Abstract
This manuscript describes the responses and correlates of outpatients with schizophrenia spectrum disorders to a tool designed to measure comprehension before obtaining informed consent for research participation. We used the Evaluation to Sign Consent (ESC) Form to document comprehension in 100 outpatients as part of their consent to participate in a study of an exercise intervention. The exercise intervention study is ongoing; these findings suggest that the ESC is a feasible and acceptable approach for documenting comprehension of research procedures prior to obtaining informed consent. Age 49 years and older and the receipt of intramuscular antipsychotic medication predicted the need for additional assistance to successfully complete the ESC (χ2 = 8.29, p = 0.016). Nurse researchers should consider documenting comprehension with the ESC due to its availability, time efficiency and utility.
Keywords: schizophrenia, schizoaffective disorder, research, ethics, informed consent
There is debate in the literature regarding the ability of persons with schizophrenia spectrum disorders (SSDs) to provide informed consent for research. The potential effects of the cognitive deficits associated with SSDs on the processes involved in providing meaningful consent for research participation, necessitate additional attention to documentation of research recruitment procedures, in order to ensure the adequacy of such consents 1. Research is needed on processes to protect human rights while fulfilling our obligation to conduct the research necessary to gather information to provide a foundation for evidence-based treatments. The purpose of this report is to describe the responses and correlates of the Evaluation to Sign Consent Form (ESC) 2 in a group of outpatients with SSDs. The research questions were:
Is the ESC acceptable to persons with SSDs?
What is the time required for administration of the ESC to persons with SSDs?
What are the responses of persons with SSDs to individual ESC items?
Do differences exist between persons with SSDs who require prompting on the ESC, and persons with SSDs who do not require prompting?
What characteristics of persons with SSDs predict the need for prompts on the ESC?
SSDs include both schizophrenia and schizoaffective disorder. There is considerable diagnostic overlap between the disorders; both include positive symptoms such as hallucinations and delusions 3. Further, recent research indicates that persons with these disorders share significant similarities on basic cognitive measures such as executive functioning 4 and associative learning 5. The presence of these deficits raises questions regarding whether persons with SSDs possess the capacity to provide ethically valid informed consent for research 2, 6. Few published studies have examined research consent capacity in schizophrenia, ours is only the second investigation to examine research informed consent in persons schizoaffective disorder.
Review of Literature
Informed consent for research may be seen as part of a broader concept known as decisional capacity. Decisional capacity may be related to research participation, treatment planning or everyday decision-making, and involves four components 7. The first is whether the person can comprehend the nature of the information relevant to the consent. Second is whether the person understands how the information applies to their condition and third if the person can reason through the information provided. These three aspects of decisional capacity encompass the comprehension aspect of informed consent. The fourth element of decisional capacity is related to the voluntary aspect of informed consent, and addresses the absence of actual or implied coercion.
The primary distinction between decisional capacity and informed consent is that decisional capacity refers only to the person, patient or research participant whose capacity is being described. In contrast, informed consent includes a component of providing full, relevant information, which is the responsibility of the researcher.
Early efforts to describe the ability of persons with schizophrenia to provide valid consent for research measured factual understanding of study procedures. Davidhizar and Wehlage 8 tested comprehension of research procedures by asking 12 hospitalized participants with schizophrenia to repeat and manipulate consent-related information. They concluded that all participants in the study were able to evidence a choice by expressing an interest in taking part in the study, co-operating with the interviews, and responding to all of the questions. However, there was variability in their ability to manipulate information and appreciate the applicability of the information to their situation.
Carpenter, et al 9 assessed capacity for research consent in 30 participants with schizophrenia and 24 healthy controls using the MacArthur Competence Assessment Tool-Clinical Research Version 10 and the ESC 2, and reported that performance on the two measures was moderately related to psychotic symptoms but strongly correlated with measures of cognition.
Moser et al 6 reported similar results as Carpenter, et al 9 when they used the MAC Cat-CR and the ESC to compare research consent capacity between 25 persons with schizophrenia and 25 persons with HIV. Persons with schizophrenia scored lower than those with HIV on the factual understanding of information presented and appreciation of the personal applicability of the information as measured by the MAC Cat-CR; 80% of participants with schizophrenia compared to 96% of participants with HIV demonstrated adequate understanding using the ESC.
Candilis and colleagues 11 compared the research consent performance of 52 persons with SSDs and 52 healthy controls on the MAC-Cat-CR. Similar to Carpenter 9, this investigation reported that cognitive capacity exerted the greatest impact upon decisional capacity.
We recently used the ESC to document research consent capacity in 29 outpatients with schizophrenia 12. Participants living in supervised housing were significantly more likely to require prompts to recall details after study explanations than those living alone (χ2 = 9.4, p = 0.024). Participants prescribed two antipsychotic medications were significantly more likely to require prompting than those prescribed only one (χ2 =5.12, p = 0.023).
This group of studies is limited by small sample sizes. In addition, the use of differing measures and procedures to measure decisional capacity makes between-study comparisons difficult. We found only one published study addressing capacity for research consent in persons with schizoaffective disorder. The purpose of this report is to describe responses and correlates of the ESC in a group of outpatients with SSDs.
Methods
This descriptive study is reports partial data from an ongoing project examining the responses of community dwelling persons with SSDs to an exercise intervention. This manuscript reports the responses and correlates of 100 outpatients to the ESC, which was completed before obtaining informed consent for the exercise intervention study.
Sample
Participants were recruited from outpatients receiving care at a Community Mental Health Center (CMHC) located in the Southeast. The CMHC is a regional, not for profit integrated system providing mental health services in 19 counties. Teams of psychiatrists, Master's level clinicians, nurses and Bachelor's level professionals provide comprehensive, individualized treatment to clients and families. The CMHC offers case management, outpatient, psychosocial rehabilitation, prevention, residential treatment and employment services to over 350 persons with SSDs. The ESC 2 was used to document the participant's ability to provide informed consent for a study of exercise intervention and was completed prior to signing consent forms for the exercise intervention study.
Prior to data collection, University IRB approval as well as the approval of the research committee at the CMHC, were obtained. Inclusion criteria for the exercise intervention study were: 1) a chart diagnosis of schizoaffective disorder or schizophrenia, any subtype, according to the criteria described in the Diagnostic and Statistical Manual for Mental Disorders 3, 2) English speaking, and 3) medical clearance for moderate exercise in writing from primary care provider. Exclusion criteria were: 1) mental retardation, 2) developmental delay, 3) uncorrected visual or hearing impairments, 4) hospitalization within the past 12 months for angina pectoris, myocardial infarction, or cardiac surgery of any kind, 5) congestive heart failure, 6) cardiac pacemaker, 7) heart rate > 100 or < 50 at rest, 8) uncontrolled hypertension defined as a blood pressure exceeding 140/90 on 3 consecutive readings despite adequate treatment, 9) history of spinal or hip fractures or hip or knee arthroplasty, and 10) neuromuscular or orthopedic limitations to normal, unassisted ambulation.
The CMHC's Notice of Privacy Practices, (signed by all patients), allows disclosure of protected health information for research, authorizing the initial chart reviews and communications required to identify potential participants. After potential participants were identified via chart review, researchers verified inclusion criteria, then approached potential participants regarding study participation while they were at the CMHC for regularly scheduled treatment appointments. Researchers met with interested persons in private offices at the recruitment site to explain the exercise intervention study, complete the ESC and obtain written informed consent.
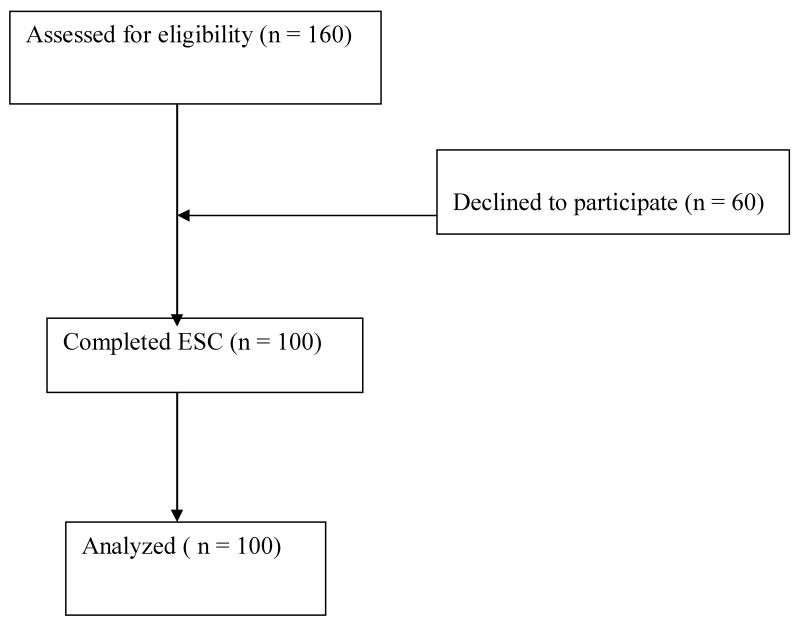
We approached a convenience sample of 160 persons who met all study participation criteria over 16 months. One hundred one participants agreed and 60 declined. Nineteen of the 60 decliners failed to specify a reason. Of those specifying a reason for declining, the most common reasons given were being too busy with other activities or being unable to perform the exercises due to perceived physical limitations. See Figure 1.
Figure 1.
Recruitment of persons with Schizophrenia Spectrum Disorders.
Measurement Tool
The ESC 2 is a 5-item questionnaire that assesses the comprehension of information required to provide ethically valid consent to participate in research. Although the ESC has been used in clinical research6,9,12, only one study has reported evidence of its reliability and validity. Resnick et al 13 administered the ESC to 346 mostly female Caucasian nursing home residents with moderate cognitive impairment according to the Mini-Mental State Examination 14. Validity of the ESC was examined based upon unidimensionality, the fit of each of the 5 items to the overall tool and item mapping. The ESC was unidimensional based upon a principal component factor analysis of the residuals. Infit statistics for all items ranged from 0.81-1.09 13; the acceptable range for these statistics is 0.6-1.4 15. Item mapping indicated a good spread of items across the continuum of ability to successfully complete the ESC. Cronbach's alpha was 0.81 and inter-rater reliability using a Pearson correlation, was 0.81 13.
The ESC is administered after education relating to study purpose, risks and procedures, but before the formal consent process; thus the ESC is specific to the study for which the potential participant is being considered. The tool includes specific cutoffs to define adequate understanding, beyond which informed consent should not be sought. Item 1(the only subjective item) reads “Alert and able to communicate with examiner”. Only if the answer to Item 1 is affirmative, is the ESC completed. The remaining items require potential participants to provide verbal information regarding study activities, as follows:
Item 2 - list any risks from study participation.
Item 3 - list at least 2 behaviors required as part of study participation.
Item 4 - explain the procedure for study withdrawal, and
Item 5 - identify procedures to follow should distress or discomfort occur in the course of the study 2.
Procedure
Initially, the complete study was explained in person in a private office including potential risks, procedures, financial remuneration and contact information for the researchers. Potential participants were given an opportunity to ask questions. Next, the ESC was administered and responses recorded by research staff. Only after successful completion of all items on the ESC was written informed consent obtained. Sociodemographic data (diagnosis, age, race, sex, and living arrangement) and a list of all prescribed medications were collected via record review immediately following informed consent.
If any ESC item was answered incorrectly, the researcher prompted by repeating the information once, then asked the question a second time. If any item was answered incorrectly the second time, informed consent was delayed by at least 24 hours, and a second trial of the ESC was begun using the identical procedures described above. During the second trial, if any potential participant answered any item incorrectly, informed consent was not obtained from that person.
Results
Participants ranged in age from 21-72 with a mean age of 46.9 years (SD = 10.2). Most participants were diagnosed with schizoaffective disorder (n = 72). The majority were female (n = 54), Caucasians (n = 56) living with family (n = 45). Numbers of medications prescribed ranged from 1-14 with a mean of 7 different medications (SD = 3.6). The most commonly prescribed antipsychotic medications were oral atypicals (n = 70). Seventy-seven persons were prescribed at least one nonpsychiatric medication, most commonly antihypertensives (n = 36), proton pump inhibitors (n = 32), or lipid lowering agents (n = 28). See Table 1.
Table 1.
Characteristics of persons with SSDs (N = 100).
| Characteristic | n (%) |
|---|---|
| Diagnosis | |
| Schizoaffective | |
| Disorder | 72(72) |
| Schizophrenia | 28 (28) |
| Sex | |
| Female | 54(54) |
| Male | 46(46) |
| Race | |
| Caucasian | 56(56) |
| African | |
| American | 43(43) |
| Asian | 1(1) |
| Living Arrangement | |
| Alone | 36(36) |
| With family | 45(45) |
| With paid | |
| Caregiver | 19(19) |
| Prescribed Medications | |
| Oral atypicals | 70(70) |
| Oral typicals | 5(5) |
| Depot Typicals | 39(39) |
| Depot Atypicals | 10(10) |
| Antidepressants | 47(47) |
| Mood stabilizers | 43(43) |
| Antianxiety | 27(27) |
| Antiparkinson | 56(56) |
| Hypnotics | 13(13) |
| Other* | 77(77) |
Note Includes medications prescribed for physical illnesses, most commonly antihypertensives (n = 36), proton pump inhibitors (n = 32), and lipid lowering agents (n = 28).
The ESC was well tolerated and acceptable to participants. Total time to complete the ESC was less than 5 minutes in all cases. All participants were judged “alert and able to communicate with examiner”, on item # 1. Without prompting, ninety-five percent of participants could list study risks correctly; eighty two percent could correctly list two study activities; ninety-one percent correctly identified study withdrawal procedure and eighty six percent identified procedures if distress was experienced during the study. After prompting, one hundred participants successfully completed all ESC items. One participant, a 47 year old female diagnosed with schizophrenia, failed to provide correct information after her two prompts; due to a scheduling conflict, researchers were unable to approach her for a second trial.
Sixty-five participants (65 %) correctly answered all items on first attempt. Twenty-one participants required prompts on one item, an additional fourteen participants required prompts on 2 items. The mean age of those needing prompts was 48.8 years; the mean age of those not needing prompts was 45.9. Independent sample t-test of this age differential was not significant.
Of the thirty-five participants requiring prompts, twenty one were prompted on one item and fourteen were prompted on two items. Five participants needed prompting on item # 2; twenty on item # 3, ten on item # 4, and 14 participants needed prompting on item # 5. Participants who needed prompts on two items were mostly male (n = 8, 57%) and Caucasian (n = 9, 64%); four were African American (28.5.3%) and one was Asian (7.5%). Their average age was 42.9 years. Characteristics of those needing prompts versus those not needing prompts are presented in Table 2.
Table 2.
Comparison of persons with SSDs not needing prompts (n = 65) versus those needing prompts (n = 35) on the ESC.
| Characteristic | Participants not needing prompts (n =65) | Participants needing prompts (n = 35) |
|---|---|---|
| n (%) | n (%) | |
| Diagnosis | ||
| Schizoaffective | ||
| Disorder | 49(75.4) | 23(65.7) |
| Schizophrenia | 16(24.6) | 12 (34.3) |
| Sex | ||
| Female | 37(56.9) | 17(48.6) |
| Male | 28(43.1) | 18(51.4) |
| Race | ||
| Caucasian | 37(56.9) | 19(54.3) |
| African | ||
| American | 28(43.1) | 15(42.8) |
| Asian | 0(0) | 1(2.9) |
| Living Arrangement | ||
| Alone | 22(33.9) | 14(40) |
| With family | 32(49.2) | 13(37.1) |
| With paid | ||
| Caregiver | 11(16.9) | 8(22.9) |
| Prescribed Medications | ||
| Oral atypicals | 50(76.9) | 20(57.1) |
| Oral typicals | 4(6.2) | 1(2.9) |
| Depot Typicals | 24(36.9) | 15(42.9) |
| Depot Atypicals | 5(7.7) | 5(14.3) |
| Antidepressants | 31(47.7) | 16(45.7) |
| Mood stabilizers | 27(41.5) | 16(45.7) |
| Antianxiety | 16(24.6) | 11(31.4) |
| Antiparkinson | 24(36.9) | 22(62.8) |
| Hypnotics | 10(!5.4) | 3(8.5) |
| Other* | 50(76.9) | 27(77.1) |
Note Includes medications prescribed for physical illnesses, most commonly antihypertensives (n = 36), proton pump inhibitors (n = 32), and lipid lowering agents (n = 28).
Due to the level of measurement nonparametric tests were used to measure associations between observed variables and the need for prompts. To permit Chi square analysis, age was categorized as either 48 and under or 49 and over and educational level was characterized as less than high school, or high school graduate and over. Table 3 summarizes the results of this analysis. Persons 49 years of age and older were significantly more likely to require prompting than those aged 48 and younger (p < 0.05). Thirty nine percent (n =- 11) of participants with schizophrenia required prompts, in contrast to 31%(n = 22) of participants with schizoaffective disorder (N/S). A higher percentage of males (n = 18, 51.4%) required prompting than females (n = 17, 48.6%) but this difference was not statistically significant.
Table 3.
Chi Square tests of association in persons with SSDs (N = 100).
| Needed Prompt (yes/no) | Age(48 & under/49 & over) | Sex | Race | Living Arrangement | |
|---|---|---|---|---|---|
| Needed prompt (yes/no) | 1.0 | ||||
| Age(48 & Under/49 & over) | 5.78* | 1.0 | |||
| Sex | 2.86 | 0.008 | 1.0 | ||
| Race | 1.09 | 2.01 | 1.37 | 1.0 | |
| Living Arrangement | 2.60 | 14.28** | 14.58** | 1.69 | 1.0 |
Note: p < 0.05
p <0.01
We used an ordinal logistic regression to test the predictive validity of various participant characteristics upon the numbers of prompts required to successfully complete the ESC. The model including age (48 and younger or 49 and over) and the prescription of intramuscular antipsychotic medication demonstrated predictive capability for the number of prompts required (χ2 =8.29, df 2, p = 0.016). The age category “48 and younger” was associated with fewer prompts on the ESC as compared to the “49 and older” category (p = 0.043). The receipt of intramuscular antipsychotic medication was associated with greater numbers of prompts compared with not receiving intramuscular medication (p = 0.047). See Table 4.
Table 4.
Ordinal logistic regression analysis: numbers of prompts in persons with SSDs (N = 100).
| Item | Estimate | Std Error | Wald | df | p |
|---|---|---|---|---|---|
| Intramuscular Medication (yes) | 0.893 | 0.438 | 4.15 | 1 | 0.03 |
| Age (48 & younger) | -.892 | 0.429 | 4.32 | 1 | 0.04 |
Discussion
We used the ESC to document comprehension as part of the informed consent procedure for a research study of exercise intervention in persons with SSDs. This study is among the first to describe responses and correlates to the ESC in persons with schizoaffective disorder. Our data indicate that persons with SSDs are willing to respond to questions documenting comprehension, and the majority of this sample did so without difficulty.
Combs et al 16 and Jeste et al 17 observed improvement in research informed consent in persons with schizophrenia after cues were provided during enhanced consent procedures; similar to our observation that persons with SSDs were able to provide correct answers to queries about study procedures after prompting. Our prompting procedure is similar to the corrective feedback condition of Eyler and colleagues 18; however in contrast to our observations, those investigators found no difference between corrective feedback and standard consent procedure. This may be because all participants in their sample were living in professionally supervised care homes, while only 19% of our sample did so. Thus, their participants may have responded less well to corrective feedback, owing to memory or other factors that made placement in supervised housing necessary.
In contrast to this investigation, our prior study reported that participants prescribed two antipsychotic medications were significantly more likely to require prompts than those prescribed only one, and that persons living in supervised housing were significantly more likely to require prompts than those living alone 12. The differing diagnoses in the two investigations and small sample size in the prior inquiry are possible explanations for these differences.
Our finding of a significant association between intramuscular medication and the need for prompts raises questions about the influence of symptomatology upon research consent capacity. Since intramuscular medications are usually reserved for persons with high symptom levels, one might surmise an association between high symptom level and reduced consent capacity as demonstrated by the ESC. However, several other researchers have concluded that cognition, not symptomatology, exerts the greatest influence upon consent capacity 6, 11, 19. This finding may be partially explained by our sample characteristics. The intramuscular formulations prescribed to our participants were mostly typical antipsychotics; these participants were not receiving the cognitive benefits associated with atypical antipsychotic medications 19, 20. This point raises important ethical considerations in treatment decisions regarding choice of antipsychotic medication and the potential cognitive benefits of atypicals.
Selection bias may threaten the internal validity of this study, as we experienced a 37.5% rate of refusal to participate. This is similar to rates of refusal in our other studies of both inpatients and outpatients with SSDs 21-23. Because the majority of participants failed to state a reason for refusal, we can only speculate about this state of affairs. If those with higher degrees of instability refused, the study may be biased toward persons who were more able to successfully complete the ESC. Our small sample size increases the risk of Type II error, introducing the possibility of nonidentification of significant relationships due to lack of power. The ESC is limited in that it addresses only the comprehension aspect of informed consent, however its strengths are its ease of use and efficiency of administration. This is only the second published study to describe responses and correlates of a research comprehension measure (the ESC) in persons with schizoaffective disorder; hence, results must be viewed with caution.
Conclusions and Future Directions
This study is among the first to examine comprehension for research consent in persons with schizoaffective disorder. We recommend the ESC be considered to document comprehension before research is conducted with persons with SSDs. It is brief, informative and well tolerated. It is ethically advisable for researchers to take steps to enhance participant comprehension in order to provide appropriate protection to this vulnerable group of research participants. However, information is needed on other issues as well. Researchers are in need of descriptive information to assist in identifying in advance those subgroups of persons with SSDs that might need additional support to provide meaningful informed consent. In addition, longitudinal data are needed to document possible fluctuations in consent capacity. Informed consent for research is currently conceptualized as a one-time assessment, which may not be ideal for persons with SSDs due to the nature of these illnesses and the likelihood of symptom variability over time.
Acknowledgments
This study was supported by a grant from the National Institutes of Health, R03 MH079047-02
Footnotes
(Parts of this text first presented as “Ethics of Research Informed Consent in Persons with Schizophrenia Spectrum Disorders” at The International Centre for Nursing Ethics Conference “Nursing Ethics and Health Care Policy: Bridging Local, National and International Perspectives”, held at Yale University, New Haven, CT, July 17-19, 2008)
References
- 1.Anderson KK, Mukherjee SD. The need for additional safeguards in the informed consent process in schizophrenia research. JMedEthics. 2006;33(11):647–650. doi: 10.1136/jme.2006.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeRenzo EG, Conley RR, Love R. Assessment of capacity to give consent to research participation: State-of –the-art and beyond. JHealthCareLawPolicy. 1998;1:66–87. [PubMed] [Google Scholar]
- 3.American Psychiatric Association (APA) Diagnostic and statistical Manual of mental disorders. 4th. Washington, DC: Author; 2000. text revision. [Google Scholar]
- 4.Premkumar P, Cooke MA, Fannon D, Peters S, Michel TM, Aasen I, Murray RM, Kuipers E, Kumari V. Misattribution bias of threat related facial expression is related to a longer duration of illness and poor executive function in schizophrenia and schizoaffective disorder. European Psychiatry. 2008;23(1):14–19. doi: 10.1016/j.eurpsy.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacchetti F, Galluzzo A, Panariello A, Parrinello G, Cappa SF. Self-ordered pointing and visual conditioning associated learning tasks in drug free schizophrenia spectrum disorder patients. BMCPsychiatry. 2008;23(8):6. doi: 10.1186/1471-244X-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser DJ, Schultz SK, Arndt S, Benjamin ML, Fleming FW, Brems MN, Paulsen JS, Appelbaum PS, Andreasen NC. Capacity to provide informed consent for participation in schizophrenia and HIV research. AmJPsychiatry. 2002;159:1201–1207. doi: 10.1176/appi.ajp.159.7.1201. [DOI] [PubMed] [Google Scholar]
- 7.Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: An overview. SchizophrBull. 2006;32(1):121–8. doi: 10.1093/schbul/sbj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidhizar R, Wehlage D. Can the client with chronic schizophrenia consent to nursing research? JAdvNursing. 1984;9:381–390. doi: 10.1111/j.1365-2648.1984.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter WT, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS. Decisional capacity for informed consent in schizophrenia research. ArchGenPsychiatry. 2000;57(6):533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum PS, Grisso T. The MacArthur Competence Assessment Tool-Clinical Research. Sarasota FL: Professional Resource Press; 1996. [Google Scholar]
- 11.Candilis PJ, Fletcher KE, Geppert CMA, Lidz CW, Appelbaum PS. A direct comparison of research decision making capacity: Schizophrenia/schizoaffective, medically ill and non-ill subjects. SchizophrResearch. 2007;99(1-3):350–358. doi: 10.1016/j.schres.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beebe LH, Smith K. Examining informed consent in persons with schizophrenia. IssuesMentHealthNurs. 2008;29(4):397–41. doi: 10.1080/01612840801904472. [DOI] [PubMed] [Google Scholar]
- 13.Resnick B, Gruber-Baldini AL, Pretzer-Aboff I, Galik E, Buie VC, Russ K, Zimmerman S. Reliability and validity of the evaluation to sign consent measure. TheGerontologist. 2007;47(1):69–77. doi: 10.1093/geront/47.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Folstein M, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. JPsychiatrRes. 1975;2:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Smith E, Smith RM. Introduction to Rasch Measurement. Maple Grove, MN: JAM Press; 2004. [Google Scholar]
- 16.Combs DR, Adams SD, Wood TD, Basso MR, Gouvier WD. Informed consent in schizophrenia: the use of cues in the assessment of understanding. SchizophrResearch. 2005;77(1):59–63. doi: 10.1016/j.schres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Jeste DV, Palmer BW, Golshan S, Eyler LT, Dunn LB, Meeks T, Glorioso D, Fellows I, Kraemer H, Appelbaum PS. Multimedia consent for research in people with schizophrenia and normal subjects: A randomized controlled trial. SchizophrBull. 2009;35(4):7719–729. doi: 10.1093/schbul/sbm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyler LT, Mirzakhanian H, Jeste DV. A preliminary study of interactive questioning methods to assess and improve understanding of informed consent among patients with schizophrenia. SchizophrResearch. 2005;75(2-3):193–198. doi: 10.1016/j.schres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. BiolPsychiatry. 2002;51:969–971. doi: 10.1016/s0006-3223(02)01399-9. [DOI] [PubMed] [Google Scholar]
- 20.Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, Miller AL. Does cognitive function improve with quetiapine in comparison to haloperidol? SchizophrResearch. 2002;53:239–248. doi: 10.1016/s0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 21.Beebe LH. Community nursing support for schizophrenic clients. ArchPsychiatrNur. 2001;15(5):214–222. doi: 10.1053/apnu.2001.27018. [DOI] [PubMed] [Google Scholar]
- 22.Beebe LH, Tian L. TIPS: Telephone Intervention – Problem Solving for persons with Schizophrenia. IssuesMentHlthNurs. 2004;25(3):317–329. doi: 10.1080/01612840490274804. [DOI] [PubMed] [Google Scholar]
- 23.Beebe LH, Tian L, Goodwin A, Morris N, Swant-Allen S, Kuldau J. Effects of exercise on the mental and physical health parameters of persons with schizophrenia. IssuesMentHlthNurs. 2005;26(6):661–676. doi: 10.1080/01612840590959551. [DOI] [PubMed] [Google Scholar]