response to hormone concentration changes experienced during the mid to late luteal phase29, we were unable to examine this potential delayed response due to the limited days examined.
Collectively, these findings indicate that cyclic variations in joint laxity are not uniform among women, or in their timing across the menstrual cycle, and therefore the pattern of laxity changes may be unique to each female. This lack of uniformity across individuals and cycle phase may in part explain why some have observed cyclic changes in AKL16–19 while others have not2,25,27,28,33, particularly when compared in smaller samples using specific days to represent a given phase. Unfortunately, the large degree of individual variability in the magnitude and timing of these changes makes it difficult to develop appropriate study designs to capture these cyclic variations. While we tracked females over multiple days to better capture individual cyclic variations, these procedures are very expensive and time intensive, and thus lack routine application to research or clinical practice. Alternative methods must be developed if we are to capture this individual variability and advance our understanding of how differences in the magnitude of these cyclic variations may be linked to at risk knee joint neuromechanics and ACL injury. To that end, efforts are ongoing to determine the hormone profiles associated with these cyclic changes, and whether an algorithm can be developed to more readily identify those who experience substantial cyclic variations in joint laxity based on a few key laxity and hormone measures.
Although there is evidence to suggest that hormones may represent a primary pathway by which these laxity measures change over time29,30, other studies have not observed a relationship between hormone concentration changes and knee laxity changes when compared across specific days.18,26 Further, the lack of uniform changes in laxity across the menstrual cycle suggest that other factors may interact with hormones or otherwise act independently to mediate these changes across the cycle. Such factors may include cyclic variations in an individual’s weight or fluid retention, and changes in muscle stiffness properties across the cycle. While changes in weight or fluid retention have not been studied relative to changes in knee laxity, Eiling et al34 compared AKL and musculotendinous stiffness at 3 time points in the cycle. While they observed no change in AKL (they did not measure GR or GJL), they reported decreased musculotendinous stiffness when tested on a day near ovulation compared to tests performed on days of the cycle representing the first day of menses, mid follicular and mid luteal phases. Because GR represents a combination of ligament, muscular and capsular restraints, this measure may be more sensitive to musculotendinous stiffness changes than AKL (where the ACL is the primary restraint to this motion), thus explaining only a moderate correlation between these two measures (0.43). Given the myriad of factors that have the potential to change cyclically, more work is needed to understand the complete set of variables that are predictive of these changes.
Anatomical contributions to these measures may also influence the extent to which one laxity variable changes relative to another within an individual across the cycle. For example, the origin or cause of GR is thought to be due to a bony deformation of the proximal tibia (e.g. a reduced/reversed posterior tibial slope), excessive capsuloligamentous laxity, or a combination of both.35–37 If the cause is structural in nature, GR may be less sensitive to changes in hormone concentrations (or other potential factors varying cyclically) than AKL and GJL. Alternatively, if GR is primarily of capsuloligamentous origin, it seems reasonable that sex hormones would equally impact the soft tissues restraining knee hyperextension (GR), anterior tibial translation (AKL), as well as motions at other joints (GJL), resulting in more congruent changes across measures.
Finally, the nature of the measure may also contribute to low correlations in changes between the three measures. With respect to GJL, cyclic changes were relatively small and only observed in 32 of the 66 subjects (figure 1). As GJL is a criterion score rather than a continuous variable, changes may only be observed if the baseline range of motion is already close to the criterion value. For example, a 10° increase in 5th finger extension would only result in a change in score if the individual already had 80°+ of motion. This may very well explain the lower correlations noted between GJL with AKL (−0.07) and GR (0.16), as compared to AKL and GR (0.43), which both represent continuous variables. These findings may also call into question the validity of using GJL as a measure to identify cyclic variations in joint laxity. Of the 32 cases where cyclic changes in GJL were observed, 5th finger extension (26 of 32 cases), thumb abduction (14 of 32 cases), and trunk flexion (9 of 32 cases) most often contributed to these changes. Active elbow hyperextension and postural knee hyperextension rarely changed (2 of 32 cases), most likely because the capsuloligamentous structures at these joints were not stressed to the same extent. Based on these data, 5th finger extension may be the most sensitive GJL sub score to detect these cyclic variations. Even with these limitations, baseline line GJL still remains an important variable to consider in future studies given its reported association with ACL injury risk.6,9,13 It may be that because this measure is less likely to change across the cycle, it can offer a more stable measure of absolute joint laxity when measured prospectively and the time in the cycle cannot be controlled.
In summary, substantial, similar, and reproducible cyclic changes in AKL, GR and GJL were observed across the menstrual cycle, with the magnitude and pattern of cyclic changes varying considerably among females. However, the magnitude of change in joint laxity that is required to modify an individual’s joint function and injury risk profile remains unknown, and work is ongoing to understand how both absolute baseline and cyclic variations in joint laxity influence weight bearing knee joint neuromechanics. Should cyclic changes in AKL, GR and GJL be of sufficient magnitude to alter an individual’s knee joint neuromechanics, it will be important to account for these cyclic changes in future injury risk studies. It will also be important to better understand the specific physiological mechanisms that mediate knee joint laxity changes across the cycle, as the effects of sex hormones (and other mediating factors) on ACL structure, metabolism and mechanical properties, as well as that of other surrounding soft tissue restraints, are currently unknown.38,39
Acknowledgments
This project was supported by Award Number R01AR053172 from the NIH-NIAMS, and through a cooperative agreement [NICHD/NIH U54 HD28934] as part of the Specialized Cooperative Centers Program in Reproductive Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
References
- 1.Griffin LY, Albohm MJ, Arendt EA, et al. Update on ACL Prevention: Theoretical and Practical Guidelines. Am J Sports Med. 2006;34:1512–1532. [Google Scholar]
- 2.Beynnon BD, Bernstein I, Belisle A, et al. The Effect of Estradiol and Progesterone on Knee and Ankle Joint Laxity. Am J Sports Med. 2005;33:1298–1304. doi: 10.1177/0363546505275149. [DOI] [PubMed] [Google Scholar]
- 3.Rosene JM, Fogarty TD. Anterior tibial translation in collegiate athletes with normal anterior cruciate ligament integrity. J Athl Train. 1999;34:93–98. [PMC free article] [PubMed] [Google Scholar]
- 4.Rozzi SL, Lephart SM, Gear WS, et al. Knee joint laxity and neuromuscular characteristics of male and female soccer and basketball players. Am J Sports Med. 1999;27:312–319. doi: 10.1177/03635465990270030801. [DOI] [PubMed] [Google Scholar]
- 5.Trimble MH, Bishop MD, Buckley BD, et al. The relationship between clinical measurements of lower extremity posture and tibial translation. Clin Biomech. 2002;17:286–290. doi: 10.1016/s0268-0033(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 6.Uhorchak JM, Scoville CR, Williams GN, et al. Risk factors associated with non-contact injury of the anterior cruciate ligament. Am J Sports Med. 2003;31:831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen AD, Shultz SJ. Sex Differences in Lower Extremity Posture. J Orthop Sports Phys Ther. 2007;37:389–398. doi: 10.2519/jospt.2007.2487. [DOI] [PubMed] [Google Scholar]
- 8.Grana WA, Moretz JA. Ligamentous laxity in secondary school athletes. JAMA. 1978;240:1975–1976. [PubMed] [Google Scholar]
- 9.Scerpella TA, Stayer TJ, Makhuli BZ. Ligamentous laxity and non-contact anterior cruciate ligament tears: A gender based comparison. Orthopaedics. 2005;28:656–660. doi: 10.3928/0147-7447-20050701-12. [DOI] [PubMed] [Google Scholar]
- 10.Larsson LG, Baum J, Mudholkar GS. Hypermobility: features and differential incidence between the sexes. Arthrit Rheum. 1987;30:1426–1430. doi: 10.1002/art.1780301216. [DOI] [PubMed] [Google Scholar]
- 11.Kramer LC, Denegar CR, Buckley WE, et al. Factors associated with anterior cruciate ligament injury: history in female athletes. J Sports Med Phys Fit. 2007;47:446–454. [PubMed] [Google Scholar]
- 12.Loudon JK, Jenkins W, Loudon KL. The relationship between static posture and ACL injury in female athletes. J Orthop Sports Phys Ther. 1996;24:91–97. doi: 10.2519/jospt.1996.24.2.91. [DOI] [PubMed] [Google Scholar]
- 13.Ramesh R, VonArx O, Azzopardi T, et al. The risk of anterior cruciate ligament rupture with generalised joint laxity. J Bone Jt Surg. 2005;87-B:800–803. doi: 10.1302/0301-620X.87B6.15833. [DOI] [PubMed] [Google Scholar]
- 14.Woodford-Rogers B, Cyphert L, Denegar CR. Risk factors for anterior cruciate ligament injury in high school and college athletes. J Athl Train. 1994;29:343–346. [PMC free article] [PubMed] [Google Scholar]
- 15.Myer GD, Ford KR, Paterno MV, et al. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36:1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deie M, Sakamaki Y, Sumen Y, et al. Anterior knee laxity in young women varies with their menstrual cycle. International Orthopaedics. 2002;26:154–156. doi: 10.1007/s00264-001-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitz NA. Hormonal changes throughout the menstrual cycle and increased anterior cruciate ligament laxity in females. J Athl Train. 1999;343:144–149. [PMC free article] [PubMed] [Google Scholar]
- 18.Park SK, Stefanyshyn DJ, Loitz-Ramage B, et al. Changing Hormone Levels During the Menstrual Cycle Affect Knee Laxity and Stiffness in Healthy Female Subjects. Am J Sports Med. 2009;37:588–598. doi: 10.1177/0363546508326713. [DOI] [PubMed] [Google Scholar]
- 19.Shultz SJ, Kirk SE, Sander TC, et al. Sex differences in knee laxity change across the female menstrual cycle. J Sports Med Phys Fit. 2005;45:594–603. [PMC free article] [PubMed] [Google Scholar]
- 20.Arendt EA, Bershadsky B, Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. Journal of gender specific medicine. 2002;5:19–26. [PubMed] [Google Scholar]
- 21.Beynnon BD, Johnson RJ, Braun S, et al. The relationship between menstrual cycle phase and anterior cruciate ligament injury: A case-control study of recreational alpine skiers. Am J Sports Med. 2006;34:757–764. doi: 10.1177/0363546505282624. [DOI] [PubMed] [Google Scholar]
- 22.Myklebust G, Engebretsen L, Braekken IH, et al. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sports Med. 2003;13:71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Slauterbeck JR, Fuzie SF, Smith MP, et al. The menstrual cycle, sex hormones, and anterior cruciate ligament injury. J Athl Train. 2002;37:275–280. [PMC free article] [PubMed] [Google Scholar]
- 24.Wojtys EM, Huston L, Boynton MD, et al. The effect of menstrual cycle on anterior cruciate ligament in women as determined by hormone levels. Am J Sports Med. 2002;30:182–188. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]
- 25.Belanger MJ, Moore DC, Crisco JJ, et al. Knee laxity does not vary with the menstrual cycle, before or after exercise. Am J Sports Med. 2004;32:1150–1157. doi: 10.1177/0363546503261360. [DOI] [PubMed] [Google Scholar]
- 26.Hertel J, Williams NI, Olmstead-Kramer LC, et al. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:817–822. doi: 10.1007/s00167-006-0047-4. [DOI] [PubMed] [Google Scholar]
- 27.Pollard CD, Braun B, Hamill J. Influence of gender, estrogen and exercise on anterior knee laxity. Clin Biomech. 2006;21:1060–1066. doi: 10.1016/j.clinbiomech.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Van Lunen BL, Roberts J, Branch D, et al. Association of menstrual cycle hormone changes with anterior cruciate ligament laxity measurements. J Athl Train. 2003;38:298–303. [PMC free article] [PubMed] [Google Scholar]
- 29.Shultz SJ, Sander TC, Kirk SE, et al. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36:1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shultz SJ, Gansneder BG, Sander TC, et al. Absolute Hormone Levels Predict the Magnitude of Change in Knee Laxity Across the Menstrual Cycle. J Orthop Res. 2006;24:124–131. doi: 10.1002/jor.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Annals Rheum Dis. 1973;32:413–418. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SK, Stefanyshyn DJ, Ramage B, et al. Alterations in Knee Joint Laxity During the Menstrual Cycle in Healthy Women Leads to Increases in Joint Loads During Selected Athletic Movements. Am J Sports Med. 2009;37:1169–1177. doi: 10.1177/0363546508330146. [DOI] [PubMed] [Google Scholar]
- 33.Karageanes SJ, Blackburn K, Vangelos ZA. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin J Sports Med. 2000;10:162–168. doi: 10.1097/00042752-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Eiling W, Bryant AL, Petersen W, et al. Effects of menstrual cycle hormone fluctuations on musculoskeletal stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15:126–132. doi: 10.1007/s00167-006-0143-5. [DOI] [PubMed] [Google Scholar]
- 35.Dejour D, Bonin N, Locatelli E. Tibial antirecurvatum osteotomies. Oper Tech Sports Med. 2000;8:67–70. [Google Scholar]
- 36.Laura G, Berruto M, Bianchi M. Genu recurvatum following distal epiphysiodesis of the femur: X-ray evaluation and therapeutical approach. Ital J Orthop Traumatol. 1992;18:505–514. [PubMed] [Google Scholar]
- 37.Piriou P, Garreau C, Combelles F, et al. Original technique for the treatment of ligament-related genu recurvatum: preliminary results. Knee Surg Sports Traumatol Arthrosc. 2002;10:260–264. doi: 10.1007/s001670100243. [DOI] [PubMed] [Google Scholar]
- 38.Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42:394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shultz SJ, Schmitz RJ, Nguyen AD. ACL Injuries: The Gender Bias: Research Retreat IV April 3 to 5, 2008 Greensboro, NC. J Athl Train. 2008;43:530–537. doi: 10.4085/1062-6050-43.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]